Professional Documents
Culture Documents
K Sembulingam Essentials of Medical Physiology 6th 008
Uploaded by
Hajara Abubakari SadiqOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
K Sembulingam Essentials of Medical Physiology 6th 008
Uploaded by
Hajara Abubakari SadiqCopyright:
Available Formats
Chapter
Body Fluids
6
INTRODUCTION
SIGNIFICANCE
COMPARTMENTS
COMPOSITION
MEASUREMENT
CONCENTRATION
MAINTENANCE OF WATER BALANCE
APPLIED PHYSIOLOGY
INTRODUCTION IN TRANSPORT MECHANISM
Body is formed by solids and fluids. Fluid part is more Body water forms the transport medium by which
than two third of the whole body. Water forms most of nutrients and other essential substances enter the cells;
the fluid part of the body. and unwanted substances come out of the cells. Water
In human beings, the total body water varies from forms an important medium by which various enzymes,
45% to 75% of body weight. In a normal young adult hormones, vitamins, electrolytes and other substances
male, body contains 60% to 65% of water and 35% to are carried from one part to another part of the body.
40% of solids. In a normal young adult female, the water
IN METABOLIC REACTIONS
is 50% to 55% and solids are 45% to 50%. In females,
water is less because of more amount of subcutaneous Water inside the cells forms the medium for various
adipose tissue. In thin persons, water content is more metabolic reactions, which are necessary for growth and
than that in obese persons. In old age, water content functional activities of the cells.
is decreased due to increase in adipose tissue. Total
quantity of body water in an average human being IN TEXTURE OF TISSUES
weighing about 70 kg is about 40 L. Water inside the cells is necessary for characteristic
form and texture of various tissues.
SIGNIFICANCE OF BODY FLUIDS
IN TEMPERATURE REGULATION
IN HOMEOSTASIS
Water plays a vital role in the maintenance of normal
Body cells survive in the fluid medium called internal body temperature.
environment or ‘milieu interieur’. Internal environment
contains substances such as glucose, amino acids, COMPARTMENTS OF BODY FLUIDS –
lipids, vitamins, ions, oxygen, etc. which are essential
DISTRIBUTION OF BODY FLUIDS
for growth and functioning of the cell. Water not only
forms the major constituent of internal environment but Total water in the body is about 40 L. It is distributed into
also plays an important role in homeostasis. two major compartments:
52 Section 2 t Blood and Body Fluids
1. Intracellular fluid (ICF): Its volume is 22 L and it
forms 55% of the total body water
2. Extracellular fluid (ECF): Its volume is 18 L and it
forms 45% of the total body water.
ECF is divided into 5 subunits:
i. Interstitial fluid and lymph (20%)
ii. Plasma (7.5%)
iii. Fluid in bones (7.5%)
iv. Fluid in dense connective tissues like cartilage
(7.5%)
v. Transcellular fluid (2.5%) that includes:
a. Cerebrospinal fluid
b. Intraocular fluid
c. Digestive juices
d. Serous fluid – intrapleural fluid, pericardial
fluid and peritoneal fluid
e. Synovial fluid in joints
f. Fluid in urinary tract.
Volume of interstitial fluid is about 12 L. Volume of
plasma is about 2.75 L. Volume of other subunits of
ECF is about 3.25 L. Water moves between different
compartments (Fig. 6.1).
COMPOSITION OF BODY FLUIDS
Body fluids contain water and solids. Solids are organic
and inorganic substances.
FIGURE 6.1: Body fluid compartments and movement of fluid
between different compartments. Other fluids = Transcellular
ORGANIC SUBSTANCES
fluid, fluid in bones and fluid in connective tissue.
Organic substances are glucose, amino acids and other
proteins, fatty acids and other lipids, hormones and
enzymes. INDICATOR DILUTION METHOD
Principle
INORGANIC SUBSTANCES
A known quantity of a substance such as a dye is
Inorganic substances present in body fluids are sodium, administered into a specific body fluid compartment.
potassium, calcium, magnesium, chloride, bicarbonate, These substances are called the marker substances or
phosphate and sulfate. indicators. After administration into the fluid compart-
ECF contains large quantity of sodium, chloride, ment, the substance is allowed to mix thoroughly with
bicarbonate, glucose, fatty acids and oxygen. ICF the fluid. Then, a sample of fluid is drawn and the
contains large quantities of potassium, magnesium, concentration of the marker substance is determined.
phosphates, sulfates and proteins. The pH of ECF is Radioactive substances or other substances whose
7.4. The pH of ICF is 7.0. Differences between ECF and concentration can be determined by using colorimeter
ICF are given in Table 6.1. are generally used as marker substances (Table 6.2).
