Professional Documents
Culture Documents
Manufacturing Butyl Acetate Ester
Uploaded by
sumitOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Manufacturing Butyl Acetate Ester
Uploaded by
sumitCopyright:
Available Formats
Subject: P.T. Expt.
No:7
EXPERIMENT NO. 7
MANUFACTURING OF BUTYL ACETATE BY ESTERIFICATION REACTION
1.0 Aim of Experiment:
To manufacture butyl acetate ester.
2.0 Context:
Subject: Petrochemical Technology.
2.1 Chapter:
Unit process in refineries.
2.2 Topic:
Esterification
3.0 Prior Concepts:
Carboxylic derivatives, types of esters.
4.0 New Concept:
Butyl Ester production method.
5.0 Learning Objectives:
Manufacture Butyl Acetate Ester by Esterification process.
5.1 Theory:
Esters are derivatives of carboxylic acid. Esterification of carboxylic acid is
done with the alcohol in presence of sulphuric acid. Esterification reaction is one of the
most important industrial reactions. Esters are widely used in chemical industry such as a
solvent for plastics, liquors, resins, gums, and coatings. Butyl acetate is commonly
synthesized through esterification of acetic acid with n -butanol in the presence of a
suitable acid catalyst by reversible and kinetically controlled reaction. Esters are a class
of organic compounds which, unlike many organics, have pleasant odors. In fact, many
of the "artificial flavors" used in food products are esters - very pure esters. Esters occur
naturally but can also be synthesized in the lab. To synthesize an ester, you must start
with two other organic compounds - an alcohol and an acid. The ester is formed when
dehydration occurs. The alcohol and acid react to form the ester and a water molecule.
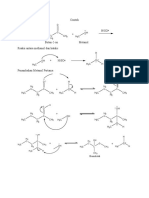
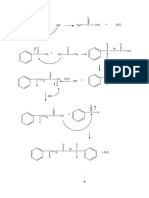
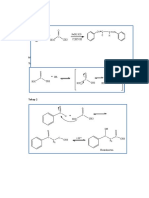
5.2 Reaction:
Chemical Engineering Dept., P.V.P.I.T., Budhgaon.
Subject: P.T. Expt.
No:7
OH
O H +
O H+ H
H2
+ + C C OH
C
C +
H3C OH H3C O H
H3C O H
H3C O H
CH2
H3C
H H H
O O+ OH
+
O
H2O + C OH
C C H+
C OH
H3C O H3C O H3C O
H3C O
CH2 CH2 CH2
H3C H3C CH2 H3C
H3C
Some esters that can be synthesized include methyl salicylate (oil of wintergreen),
isoamyl butyrate (pear), isoamyl acetate (banana), ethyl acetate (fruity), and ethyl
salicylate (sweet smell).
6.0 Apparatus:
Two necked round bottom flask, condenser, weigh balance, thermometer, separating
funnel, heating mantle.
6.1 Reagents:
Glacial acetic acid, butyl alcohol, sulphuric acid.
7.0 Stepwise Procedure:
1) Place a mixture of 1mole i.e 60 gm glacial acetic acid and 2 moles i.e 148 gm of
butyl alcohol solution in round bottom flask and add 4-5 drops of sulphuric acid as
catalyst.
2) Attatch a reflux condenser to flask and heat the flask with total reflux using
heating mantle.
3) Add peaces of porcelein tiles to avoid bumping and overheating of the mixture
and
temperature should be maintained at 65 °C for 1 hour.
Chemical Engineering Dept., P.V.P.I.T., Budhgaon.
Subject: P.T. Expt.
No:7
4) After 1 hour stop the heating and cool the mixture at ambient temperature.
5) Cooled mixture is transfer in separating funnel and add 50 ml water in it to separate
the ester layer at bottom and unreacted acid and alcohol layer at top.
6) Collect the butyl ester in beaker measure its volume, weight and density and also smell
and colour of ester.
