Professional Documents
Culture Documents
Erythropoietin CASE STUDY
Uploaded by
VVBCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Erythropoietin CASE STUDY
Uploaded by
VVBCopyright:
Available Formats
EPO CASE STUDY
Product case study: Neorecormon
Neorecormon (tradename, also known as epoetin beta) is a recombinant human EPO first approved
for medical use in the EU in 1997.
It is indicated for the treatment of anaemia associated with various medical conditions, most
commonly chronic renal failure and cancer patients receiving chemotherapy.
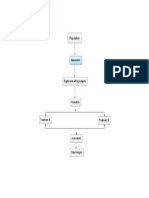
Neorecormon is produced by recombinant DNA technology in a CHO cell line and is manufactured as
outlined in Figure below. It is presented in lyophilized format at various strengths (500–10 000
IU/vial) and contains phosphate buffer, sodium chloride, calcium chloride, urea, polysorbate and
various amino acids as excipients.
The product displays a terminal half-life of 4–12 h and 8–22 h after i.v. and s.c. administration
respectively. Dosage regime is dependent upon the exact disease condition, but generally involves
administration once/several times weekly. Various clinical trials investigating various indications
proved product efficacy in the treatment and prevention of anaemia, with increased haemocrit
values observed.
Common side effects include increased blood pressure, increased respiratory infections and
increased platelet counts. Serious (rare) side effects were most often related to cardiovascular
complications. Neorecormon is marketed by Roche.
You might also like
- Drug Interaction of Dental DrugsDocument14 pagesDrug Interaction of Dental DrugsAnubhuti SabhlokNo ratings yet
- Drug StudyDocument19 pagesDrug StudyCalimlim KimNo ratings yet
- PHM - Hematologic DrugsDocument3 pagesPHM - Hematologic DrugsJeanne Rodiño100% (3)
- Mcqs-Cleanrooms: PIC/S GMP Guide (PE 009-12)Document1 pageMcqs-Cleanrooms: PIC/S GMP Guide (PE 009-12)VVB0% (1)
- MCQ Transdermal Drug DeliveryDocument2 pagesMCQ Transdermal Drug DeliveryVVB100% (2)
- Erythropoietin (EPO) : Dr. Zulfkar Qadrie SR., PharmacologyDocument28 pagesErythropoietin (EPO) : Dr. Zulfkar Qadrie SR., PharmacologyZulfkar Latief QadrieNo ratings yet
- EbpDocument3 pagesEbpJharene BasbañoNo ratings yet
- Drug StudiesDocument16 pagesDrug Studiesvitcloud23100% (2)
- Erythropoietin 2Document12 pagesErythropoietin 2jolie_victoriaNo ratings yet
- EPO PresentationDocument9 pagesEPO PresentationJoy SmokeNo ratings yet
- ESA in CKD - Review ArticleDocument27 pagesESA in CKD - Review ArticleBhavani KirthiNo ratings yet
- Perea (Ibuprofeno 5 MG ML)Document22 pagesPerea (Ibuprofeno 5 MG ML)Paulina AlonsoNo ratings yet
- ChoiDocument19 pagesChoiLuciana RafaelNo ratings yet
- A Review On ErythropoietinDocument14 pagesA Review On ErythropoietinZulfkar Latief QadrieNo ratings yet
- Allopurinol-Induced Drug Reactions With Eosinophilia and Systemic Symptoms Syndrome With Interstitial NephritisDocument12 pagesAllopurinol-Induced Drug Reactions With Eosinophilia and Systemic Symptoms Syndrome With Interstitial NephritisJoc HerreraNo ratings yet
- Perioperative Management of Porphyria: A Case: Key Words: Porphyria, Perioperative Man-Agement, Hemodynamic InstabilityDocument3 pagesPerioperative Management of Porphyria: A Case: Key Words: Porphyria, Perioperative Man-Agement, Hemodynamic InstabilityMarcela GodoyNo ratings yet
- Chemical Information Review Document For Evening Primrose Oil (Oenothera Biennis L.) (CAS No. 90028-66-3) - Evening - Primrose - Nov2009Document44 pagesChemical Information Review Document For Evening Primrose Oil (Oenothera Biennis L.) (CAS No. 90028-66-3) - Evening - Primrose - Nov2009patgarettNo ratings yet
- Holoxan Pi PDFDocument18 pagesHoloxan Pi PDFKarol IonasNo ratings yet
- Flolan Drug Monograph - Munoz-3Document3 pagesFlolan Drug Monograph - Munoz-3Muhammad Muneeb KhawarNo ratings yet