MEASUREMENT OF BODY FLUID VOLUME Formula to Measure the Volume of Fluid by
Total body water and the volume of different compart- Indicator Dilution Method
ments of the body fluid are measured by indicator dilution Quantity of fluid in the compartment is measured using
method or dye dilution method. the formula:
Chapter 6 t Body Fluids 53
2. Must mix with the fluid compartment thoroughly
within reasonable time
V = Volume of fluid in the compartment. 3. Should not be excreted rapidly
M = Mass or total quantity of marker substance 4. Should be excreted from the body completely within
injected. reasonable time
C = Concentration of the marker substance in the 5. Should not change the color of the body fluid
sample fluid 6. Should not alter the volume of the body fluid.
Correction factor Marker Substances Used to
Measure Fluid Compartments
Some amount of marker substance is lost through
urine during distribution. So, the formula is corrected as Marker substances used to measure different fluid
follows: compartment are listed in Table 6.2.
M – Amount of substance excreted
Volume = MEASUREMENT OF TOTAL BODY WATER
C
Volume of total body water (fluid) is measured by using
a marker substance which is distributed through all the
Uses of Indicator Dilution Method
compartments of body fluid. Such substances are listed
Indicator dilution or dye dilution method is used to in Table 6.2.
measure ECF volume, plasma volume and the volume Deuterium oxide and tritium oxide mix with fluids
of total body water. of all the compartments within few hours after injection.
Since plasma is part of total body fluid, the concentration
Characteristics of Marker Substances of marker substances can be obtained from sample of
Dye or any substance used as a marker substance plasma. The formula for indicator dilution method is
should have the following qualities: applied to calculate total body water.
1. Must be nontoxic Antipyrine is also used to measure total body water.
But as it takes longer time to penetrate various fluid
TABLE 6.1: Differences between extracellular fluid (ECF) compartments, the value obtained is slightly low.
and intracellular fluid (ICF)
MEASUREMENT OF EXTRACELLULAR
Substance ECF ICF FLUID VOLUME
Sodium 142 mEq/L 10 mEq/L
Substances which pass through the capillary membrane
Calcium 5 mEq/L 1 mEq/L but do not enter the cells, are used to measure ECF
Potassium 4 mEq/L 140 mEq/L volume. Such marker substances are listed in Table 6.2.
Magnesium 3 mEq/L 28 mEq/L These substances remain only in ECF and do not enter
Chloride 103 mEq/L 4 mEq/L the cell (ICF). When any of these substances is injected
into blood, it mixes with the fluid of all subcompartments
Bicarbonate 28 mEq/L 10 mEq/L
of ECF within 30 minutes to 1 hour. Indicator dilution
Phosphate 4 mEq/L 75 mEq/L method is applied to calculate ECF volume. Since ECF
Sulfate 1 mEq/L 2 mEq/L includes plasma, the concentration of marker substance
Proteins 2 g/dL 16 g/dL can be obtained in the sample of plasma.
Amino acids 30 mg/dL 200 mg/dL Some of the marker substances like sodium, chloride,
Glucose 90 mg/dL 0-20 mg/dL
inulin and sucrose diffuse more evenly throughout all
subcompartments of ECF. So, the measured volume of
Lipids 0.5 g/dL 2-95 g/dL
ECF by using these substances is referred as sodium
Partial pressure of space, chloride space, inulin space and sucrose space.