8.0 Observations:
1. Weight of Butyl ester = ……..gm
2. Volume of Buytl ester = ….....ml
3. Density of Butyl ester = ……..gm/ml
4. Smell and colour of butyl ester = ……………………………………
9.0 Result and Conclusion:
Esterification is to be done with butyl alcohol and acetic acid in presence of sulphuric
acid catalyst and the amount of butyl ester produced is ……….gm.
Chemical Engineering Dept., P.V.P.I.T., Budhgaon.
You might also like
- Esterfication MechanismDocument1 pageEsterfication MechanismrasikamuhandiramgeNo ratings yet
- Reaksi antara Metanol dan Butan-2-on menghasilkan Ketal 2,2-dimetoksibutanaDocument2 pagesReaksi antara Metanol dan Butan-2-on menghasilkan Ketal 2,2-dimetoksibutanaElis TianiNo ratings yet
- Reaksi antara Metanol dan Butan-2-on menghasilkan Ketal 2,2-dimetoksibutanaDocument2 pagesReaksi antara Metanol dan Butan-2-on menghasilkan Ketal 2,2-dimetoksibutanaElis TianiNo ratings yet
- Esterification: Experiment 3. Ester Formation: Preparation of BenzocaineDocument3 pagesEsterification: Experiment 3. Ester Formation: Preparation of BenzocaineJasreen ArieshaNo ratings yet
- Esterification: Experiment 3. Ester Formation: Preparation of BenzocaineDocument3 pagesEsterification: Experiment 3. Ester Formation: Preparation of BenzocaineSurya kumar AhirwarNo ratings yet
- Reductions PPT 29-08-2020Document12 pagesReductions PPT 29-08-2020jkc collegeNo ratings yet
- MEKANISMEDocument2 pagesMEKANISMEEry NourikaNo ratings yet
- OCH:: C H CH Ohch:+ O C H OCH - CH OHDocument1 pageOCH:: C H CH Ohch:+ O C H OCH - CH OHYash ShindeNo ratings yet
- Preparation of Asperine and Oil of WintergreenDocument4 pagesPreparation of Asperine and Oil of WintergreenKaye ArcherNo ratings yet
- Doc-20170131-Wa0159 1 1Document9 pagesDoc-20170131-Wa0159 1 1rashidNo ratings yet
- UNSTABLE FUNCTIONAL GROUPS IN ORGANIC by S.K.sinha See Chemistry Animations atDocument1 pageUNSTABLE FUNCTIONAL GROUPS IN ORGANIC by S.K.sinha See Chemistry Animations atmyiitchemistry100% (2)
- Edited MonsantoDocument9 pagesEdited MonsantoChristiana ChukwubuezeNo ratings yet
- Rapport Ester HT21Document8 pagesRapport Ester HT21Elizabeth queenNo ratings yet
- CY2102Document2 pagesCY2102Prarabdha SharmaNo ratings yet
- Alkyl Halides & Aryl Halides-02 - Solved ProblemsDocument13 pagesAlkyl Halides & Aryl Halides-02 - Solved ProblemsRaju SinghNo ratings yet
- MechanismDocument2 pagesMechanismRyan BoodramlallNo ratings yet
- Mekanisme RX DibenzalasetonDocument2 pagesMekanisme RX DibenzalasetonWulan safitriNo ratings yet
- Answers To AssignmentDocument1 pageAnswers To AssignmentIgbereyivwe TejiriNo ratings yet
- Exp 7 Preparation of AlkenesDocument14 pagesExp 7 Preparation of AlkenesGeorge PiliposyanNo ratings yet
- Trabajo de Química OrgánicaDocument1 pageTrabajo de Química OrgánicaPaulina Mota MacipNo ratings yet
- Types Reactions 08 WebDocument43 pagesTypes Reactions 08 Webapi-3706290No ratings yet
- Coursebook Answers Chapter 18 Asal ChemistryDocument4 pagesCoursebook Answers Chapter 18 Asal ChemistryMarin PesicNo ratings yet
- Chemistry Xam IdeaDocument9 pagesChemistry Xam Ideagowrimanohar1975No ratings yet
- Aldol ReactionDocument4 pagesAldol ReactionhafizNo ratings yet
- Polymer Bound Oxidation ReportDocument7 pagesPolymer Bound Oxidation ReportMomin ShahNo ratings yet