- Updated Advice On Use of High-Dose IbuprofenDocument4 pagesUpdated Advice On Use of High-Dose IbuprofenVeerawit TorsongneanNo ratings yet
- Oxandrolone TabletsDocument5 pagesOxandrolone Tabletsfaqed ilzakiraNo ratings yet
- Nephrotic SyndromeeDocument28 pagesNephrotic SyndromeeRiteka SinghNo ratings yet
- Effects of Dietary Polyphenols On Gene Expression in Human Vascular Endothelial CellsDocument6 pagesEffects of Dietary Polyphenols On Gene Expression in Human Vascular Endothelial CellsVivi GaviriaNo ratings yet
- Bookshelf NBK548889Document18 pagesBookshelf NBK548889Rizqan Fahlevvi AkbarNo ratings yet
- Archer Review Note 5Document11 pagesArcher Review Note 5karan SinghNo ratings yet
- Ijcep 0021902Document10 pagesIjcep 0021902Tri UtomoNo ratings yet
- What Is Lupus NephritisDocument2 pagesWhat Is Lupus NephritisNozi MavundlaNo ratings yet
- Agranulocytosis An Adverse Effect of Allopurinol TDocument4 pagesAgranulocytosis An Adverse Effect of Allopurinol TSYARIF HIDAYATULLAHNo ratings yet
- Ncologist: Erythropoiesis-Stimulating Agents in Renal MedicineDocument6 pagesNcologist: Erythropoiesis-Stimulating Agents in Renal MedicineSergio RodriguezNo ratings yet
- Side Effects: GeneralDocument10 pagesSide Effects: GeneralArianda Nurbani WidyaputriNo ratings yet
- TheEffectsofNaturalAntioxDocument8 pagesTheEffectsofNaturalAntioxLazuardi RaydithoNo ratings yet
- IbuprofrnDocument5 pagesIbuprofrnVivi OpierNo ratings yet
- Amiodarone-Induced Hypothyroidism With EPO-resistant Anemia in A Patient With Chronic Renal FailureDocument3 pagesAmiodarone-Induced Hypothyroidism With EPO-resistant Anemia in A Patient With Chronic Renal Failureeki_megaraniNo ratings yet
- Two Toxicologic Emergencies: Case Studies inDocument4 pagesTwo Toxicologic Emergencies: Case Studies insiddharsclubNo ratings yet
- AECOPD GuidelineDocument8 pagesAECOPD GuidelineRonlie RonneyNo ratings yet
- Cefradime, Paracetamol, KetorolacDocument10 pagesCefradime, Paracetamol, KetorolacMae_Sheryn_Lip_1795No ratings yet
- Preoperative Assessment and PremedicationDocument10 pagesPreoperative Assessment and PremedicationHelene AlawamiNo ratings yet
- Handouts Management of Anemia in CKDDocument5 pagesHandouts Management of Anemia in CKDduelcrossNo ratings yet
- Drugs and Kidney Dec 28 2012Document43 pagesDrugs and Kidney Dec 28 2012Vin BitzNo ratings yet
- Modulación Inflamatoria en El Shock TraumáticoDocument5 pagesModulación Inflamatoria en El Shock TraumáticoFarmacia Del BosqueNo ratings yet
- Esa Anemia CKDDocument6 pagesEsa Anemia CKDmonia agni wiyatamiNo ratings yet
- HyrdrocortisoneDocument7 pagesHyrdrocortisoneRoseben SomidoNo ratings yet
- 1 s2.0 S0301472X08003883 MainDocument12 pages1 s2.0 S0301472X08003883 MainHồ Đại NghĩaNo ratings yet
- TIVA in CKDDocument7 pagesTIVA in CKDfixheartNo ratings yet
- Inotropes: Learning ObjectivesDocument7 pagesInotropes: Learning ObjectivesOrion JohnNo ratings yet
- Q1 Hepatorenal Protective Efficacy of Flavonoids From Ocimum BasilicumDocument13 pagesQ1 Hepatorenal Protective Efficacy of Flavonoids From Ocimum BasilicumPutriSakinahNo ratings yet
- Drug StudyDocument10 pagesDrug StudyJeoffrey MatiasNo ratings yet
- Epoetin AlfaDocument4 pagesEpoetin AlfachoyuminNo ratings yet
- Etoposide Consumer InformationDocument13 pagesEtoposide Consumer InformationEdson KarundengNo ratings yet
- Internal Medicine Form 2:: Answers With Explanations From UptodateDocument11 pagesInternal Medicine Form 2:: Answers With Explanations From UptodateSelena GajićNo ratings yet
- Sciencedaily (June 10, 2010) - A Drug Commonly Used To Treat Gout May Help MaintainDocument4 pagesSciencedaily (June 10, 2010) - A Drug Commonly Used To Treat Gout May Help MaintainAmbbiga AmirthamNo ratings yet
- Anemia in Kidney DiseaseDocument6 pagesAnemia in Kidney DiseaseBazz DanteNo ratings yet
- 3 and 4 Management of Medically CompromisedDocument63 pages3 and 4 Management of Medically Compromisedranareda499No ratings yet