35 mm Hg 20 mm Hg
oxygen
Partial pressure of Example for Measurement of ECF Volume
46 mm Hg 50 mm Hg
carbon dioxide
Quantity of sucrose injected (Mass) : 150 mg
Water 15 to 20 L (18) 20 to 25 L (22)
Urinary excretion of sucrose : 10 mg
pH 7.4 7.0 Concentration of sucrose in plasma : 0.01 mg/mL
54 Section 2 t Blood and Body Fluids
TABLE 6.2: Marker substances used to measure body
fluid compartments
Mass – Amount lost in urine Fluid compartment Marker substances
Sucrose space =
Concentration of sucrose in plasma 1. Deuterium oxide (D2O)
Total body water 2. Tritium oxide (T2O)
150 – 10 mg 3. Antipyrine
=
0.01 mg/mL 1. Radioactive sodium, chloride,
= 14,000 mL bromide, sulfate and thiosulfate.
Extracellular fluid 2. Non-metabolizable saccharides
Therefore, the ECF volume = 14 L. like inulin, mannitol, raffinose
and sucrose
MEASUREMENT OF PLASMA VOLUME 1. Radioactive iodine (131I)
Plasma
2. Evans blue (T-1824)
The substance which binds with plasma proteins strongly
and diffuses into interstitium only in small quantities or
OSMOLARITY
does not diffuse is used to measure plasma volume.
Such substances are listed in Table 6.2. Osmolarity is another term to express the osmotic
(Measurement of plasma volume and blood volume concentration. It is the number of particles (osmoles) per
is explained in chapter 23). liter of solution (osmoles/L).
Osmotic pressure in solutions depends upon
MEASUREMENT OF INTERSTITIAL osmolality. However, in practice, the osmolarity and
FLUID VOLUME not osmolality is considered to determine the osmotic
pressure because of the following reasons:
Volume of interstitial fluid cannot be measured directly. It
i. Measurement of weight (kilogram) of water in
is calculated from the values of ECF volume and plasma
solution is a difficult process
volume.
ii. Difference between osmolality and osmolarity is
Interstitial fluid volume = very much negligible and it is less than 1%.
ECF volume – Plasma volume Often, these two terms are used interchangeably.
Change in osmolality of ECF affects the volume of
MEASUREMENT OF INTRACELLULAR both ECF and ICF. When osmolality of ECF increases,
FLUID VOLUME water moves from ICF to ECF. When the osmolality
Volume of ICF cannot be measured directly. It is calcula- decreases in ECF, water moves from ECF to ICF. Water
ted from the values of total body water and ECF. movement continues until the osmolality of these two
fluid compartments becomes equal.
ICF volume = Total fluid volume – ECF volume.
Mole and Osmole
CONCENTRATION OF BODY FLUIDS
A mole (mol) is the molecular weight of a substance in
Concentration of body fluids is expressed in three gram. Millimole (mMol) is 1/1000 of a mole. One osmole
ways: (Osm) is the expression of amount of osmotically active
1. Osmolality particles. It is the molecular weight of a substance in
2. Osmolarity grams divided by number of freely moving particles
3. Tonicity. liberated in solution of each molecule. One milliosmole
(mOsm) is 1/1000 of an osmole.
OSMOLALITY
TONICITY
Measure of a fluid’s capability to create osmotic pressure
is called osmolality or osmotic (osmolar) concentration Usually, movement of water between the fluid
of a solution. In simple words, it is the concentration of compartments is not influenced by small molecules like
osmotically active substance in the solution. Osmolality urea and alcohol, which cross the cell membrane very
is expressed as the number of particles (osmoles) per rapidly. These small molecules are called ineffective
kilogram of solution (osmoles/kg H2O). osmoles. On the contrary, the larger molecules like
Chapter 6 t Body Fluids 55
sodium and glucose, which cross the cell membrane
slowly, can influence the movement of water. Therefore,
such molecules are called effective osmoles. Osmolality
that causes the movement of water from one compartment
to another is called effective osmolality and the effective
osmoles are responsible for this.
Tonicity is the measure of effective osmolality. In
terms of tonicity, the solutions are classified into three
categories:
i. Isotonic fluid
ii. Hypertonic fluid
iii. Hypotonic fluid. FIGURE 6.2: Effect of isotonic, hypertonic and hypotonic
solutions on red blood cells
i. Isotonic Fluid
Fluid which has the same effective osmolality (tonicity) APPLIED PHYSIOLOGY
as body fluids is called isotonic fluid. Examples are 0.9%
sodium chloride solution (normal saline) and 5% glucose DEHYDRATION
solution.