- Qu 2014Document18 pagesQu 2014Melsi SihombingNo ratings yet
- Cells and Sugars 2-Ionisation+Reactions-studentDocument8 pagesCells and Sugars 2-Ionisation+Reactions-studenttyhbbhhNo ratings yet
- Mini Presentation of IBu-For Prof - SankararamanDocument16 pagesMini Presentation of IBu-For Prof - SankararamanCreative ThinkerNo ratings yet
- PS2 Carboxylic Acids and DerivativesDocument2 pagesPS2 Carboxylic Acids and Derivativesscarllee rogerNo ratings yet
- Mecanismo de Los Derivados Muestra 3Document5 pagesMecanismo de Los Derivados Muestra 3MELISSA Peña OrtizNo ratings yet
- B PharmDocument23 pagesB PharmpurnimaNo ratings yet
- Glyc o SidesDocument22 pagesGlyc o Sidessiddra khalidNo ratings yet
- Aldol ReactionDocument4 pagesAldol ReactionRavi Seedath100% (1)
- Acids and BasesDocument30 pagesAcids and BasesSwagata SahaNo ratings yet
- Synthesis of Aspirin and Wintegreen Spring 2006Document6 pagesSynthesis of Aspirin and Wintegreen Spring 2006apzzzzNo ratings yet
- Chapter 5 Activity AnswersDocument9 pagesChapter 5 Activity AnswersSF MasturahNo ratings yet
- Advanced Biochemistry: The Krebs CycleDocument11 pagesAdvanced Biochemistry: The Krebs CycleMaritsa PerHerNo ratings yet
- 0331 S 05 BioenergyDocument44 pages0331 S 05 BioenergyDaisyNo ratings yet
- Jurnal Kinetika KimiaDocument7 pagesJurnal Kinetika KimiaAsri RahmaNo ratings yet
- 100S120 CS19L01Document38 pages100S120 CS19L01b101112154No ratings yet
- An Aldol Condensation To Synthesize ChalconesDocument6 pagesAn Aldol Condensation To Synthesize ChalconesAssyakurNo ratings yet
- 03-RAZOES PARA A DESTRUICAO DE ACUCAR Cópia 2Document34 pages03-RAZOES PARA A DESTRUICAO DE ACUCAR Cópia 2Rafael PaulinoNo ratings yet
- Kraft Reactin-Dye SynthesisDocument7 pagesKraft Reactin-Dye Synthesisgulft1777No ratings yet
- Xercise: Socl Pyridine, O CH CL MG (CH) O O CH MGCLDocument5 pagesXercise: Socl Pyridine, O CH CL MG (CH) O O CH MGCLPriyanshu RajNo ratings yet
- Dap An Lev BDocument59 pagesDap An Lev BStormy StudiosNo ratings yet
- OXIdation and REDuction CompleteDocument81 pagesOXIdation and REDuction CompleteMateen AliNo ratings yet
- Unit 4, CHEMISTRY OF CARBOHYDRATESDocument31 pagesUnit 4, CHEMISTRY OF CARBOHYDRATESDessalegn Bekele AlemayehuNo ratings yet
- Hidro KarbonDocument43 pagesHidro KarbonElisabet NoviantiNo ratings yet
- Reaction Mechanism: C C H H H O Hgso H SO CHO H CDocument6 pagesReaction Mechanism: C C H H H O Hgso H SO CHO H CFATHIMA THANHA T NNo ratings yet
- 2022 Edited Chemistry of Carbohydrate 2Document33 pages2022 Edited Chemistry of Carbohydrate 2kel GetanehNo ratings yet
- Overview of Alcohol Production 07042020Document66 pagesOverview of Alcohol Production 07042020PujitNo ratings yet
- 102 Lecture Ch13Document36 pages102 Lecture Ch13macybnzNo ratings yet
- Cannizzaro Reaction: Reaction of The Day - 06Document4 pagesCannizzaro Reaction: Reaction of The Day - 06Raufa HussainNo ratings yet
- Mechanisms 1-10: CHEM 725: Davey 1Document7 pagesMechanisms 1-10: CHEM 725: Davey 1Bradley DaveyNo ratings yet
- Diels-Alder Reaction Practice ProblemsDocument2 pagesDiels-Alder Reaction Practice ProblemsBoas Wayne100% (1)
- TutorialDocument27 pagesTutorialSiti NuraqidahNo ratings yet
- BCH 210 CarbohydrateDocument28 pagesBCH 210 CarbohydratedarrielabuaNo ratings yet
