Professional Documents
Culture Documents
31.3 Travel - Medicine - Overview
31.3 Travel - Medicine - Overview
Uploaded by
MSKCOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
31.3 Travel - Medicine - Overview
31.3 Travel - Medicine - Overview
Uploaded by
MSKCCopyright:
Available Formats
College of Pharmacy and Nutrition
University of Saskatchewan
Saskatoon SK S7N 5C9
www.medsask.usask.ca
Vol. 31, No. 3 May 2014
Travel Vaccines
There is a bug going around Canada right now. It’s called the ‘travel bug’, and it gives people the compelling urge to
travel far away to exotic locations. While this bug is harmless, there are many other viruses and bugs at these locations that
warrant protection for ourselves. Modern day pharmaceuticals have allowed us to prevent many deadly diseases that can
be acquired abroad, but they only work when taken appropriately. Therefore, it’s important as health care professionals to
encourage the correct use of vaccines and antibiotics when patients travel to hazardous locations.
When a patient asks about travel vaccines, the health care professional may want to verify routine vaccinations
(tetanus, diphtheria, pertussis, pneumococcus, etc.) are up to date [1]. These immunizations are strongly recommended by
the World Health Organization [2] and the Public Health Agency of Canada [1] to prevent unnecessary illness when travelling
abroad. The patient should also be aware that it is advisable to visit a travel clinic at least two to three months prior to
travel to ensure they can receive their full course of vaccinations before departure [3]. If the patient is inquiring about what
vaccinations are needed for a country of interest, visit the International Association for Medical Assistance to Travelers
(IAMAT) [4] or Health Canada Travel Guide [5] to find a list of mandatory and recommended vaccines for each country. As a
pharmacist, it is also helpful to recommend your patients follow the 5 ‘I’s when traveling [6].
Insects: Use DEET 30% and nets to repel mosquitoes, or go outside when insects are less active
Ingestions: Wash your hands frequently and avoid food and water that may be contaminated
Indiscretions: Use protection in a sexual encounter and use clean needles
Injuries: Wear a seatbelt when travelling, and avoid wild animals or situations that may be dangerous
Insurance: Obtain coverage for when it is needed
Vaccine Pearls:
Most guidelines for travel are centered on the average healthy adult, yet many do not meet this criteria
Immunocompromised patients can be characterized by; Active HIV infection, use of immune modulating drugs
(infliximab, methotrexate, etc.), ≥20mg/day of prednisone or its equivalent for 14 days or more, cancer patients,
transplant patients, etc. [1]
o If immunocompromised, risks can still be weighed against the benefits if they have not taken a medication that
suppresses the immune system for ≥3 months, or are recovering from cancer [1]
o Even inactive vaccines may not be fully effective in immunocompromised individuals [7]
Pregnant women, young children, or elderly individuals should be counselled on the limitations of safety and efficacy in
each vaccine regarding age and pregnancy concerns on a case by case basis [8]
Telephone: 1-800-667-3425 (SK); 966-6340 (Saskatoon)
Fax: (306) 966-2286 Text: (306) 260-3554
While most vaccinations can be given together, some require separate administration:
o The oral typhoid vaccine may diminish the therapeutic effect of Dukoral, and should be separated by at least 8
hours [9]
o Administration of Rabies Immune Globulin (RIG) may diminish effect of live vaccines with the exception of the
Yellow Fever vaccine. Live organism vaccines should be withheld for up to 6 months following globulin
administration. RIG administration after a live vaccine varies in timeframe, but its benefit will usually outweigh
risks of a diminished immune response [9]
o Whenever possible, injected live virus vaccines should be given ≥28 days apart or ≥30 for Yellow Fever vaccine
[10]
Missing a dose does not always give cause to restart a vaccination series:
o Scheduled doses may be missed due to forgetfulness, inability to get to a clinic, or a drug shortage to name a
few. Available data suggests that intervals between doses longer than what’s recommended do not affect
seroconversion rate or titer volume when the schedule is complete. Therefore it is not necessary to restart a
series or add doses if the interval is extended. The only exception to this is the oral typhoid vaccine, which
should be restarted if the four dose schedule is not completed within three weeks [10]
Using different brands of vaccines to complete a series can be possible in some cases:
o Vaccination schedules should be completed with the same brand if possible. However, a vaccine may be
considered interchangeable when:
Authorized with the same indications and equal schedules
Authorized for the same population
Contains comparable antigens
Similar in safety, immunogenicity, and efficacy [11]
o Visit http://www.phac-aspc.gc.ca/publicat/cig-gci/p01-06-eng.php for specific information pertaining to
individual vaccines
Receiving protection against hepatitis A and B after patient has only had one dose of a univalent vaccine:
o If a patient has already received one dose or multiple doses of a univalent vaccine for hepatitis A or B, they can
still receive a combination hepatitis series. Repeating a hepatitis vaccine series is not harmful [12]
Many different vaccines may be required based on which routine vaccinations have been obtained in the past and
which diseases are prevalent in the destination country. The following is a discussion of some common vaccinations that
may be provided in community pharmacies. A supplemental vaccination table is available at the end of the document.