Red blood cells or other cells placed in isotonic fluid Definition
(normal saline) neither gain nor lose water by osmosis Dehydration is defined as excessive loss of water from
(Fig. 6.2). This is because of the osmotic equilibrium the body. Body requires certain amount of fluid intake
between inside and outside the cell across the cell daily for normal functions. Minimum daily requirement
membrane. of water intake is about 1 L. This varies with the age
and activity of the individual. The most active individuals
ii. Hypertonic Fluid need 2 to 3 L of water intake daily. Dehydration occurs
Fluid which has greater effective osmolality than the when fluid loss is more than what is consumed.
body fluids is called hypertonic fluid. Example is 2%
sodium chloride solution. Classification
When red blood cells or other cells are placed in Basically, dehydration is of three types:
hypertonic fluid, water moves out of the cells (exosmosis) 1. Mild dehydration: It occurs when fluid loss is about
resulting in shrinkage of the cells (crenation). Refer 5% of total body fluids. Dehydration is not very
Figure 6.2 serious and can be treated easily by rehydration.
2. Moderate dehydration: It occurs when fluid loss
iii. Hypotonic Fluid is about 10%. Dehydration becomes little serious
Fluid which has less effective osmolality than the body and immediate treatment should be given by
fluids is called hypotonic fluid. Example is 0.3% sodium rehydration.
chloride solution. 3. Severe dehydration: It occurs when fluid loss is
When red blood cells or other cells are placed in about 15%. Dehydration becomes severe and
hypotonic fluid, water moves into the cells (endosmosis) requires hospitalization and emergency treatment.
and causes swelling of the cells (Fig. 6.2). Now the red When fluid loss is more than 15%, dehydration
blood cells become globular (sphereocytic) and get becomes very severe and life threatening.
ruptured (hemolysis). On the basis of ratio between water loss and sodium
loss, dehydration is classified into three types:
MAINTENANCE OF WATER BALANCE 1. Isotonic dehydration: Balanced loss of water and
sodium as in the case of diarrhea or vomiting.
Body has several mechanisms which work together to 2. Hypertonic dehydration: Loss of more water than
maintain the water balance. The important mechanisms sodium as in the case of fever.
involve hypothalamus (Chapters 4, 149) and kidneys 3. Hypotonic dehydration: Loss of more sodium than
(Chapter 53). water as in the case of excess use of diuretics.
56 Section 2 t Blood and Body Fluids
Causes Aging Effects on Dehydration
1. Severe diarrhea and vomiting due to gastrointestinal Elders are at higher risk for dehydration even if they
disorders are healthy. It is because of increased fluid loss and
2. Excess urinary output due to renal disorders decreased fluid intake. In some cases, severe dehydra-
3. Excess loss of water through urine due to endocrine tion in old age may be fatal.
disorders such as diabetes mellitus, diabetes
insipidus and adrenal insufficiency Treatment
4. Insufficient intake of water Treatment depends upon the severity of dehydration. In
5. Prolonged physical activity without consuming mild dehydration, the best treatment is drinking of water
adequate amount of water in hot environment and stopping fluid loss. However, in severe dehydration
6. Excess sweating leading to heat frustration drinking water alone is ineffective because it cannot
(extreme loss of water, heat and energy). Severe compensate the salt loss. So the effective treatment for
sweating and dehydration occur while spending severe dehydration is oral rehydration therapy.
longer periods on regular basis in the saunas
7. Use of laxatives or diuretics in order to lose weight Oral rehydration therapy
quickly. This is common in athletes. Oral rehydration therapy (ORT) is the treatment for
dehydration in which a oral rehydration solution (ORS)
Signs and Symptoms is administered orally. ORS was formulated by World
Health Organization (WHO). This solution contains
Mild and moderate dehydration
anhydrous glucose, sodium chloride, potassium chloride
1. Dryness of the mouth and trisodium citrate.
2. Excess thirst In case of very severe dehydration, proper treatment
3. Decrease in sweating is the intravenous administration of necessary water and
4. Decrease in urine formation electrolytes.
5. Headache
6. Dizziness WATER INTOXICATION OR OVERHYDRATION
7. Weakness
8. Cramps in legs and arms. Definition
Severe dehydration Water intoxication is the condition characterized by great
increase in the water content of the body. It is also called
1. Decrease in blood volume overhydration, hyperhydration, water excess or water
2. Decrease in cardiac output poisoning.
3. Low blood pressure
4. Hypovolemic cardiac shock Causes
5. Fainting.
Water intoxication occurs when more fluid is taken than
Very severe dehydration that can be excreted. Water intoxication due to drinking
excess water is rare when the body’s systems are
1. Damage of organs like brain, liver and kidneys
functioning normally. But there are some conditions that
2. Mental depression and confusion
can produce water intoxication.