- OH A, A Is: : CHN 1 EqvDocument4 pagesOH A, A Is: : CHN 1 EqvAtharva GanjuNo ratings yet
- Invitation EtterDocument1 pageInvitation EttersumitNo ratings yet
- Paushtech Event ParticipantsDocument1 pagePaushtech Event ParticipantssumitNo ratings yet
- Overall Allotment R1+R2+MA - Open Elective III As On 26.07.2023Document50 pagesOverall Allotment R1+R2+MA - Open Elective III As On 26.07.2023sumitNo ratings yet
- Chemical Engineering Assignment Q No. 1: SolutionDocument6 pagesChemical Engineering Assignment Q No. 1: SolutionsumitNo ratings yet
- Masters DataDocument72 pagesMasters DatasumitNo ratings yet
- ApplicationDocument1 pageApplicationsumitNo ratings yet
- Chemical Engineering Assignment Q No. 1: SolutionDocument6 pagesChemical Engineering Assignment Q No. 1: SolutionsumitNo ratings yet
- Packaging TechnologyDocument5 pagesPackaging TechnologysumitNo ratings yet
- AD Patil Sir SynopsisDocument13 pagesAD Patil Sir SynopsissumitNo ratings yet
- Interview FormDocument2 pagesInterview FormsumitNo ratings yet
- Pneumatic Proportional ControllerDocument5 pagesPneumatic Proportional ControllersumitNo ratings yet
- Mech Monitering 2019Document21 pagesMech Monitering 2019sumitNo ratings yet
- ME Chemical Engineering Thesis on Heat Exchanging Reactor DesignDocument12 pagesME Chemical Engineering Thesis on Heat Exchanging Reactor DesignsumitNo ratings yet
- ApplicantProfile2023-07-11 - 23 35 57Document2 pagesApplicantProfile2023-07-11 - 23 35 57sumitNo ratings yet
- Comparative Study of Dye Removal by Adsorption Method and Nanocrystalline Tio Thin FilmsDocument11 pagesComparative Study of Dye Removal by Adsorption Method and Nanocrystalline Tio Thin FilmssumitNo ratings yet
- Initial Pages S B LagareDocument4 pagesInitial Pages S B LagaresumitNo ratings yet
- Chemical Engieering SME Test - 2021 - NEW OneDocument2 pagesChemical Engieering SME Test - 2021 - NEW OnesumitNo ratings yet
- Journal for Research article on various topicsDocument7 pagesJournal for Research article on various topicssumitNo ratings yet
- Renuka Chemicals ProjectDocument3 pagesRenuka Chemicals ProjectsumitNo ratings yet
- Dept. of Chemical Engineering: Class-S.Y. (Chemical) 2018-19 Micro - Project GroupsDocument3 pagesDept. of Chemical Engineering: Class-S.Y. (Chemical) 2018-19 Micro - Project GroupssumitNo ratings yet
- Major Equations: Major Equation Used in ModelsDocument3 pagesMajor Equations: Major Equation Used in ModelssumitNo ratings yet
- Jublient Training ReportDocument33 pagesJublient Training ReportsumitNo ratings yet
- Kinetics of Esterification Reaction Using Ion-Exchange Resin CatalystDocument5 pagesKinetics of Esterification Reaction Using Ion-Exchange Resin CatalystsumitNo ratings yet
- Sumit Mech MoniteringDocument13 pagesSumit Mech MoniteringsumitNo ratings yet
- ApplicationDocument2 pagesApplicationsumitNo ratings yet
- CHEGGDocument12 pagesCHEGGsumitNo ratings yet
- Tittle PageDocument1 pageTittle PagesumitNo ratings yet
- Process Simulation of Heat ExchangerDocument1 pageProcess Simulation of Heat ExchangersumitNo ratings yet
- List of projects-BYBDocument10 pagesList of projects-BYBsumitNo ratings yet
- Process Simulation of Heat ExchangerDocument5 pagesProcess Simulation of Heat ExchangersumitNo ratings yet