- Opioid Withdrawal in The Emergency Setting - UpToDateDocument15 pagesOpioid Withdrawal in The Emergency Setting - UpToDateSanti HerreraNo ratings yet
- ETOPOSIDEDocument4 pagesETOPOSIDEkajal guptaNo ratings yet
- USPI - Med Guide - Feldene - Piroxicam - CapsulesDocument15 pagesUSPI - Med Guide - Feldene - Piroxicam - CapsulesDini FarhatunnabilahNo ratings yet
- Phenelzine - Liver ToxicityDocument8 pagesPhenelzine - Liver Toxicitydo leeNo ratings yet
- Observation Report - Hemodialysis - Kit P. RoaquinDocument15 pagesObservation Report - Hemodialysis - Kit P. Roaquineljhayar_18No ratings yet
- Anti Epileptic Drugs - The Old and The New 2011Document10 pagesAnti Epileptic Drugs - The Old and The New 20115stringcelloNo ratings yet
- Pycnogenol Consumer/Patient Information SheetDocument1 pagePycnogenol Consumer/Patient Information SheetDeboraNainggolanNo ratings yet
- Hyperbaric Oxygenation Therapy: Molecular Mechanisms and Clinical ApplicationsFrom EverandHyperbaric Oxygenation Therapy: Molecular Mechanisms and Clinical ApplicationsNariyoshi ShinomiyaNo ratings yet
- Ethical Considerations Case StudyDocument3 pagesEthical Considerations Case StudyVVBNo ratings yet
- Clinical Pharmacology Case Study Bioequivalence & Bioavailability VVBDocument3 pagesClinical Pharmacology Case Study Bioequivalence & Bioavailability VVBVVBNo ratings yet
- CONSORT Flow Diagram EXAMPLEDocument2 pagesCONSORT Flow Diagram EXAMPLEVVBNo ratings yet
- Parallen Clinical Trial Design PDFDocument1 pageParallen Clinical Trial Design PDFVVBNo ratings yet
- Problem Set 3 Sample SizeDocument1 pageProblem Set 3 Sample SizeVVBNo ratings yet
- Ethical Considerations of Human Challenge Study - Case Study ASSIGNMENTDocument1 pageEthical Considerations of Human Challenge Study - Case Study ASSIGNMENTVVBNo ratings yet
- Three Pillars of Drug Development Case StudyDocument2 pagesThree Pillars of Drug Development Case StudyVVBNo ratings yet
- Clinical Trial CasestudyDocument2 pagesClinical Trial CasestudyVVBNo ratings yet
- Pharmaceutical Regulatory Agency NotesDocument3 pagesPharmaceutical Regulatory Agency NotesVVBNo ratings yet
- Insulin Structural AnaloguesDocument1 pageInsulin Structural AnaloguesVVBNo ratings yet
- Terminology Review ODDS RATIODocument1 pageTerminology Review ODDS RATIOVVBNo ratings yet
- Insulin Structural AnaloguesDocument1 pageInsulin Structural AnaloguesVVBNo ratings yet
- Clinical Trial Risk Calculation ProblemsDocument3 pagesClinical Trial Risk Calculation ProblemsVVBNo ratings yet
- Interferon As BiologicsDocument1 pageInterferon As BiologicsVVBNo ratings yet
- GMP QC Qa RevisionDocument1 pageGMP QC Qa RevisionVVBNo ratings yet
- Insulin Types PK ProfilesDocument1 pageInsulin Types PK ProfilesVVBNo ratings yet
- TDDS PK ProfileDocument1 pageTDDS PK ProfileVVBNo ratings yet
- TDDS Permeability Enhancement StrategyDocument1 pageTDDS Permeability Enhancement StrategyVVBNo ratings yet
- Cleanrooms PDFDocument1 pageCleanrooms PDFVVBNo ratings yet
- Transdermal Delivery Case StudyDocument4 pagesTransdermal Delivery Case StudyVVBNo ratings yet
- TDDS Patch TypesDocument1 pageTDDS Patch TypesVVBNo ratings yet
- Cleanroom RevisionDocument8 pagesCleanroom RevisionVVBNo ratings yet
- Cleanrooms: Refer To See Construction of CleanroomDocument1 pageCleanrooms: Refer To See Construction of CleanroomVVBNo ratings yet
- Biosafety LevelsDocument1 pageBiosafety LevelsVVBNo ratings yet
- Erythropoeitin: The Gene Consists of Four Introns and Fi Ve ExonsDocument1 pageErythropoeitin: The Gene Consists of Four Introns and Fi Ve ExonsVVBNo ratings yet
- Biosfety Levels PDFDocument1 pageBiosfety Levels PDFVVBNo ratings yet
- Quality Investigation Analysis Example: Quadratic Regression Decaff Coffee Example QCDocument1 pageQuality Investigation Analysis Example: Quadratic Regression Decaff Coffee Example QCVVBNo ratings yet