3. Renal failure
1. Heart failure in which heart cannot pump blood
4. Convulsions
properly
5. Coma.
2. Renal disorders in which kidney fails to excrete
enough water in urine
Dehydration in Infants
3. Hypersecretion of antidiuretic hormone as in the
Infants suffering from severe diarrhea and vomiting case of syndrome of inappropriate hypersecretion
caused by bacterial or viral infection, develop dehydra- of antidiuretic hormone (SIADH)
tion. It becomes life threatening if the lost body fluids are 4. Intravenous administration of unduly large amount
not replaced. This happens when parents are unable to of medications and fluids than the person’s body
recognize the signs. can excrete
Chapter 6 t Body Fluids 57
5. Infants have greater risk of developing water 6. Muscular symptoms such as weakness, cramps,
intoxication in the first month of life, when the filtration twitching, poor coordination and paralysis develop
mechanism of the kidney is underdeveloped and 7. Severe conditions of water intoxication result in:
cannot excrete the fluid rapidly i. Delirium (extreme mental condition characterized
6. Water intoxication is also common in children having by confused state and illusion)
swimming practice, since they are more prone to ii. Seizures (sudden uncontrolled involuntary mus-
drink too much of water while swimming cular contractions)
7. An adult (whose heart and kidneys are functioning iii. Coma (profound state of unconsciousness, in
normally) can develop water intoxication, if the which the person fails to respond to external
person consumes about 8 L of water everyday stimuli and cannot perform voluntary actions).
regularly.
Treatment
Signs and Symptoms
1. Since the brain is more vulnerable to the effects of Mild water intoxication requires only fluid restriction. In
water intoxication, behavioral changes appear first very severe cases, the treatment includes:
2. Person becomes drowsy and inattentive 1. Diuretics to increase water loss through urine
3. Nausea and vomiting occur 2. Antidiuretic hormone (ADH) receptor antagonists
4. There is sudden loss of weight, followed by weak- to prevent ADH-induced reabsorption of water from
ness and blurred vision renal tubules
5. Anemia, acidosis, cyanosis, hemorrhage and shock 3. Intravenous administration of saline to restore
are also common sodium.
You might also like
- Ramadan Planner PDFDocument44 pagesRamadan Planner PDFArooj Kanwal83% (6)
- Osmosis Observation Using PotatoesDocument3 pagesOsmosis Observation Using Potatoesapi-32815725280% (5)
- Edaic 3Document16 pagesEdaic 3DsdNo ratings yet
- Anatomy Viva QuestionsDocument1 pageAnatomy Viva QuestionsKumar KPNo ratings yet
- PAE 5 Techniques of The Treatment With Faradic Type Current-1Document35 pagesPAE 5 Techniques of The Treatment With Faradic Type Current-1Huzaifa HabibNo ratings yet
- Muscle Structure and FunctionsDocument59 pagesMuscle Structure and FunctionsThopu Umamaheswari100% (1)
- Crisp 2EDocument11 pagesCrisp 2EElavarasan0% (3)
- Pharma For PhysioDocument310 pagesPharma For PhysiosportsphysioNo ratings yet
- BSC1005 Exam 2 Review List - Chapter 4 & 5 Energy, PhotosynthesisDocument5 pagesBSC1005 Exam 2 Review List - Chapter 4 & 5 Energy, PhotosynthesismystaceeNo ratings yet
- Isotonic SolutionsDocument33 pagesIsotonic SolutionsChristine Annmarie TapawanNo ratings yet