- PV 48Document12 pagesPV 48thierrylindoNo ratings yet
- GIS 36-107 Integral Cladding Weld Overlay and Limited LooseDocument17 pagesGIS 36-107 Integral Cladding Weld Overlay and Limited LooseMarkoJovicin100% (5)
- CH 18 Solutions PDFDocument8 pagesCH 18 Solutions PDFHosa HassibNo ratings yet
- Visikol-HISTO - Clarifying Tissue For MicrosDocument30 pagesVisikol-HISTO - Clarifying Tissue For MicrosSathyaSrNo ratings yet
- TM 9-2350-230-10 M-551A1 152mm Airborne Assult VechicleDocument495 pagesTM 9-2350-230-10 M-551A1 152mm Airborne Assult Vechicleehj cho100% (2)
- AAS .PPT (Compatibility Mode)Document53 pagesAAS .PPT (Compatibility Mode)Nadia AdeliaNo ratings yet
- Sae J2295-2016Document28 pagesSae J2295-2016Andris ZaharovNo ratings yet
- CR and Galvanized Steel PDFDocument6 pagesCR and Galvanized Steel PDFthadikkaranNo ratings yet
- Dupont Zytel Htn51g35hsl Nc01Document6 pagesDupont Zytel Htn51g35hsl Nc01josebernal_mzaNo ratings yet
- AHRI Standard 410-2001 With Addenda 1 2 and 3Document67 pagesAHRI Standard 410-2001 With Addenda 1 2 and 3Oziel Reyes100% (1)
- India's Vulnerable Borders Fuel Drug TraffickingDocument60 pagesIndia's Vulnerable Borders Fuel Drug TraffickingPrabhanjan GururajNo ratings yet
- Chemistry Form 6 Sem 3 10Document29 pagesChemistry Form 6 Sem 3 10Anonymous WAnr0jvNo ratings yet
- 3 - Direct Acting Cholinergic DrugsDocument12 pages3 - Direct Acting Cholinergic DrugsHassan NaseerNo ratings yet
- Indesit Manual Wil - 62 PDFDocument16 pagesIndesit Manual Wil - 62 PDFFranceskoNo ratings yet
- NEUROTRANSMITTERSDocument15 pagesNEUROTRANSMITTERSShahran KumarNo ratings yet
- Kid para Valvula FlowtekDocument1 pageKid para Valvula FlowtekshenNo ratings yet
- Geneglace Ice GeneratorsDocument8 pagesGeneglace Ice GeneratorsGia HoàngNo ratings yet
- Materials Science and Engineering-Chapter 11Document3 pagesMaterials Science and Engineering-Chapter 11JurgenNo ratings yet
- TOEFL - Reading Comprehension - Test 2Document2 pagesTOEFL - Reading Comprehension - Test 2ruswandi_123100% (1)
- Production of PhenolDocument65 pagesProduction of Phenolchaitanyavura67% (3)
- LC analysis of impurities in gasesDocument2 pagesLC analysis of impurities in gasesAlex-Bogdan Vișa100% (1)
- CaseHard BS970-1955EN36ADocument2 pagesCaseHard BS970-1955EN36AtechzonesNo ratings yet
- Hydro EfbDocument7 pagesHydro EfbFA MonsterNo ratings yet
- Presenter: Dr. Nishant Shah M.V.Sc. (Medicine)Document104 pagesPresenter: Dr. Nishant Shah M.V.Sc. (Medicine)Santosh BhandariNo ratings yet
- ISO 817 2014 Amd 2 2021Document10 pagesISO 817 2014 Amd 2 2021EdwinMedinaBejaranoNo ratings yet
- Ventilation - Electrical Rooms PDFDocument4 pagesVentilation - Electrical Rooms PDFvalentinlupascu33No ratings yet
- ControlValves Datasheet PDFDocument60 pagesControlValves Datasheet PDFBsd FareedNo ratings yet
- Carboguard 888 PDSDocument3 pagesCarboguard 888 PDSCane CirpoNo ratings yet
- Purlin LysaghtDocument6 pagesPurlin LysaghtAnonymous MHMqCrzgTNo ratings yet
- Allstate Gasket: Section 2 Compression PackingDocument29 pagesAllstate Gasket: Section 2 Compression PackingJose NavarreteNo ratings yet
- Why We Die: The New Science of Aging and the Quest for ImmortalityFrom EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityRating: 4 out of 5 stars4/5 (3)