- PHYSIOLOGY OF HUMAN DEVELOPMENT FROM FERTILIZATION TO IMPLANTATIONDocument68 pagesPHYSIOLOGY OF HUMAN DEVELOPMENT FROM FERTILIZATION TO IMPLANTATIONانس ابوهيبةNo ratings yet
- Fundamental and Derived PositionDocument47 pagesFundamental and Derived PositionAmbusam Anita100% (1)
- 16 Back Spinal CordDocument27 pages16 Back Spinal CordAnonymous lsnDTjvNo ratings yet
- Dhatu Poshana NyayaDocument4 pagesDhatu Poshana NyayaHajusha PNo ratings yet
- The Shoulder Anatomy & Approaches: Abdulaziz F. Ahmed, MBBS PGY-2Document61 pagesThe Shoulder Anatomy & Approaches: Abdulaziz F. Ahmed, MBBS PGY-2Abdulaziz Al-Akhras100% (1)
- 07 2018 Biomedical Instrumentation - Electrical StimulationDocument48 pages07 2018 Biomedical Instrumentation - Electrical Stimulationviki mikiNo ratings yet
- CARDIAC CYCLE - Part I PDFDocument55 pagesCARDIAC CYCLE - Part I PDFChirag Dagar100% (1)
- Forensic MedicineDocument17 pagesForensic MedicineMarites L. DomingoNo ratings yet
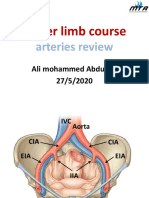
- Arteries ReviewDocument75 pagesArteries ReviewfakhirNo ratings yet
- Overview of Physiotherapy in Pakistan - History, Scope & CareerDocument4 pagesOverview of Physiotherapy in Pakistan - History, Scope & CareerHayat sharafNo ratings yet
- Pleural EffusionDocument78 pagesPleural EffusionJessa AdenigNo ratings yet
- Recommended - Books - Anatomy PDFDocument2 pagesRecommended - Books - Anatomy PDFPrasanna S50% (2)
- Basal GangliaDocument40 pagesBasal GangliaEnkefalos KardiaNo ratings yet
- 1 - Oxygen DebtDocument1 page1 - Oxygen DebtNaseeb AliNo ratings yet
- Etiology and Morphology of CalcificationDocument20 pagesEtiology and Morphology of CalcificationMuhammad Waqas Munir100% (1)
- Disease Essay-RubricDocument2 pagesDisease Essay-Rubricapi-321155161No ratings yet
- VRIDDHI AND KSHAYA LAKSHANAS OF THE THREE DOSHASDocument37 pagesVRIDDHI AND KSHAYA LAKSHANAS OF THE THREE DOSHASVenkatesan VidhyaNo ratings yet
- 2017 PNF and Manual Therapy Treatment Results of Cervical OADocument7 pages2017 PNF and Manual Therapy Treatment Results of Cervical OApainfree888100% (1)
- K Sembulingam Essentials of Medical Physiology 6th 105 PDFDocument11 pagesK Sembulingam Essentials of Medical Physiology 6th 105 PDFBlerta Deari100% (2)
- Nerve Muscle PhysiologyDocument18 pagesNerve Muscle PhysiologyDr P N N Reddy100% (2)
- Pal-Medical Physiology, 4e Flyer Nov21 FinalDocument2 pagesPal-Medical Physiology, 4e Flyer Nov21 FinalE CommerceNo ratings yet
- 52 Page PDF PF PPT Neurophysiological Effects of Spinal Manual Therapy in The Upper Extremity - ChuDocument52 pages52 Page PDF PF PPT Neurophysiological Effects of Spinal Manual Therapy in The Upper Extremity - ChuDenise MathreNo ratings yet
- Kuliah Spine TraumaDocument91 pagesKuliah Spine TraumaEvi MaisyariNo ratings yet
- Master of PhysiotherapyDocument98 pagesMaster of Physiotherapyakhilesh jhariyaNo ratings yet
- Rehabilitation After Anterior Cruciate Ligament ReconstructionDocument12 pagesRehabilitation After Anterior Cruciate Ligament ReconstructionRabbani ObbyNo ratings yet
- Logbook For PG PhysiologyDocument42 pagesLogbook For PG Physiologydevdsantosh100% (1)
- 7 PhysiologyDocument2 pages7 PhysiologyIbrahimFikryNo ratings yet
- High Voltage Galvanic Current Lecture PDFDocument22 pagesHigh Voltage Galvanic Current Lecture PDFdesp100% (1)
- Antenatal and Postnatal PPT PDFDocument15 pagesAntenatal and Postnatal PPT PDFNikita -0251 ENo ratings yet
- CartoonDocument19 pagesCartoonchintujNo ratings yet
- Anatomi PelvisDocument44 pagesAnatomi Pelvisari naNo ratings yet
- A&P - 7. Shoulder Girdle & Upper Limb Detailed Anatomy (42p)Document42 pagesA&P - 7. Shoulder Girdle & Upper Limb Detailed Anatomy (42p)andreeaNo ratings yet
- Viva Questions Final Stage Upper LimbDocument7 pagesViva Questions Final Stage Upper LimbMaria Cheema100% (1)
- OedemaDocument23 pagesOedemaTavleen KaurNo ratings yet
- 15 CerebrumDocument32 pages15 CerebrumMédecin Adrian TGNo ratings yet
- Dhatuposhannyayas 190123063316Document25 pagesDhatuposhannyayas 190123063316Pranit PatilNo ratings yet
- Em Viva PhysiologyDocument11 pagesEm Viva PhysiologyWaleed Mabood100% (1)
- Clinical Guidelines For The Physiotherapy Management of Females Aged 16-65 With Stress Urinary IncontinenceDocument72 pagesClinical Guidelines For The Physiotherapy Management of Females Aged 16-65 With Stress Urinary IncontinenceAndré RodriguesNo ratings yet
- The Upper LimbDocument100 pagesThe Upper LimbAhmed Raza KhanNo ratings yet
- Basics of Electrotherapy 2nd PDFDocument175 pagesBasics of Electrotherapy 2nd PDFPraneetha NouduriNo ratings yet
- Gastric Secretion Intestinal PhaseDocument5 pagesGastric Secretion Intestinal Phasemehdi mafakheriNo ratings yet
- Principles of Aerobic ExerciseDocument35 pagesPrinciples of Aerobic ExerciseSherin KNo ratings yet
- The Convex Concave Rule and The Lever LawDocument4 pagesThe Convex Concave Rule and The Lever LawOwais KhanNo ratings yet
- Renal PathologyDocument5 pagesRenal PathologyEmmanuel De LeonNo ratings yet
- Strength Duration Curves ProcedureDocument4 pagesStrength Duration Curves ProcedureDr GowrishankarPotturi PT67% (3)
- Types of Muscle ContractionDocument12 pagesTypes of Muscle Contractions_saikalyanNo ratings yet
- DownloadDocument11 pagesDownloadChristopher Chew Dian MingNo ratings yet
- General PhysiotherapyDocument6 pagesGeneral Physiotherapysomebody_maNo ratings yet
- 2-5 Current (Träbert) Description and ApplicationDocument3 pages2-5 Current (Träbert) Description and ApplicationNi Made Lidia SwandariNo ratings yet
- Anatomy and Embryology of the Lesser OmentumDocument32 pagesAnatomy and Embryology of the Lesser OmentumspiraldaoNo ratings yet
- Interactions between the Craniomandibular System and Cervical Spine: The influence of an unilateral change of occlusion on the upper cervical range of motionFrom EverandInteractions between the Craniomandibular System and Cervical Spine: The influence of an unilateral change of occlusion on the upper cervical range of motionNo ratings yet
- Body Fluid Compartments and Intro To PhysiologyDocument20 pagesBody Fluid Compartments and Intro To PhysiologyDoc HamsNo ratings yet
- Physiology - Darah & Cairan TubuhDocument90 pagesPhysiology - Darah & Cairan TubuhMuhammad RanggaNo ratings yet
- Curriculum Vitae - Aisha Abubakar SadiqDocument4 pagesCurriculum Vitae - Aisha Abubakar SadiqHajara Abubakari SadiqNo ratings yet
- ChemistryDocument40 pagesChemistryMr DamphaNo ratings yet
- 30 Dua'a Within QuranDocument33 pages30 Dua'a Within QuranHajara Abubakari SadiqNo ratings yet
- English Language PDFDocument11 pagesEnglish Language PDFMumini JallowNo ratings yet