- Algorithms to Live By: The Computer Science of Human DecisionsFrom EverandAlgorithms to Live By: The Computer Science of Human DecisionsRating: 4.5 out of 5 stars4.5/5 (722)
- Summary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisFrom EverandSummary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisRating: 5 out of 5 stars5/5 (8)
- Gut: the new and revised Sunday Times bestsellerFrom EverandGut: the new and revised Sunday Times bestsellerRating: 4 out of 5 stars4/5 (392)
- The Obesity Code: Unlocking the Secrets of Weight LossFrom EverandThe Obesity Code: Unlocking the Secrets of Weight LossRating: 4 out of 5 stars4/5 (5)
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingFrom EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingRating: 5 out of 5 stars5/5 (5)
- Summary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisFrom EverandSummary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisRating: 4.5 out of 5 stars4.5/5 (42)
- From Darkness to Sight: A Journey from Hardship to HealingFrom EverandFrom Darkness to Sight: A Journey from Hardship to HealingRating: 4 out of 5 stars4/5 (3)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingFrom EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingRating: 3.5 out of 5 stars3.5/5 (33)
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisFrom EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisRating: 3.5 out of 5 stars3.5/5 (2)
- To Explain the World: The Discovery of Modern ScienceFrom EverandTo Explain the World: The Discovery of Modern ScienceRating: 3.5 out of 5 stars3.5/5 (51)
- The Marshmallow Test: Mastering Self-ControlFrom EverandThe Marshmallow Test: Mastering Self-ControlRating: 4.5 out of 5 stars4.5/5 (58)
- A Brief History of Time: From the Big Bang to Black HolesFrom EverandA Brief History of Time: From the Big Bang to Black HolesRating: 4 out of 5 stars4/5 (2193)
- The Fabric of Civilization: How Textiles Made the WorldFrom EverandThe Fabric of Civilization: How Textiles Made the WorldRating: 4.5 out of 5 stars4.5/5 (57)
- Tales from Both Sides of the Brain: A Life in NeuroscienceFrom EverandTales from Both Sides of the Brain: A Life in NeuroscienceRating: 3 out of 5 stars3/5 (18)
- Alex & Me: How a Scientist and a Parrot Discovered a Hidden World of Animal Intelligence—and Formed a Deep Bond in the ProcessFrom EverandAlex & Me: How a Scientist and a Parrot Discovered a Hidden World of Animal Intelligence—and Formed a Deep Bond in the ProcessNo ratings yet
- ChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindFrom EverandChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindNo ratings yet
- Dark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseFrom EverandDark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseRating: 3.5 out of 5 stars3.5/5 (69)
- Sully: The Untold Story Behind the Miracle on the HudsonFrom EverandSully: The Untold Story Behind the Miracle on the HudsonRating: 4 out of 5 stars4/5 (103)
- A Brief History of Intelligence: Evolution, AI, and the Five Breakthroughs That Made Our BrainsFrom EverandA Brief History of Intelligence: Evolution, AI, and the Five Breakthroughs That Made Our BrainsRating: 4.5 out of 5 stars4.5/5 (4)