- Further Mathematics/Mathematics (Elective) Aims of The SyllabusDocument18 pagesFurther Mathematics/Mathematics (Elective) Aims of The SyllabusHajara Abubakari SadiqNo ratings yet
- ChemistryDocument40 pagesChemistryMr DamphaNo ratings yet
- ChemistryDocument40 pagesChemistryMr DamphaNo ratings yet
- ChemistryDocument40 pagesChemistryMr DamphaNo ratings yet
- ChemistryDocument40 pagesChemistryMr DamphaNo ratings yet
- Botany in 60 MinutesDocument42 pagesBotany in 60 MinutesGovind Mani BhattNo ratings yet
- IV FluidsDocument3 pagesIV FluidsEvangeline San Jose83% (6)
- Rancangan Pengajaran Tahunan Bio Form 4Document50 pagesRancangan Pengajaran Tahunan Bio Form 4Cik NanaNo ratings yet
- JAMB Biology Past Questions and AnswersDocument53 pagesJAMB Biology Past Questions and AnswersOma AttamahNo ratings yet
- Isotonic, HypotDocument4 pagesIsotonic, HypotSannyjale BaldozaNo ratings yet
- Cellular Transport Test Review ExplainedDocument4 pagesCellular Transport Test Review ExplainedJustin Tis0% (1)
- Lesson Plan-OsmosisDocument2 pagesLesson Plan-OsmosisZm Daza100% (2)
- 500 Medical Surgical Sample QuestionDocument323 pages500 Medical Surgical Sample QuestionElizabella Henrietta TanaquilNo ratings yet
- Department of Education: Republic of The PhilippinesDocument2 pagesDepartment of Education: Republic of The PhilippinesGlaiza Dalayoan FloresNo ratings yet
- PUC II EXPERT CHEMISTRY 12 Set of MODEL QUESTION PAPERDocument46 pagesPUC II EXPERT CHEMISTRY 12 Set of MODEL QUESTION PAPERShreyasNo ratings yet
- Complete Biology-Notes-Form-1-4-Booklet 2024Document412 pagesComplete Biology-Notes-Form-1-4-Booklet 2024MBUGUA GRAPHICSNo ratings yet
- Latest Biology Ss1Document163 pagesLatest Biology Ss1chidimma AnyaboluNo ratings yet
- Test Review - Cells, Cell Transportation and Communication: Unit ObjectivesDocument5 pagesTest Review - Cells, Cell Transportation and Communication: Unit ObjectivesCole McFarlandNo ratings yet
- Hoffman - Hematology. Basic Principles and Practice (3th Edition)Document117 pagesHoffman - Hematology. Basic Principles and Practice (3th Edition)Carmina Lepa100% (1)
- NCLEX HomeostasisDocument10 pagesNCLEX HomeostasisAngie MandeoyaNo ratings yet
- REVISION NOTES - B2.2MovementInAndOutOfTheCellDocument4 pagesREVISION NOTES - B2.2MovementInAndOutOfTheCellDavé TranNo ratings yet
- Egg Osmosis Lab ChartDocument2 pagesEgg Osmosis Lab ChartMia PascualNo ratings yet
- Diffusion and Osmosis Lab Answer Sheet Lab 5Document9 pagesDiffusion and Osmosis Lab Answer Sheet Lab 5BlonDeAmBitionTouRNo ratings yet
- 4 Movement of Substances Across The Cell Membrane: A. Concept CheckingDocument11 pages4 Movement of Substances Across The Cell Membrane: A. Concept CheckingRyo LamNo ratings yet
- Lab ReportDocument48 pagesLab ReportAthirah JeffryNo ratings yet
- Osmoregulation in EarthwormsDocument15 pagesOsmoregulation in EarthwormsValerie Sol BengzonNo ratings yet
- INORG LAB - (2) Experiment 1 - Quality ControlDocument5 pagesINORG LAB - (2) Experiment 1 - Quality ControlAyee Allyra Mocaram EspinosaNo ratings yet
- OSMOSISDocument2 pagesOSMOSISHarriet Lucille VidalNo ratings yet
- Cell TransportDocument19 pagesCell TransportOctavio ManciniNo ratings yet
- Class - Xii - Chemistry Annexure C Set 2Document7 pagesClass - Xii - Chemistry Annexure C Set 2nrusinghsamal2006No ratings yet
- Topic 3 NCM 112Document4 pagesTopic 3 NCM 112Marielle ChuaNo ratings yet