Telephone: 1-800-667-3425 (SK); 966-6340 (Saskatoon)
Fax: (306) 966-2286 Text: (306) 260-3554
Cholera & ETEC Travelers’ Diarrhea:
These acute intestinal infections are now considered more of a nuisance than a threat for travelers. Cholera is
caused by the bacterium Vibrio cholerae, and it is spread through contaminated water and food. Symptoms include mild to
moderate diarrhea with or without vomiting, potentially leading to leg cramps, severe diarrhea, dehydration, and death.
Staying hydrated is the best treatment for this infection, but antibiotics may be warranted in severe cases [2][7].
Enterotoxigenic Escherichia Coli (ETEC) accounts for 25 to 50 percent of travelers’ diarrhea, and is also transmitted through
contaminated food or water. Most episodes are self-limiting and mild in nature with gastrointestinal symptoms. Currently,
the oral vaccine Dukoral is not recommended for routine use, and it should therefore be reserved for relief workers and
health care professionals in endemic countries [1].
Dukoral, a killed oral vaccine, is about 86 percent effective against epidemic cholera for two years, and roughly 25
percent against ETEC for three months after the second immunization [1]. Two doses are given a minimum of one week
apart and a maximum of six weeks apart for adults, while three doses are recommended for children aged two to five with
the same dosing interval [2]. Food, drink, and any medication should be avoided one hour before and after each oral dose.
The immunogenicity effects of Dukoral differ between cholera and ETEC, but protection is generally seen two weeks after
the final dose, and a booster dose is recommended three months after the primary series if the traveler is under ongoing
risk [2][13]. If more than 5 years pass since last immunization or booster, the primary series should be repeated [13].
Hepatitis A/B:
Hepatitis A is a viral disease that is largely transmitted via the stool of an infected person through contaminated
food and water or close contact. Symptoms can take around a month to appear, and can range from none to severe fever,
fatigue, nausea, and vomiting with liver damage and possibly death. Most cases resolve within a few weeks, but some
infections can persist for months [1][13].
The Hepatitis B virus is contracted from direct contact with blood or other body fluids, and targets the liver. While
50 percent of adults may develop acute symptoms, roughly 90 to 95 percent will clear the virus and develop lifelong
protection. The remaining 5 to 10 percent will develop chronic Hepatitis B [1][13].
Vaccines for Hepatitis A come in a 2 dose regimen with an interval of 6 to 12 months between doses [2][14].
Immunity is seen within two to four weeks, and protection with these vaccines probably lasts 20 years or more. Hepatitis B
can be protected against with a 3 dose regimen that is given intramuscularly at 0, 1, and 6 months [13]. An accelerated
schedule is available as 0, 7, and 21 days, followed by a booster dose at 1 year for those who need to leave before a
standard schedule can be given [14]. Onset of immunity is the same as with Hepatitis A, and protection is thought to last at
least 25 years. For patients requiring coverage against both viruses, Twinrix is available in three doses given at 0, 1, and 6
months. The same rapid schedule as Hepatitis B vaccine can be utilized, and immunity also begins to develop 2 to 4 weeks
after the first dose [14].
Japanese Encephalitis:
Japanese encephalitis is spread through the bite of a Culex mosquito infected with flavivirus from a pig or wild bird
[2]
. Infection can lead to swelling of the brain with consequential nerve and brain damage. The risk is low in urban areas,
but increases as travelers visit rural and agriculture locations in Asia. Most cases of infection are asymptomatic, but some
individuals will see symptoms begin to emerge within one to two weeks after the initial bite. These symptoms include
Telephone: 1-800-667-3425 (SK); 966-6340 (Saskatoon)
Fax: (306) 966-2286 Text: (306) 260-3554
fever, headaches, vomiting, neck stiffness, confusion, and mental changes. Untreated cases can progress to paralysis,
coma, seizures, and convulsions with 20 to 30 percent mortality [13].
The vaccine for Japanese encephalitis is inactivated and administered intramuscularly in three doses: the first two
doses are separated by four weeks and the recommended booster is given at one year. Optimal protection occurs roughly
28 days after the second dose and protection lasts approximately one year. Data on concomitant administration with other
vaccines is not currently available, so this vaccine should be administered alone. Pediatric safety studies are underway;
however the vaccine is currently only licensed for those aged 18 or older [2][13].
Rabies:
Lyssavirus, the virus that causes rabies, is spread to humans through close contact with the saliva of an infected
animal. This could occur as a result of an animal bite, scratch, or lick on open skin [13]. The risks of acquiring rabies after an
exposure are not well known, but are most common if an individual is bitten multiple times or if the bites are closer to the
head. Post-exposure prophylaxis is strongly encouraged as there is no treatment for the infection. If infected, there is an
incubation period of one to three months before two different types of symptoms will develop. ‘Furious rabies’ (the more
common symptom) is characterized by feelings of anxiety, confusion, hyperactivity, hallucinations, fear of water, and
seizures. ‘Dumb rabies’ (the less common symptom) presents more so with weakness and paralysis [2]. If symptoms appear,
prognosis is very poor, and death generally follows 7 to 14 days after first presentation as the respiratory system ceases to
function [13].
Rabies can be vaccinated against in both a prophylactic and post-exposure fashion. The vaccination used is the
same, but the schedule differs. Prophylactic injections require three doses on days 0, 7, and 21 or 28. If the patient is then
exposed to rabies while travelling, two additional booster doses should be given on day 0 and 3 after contact with the
animal, or as soon as possible [1][2]. Post-bite prophylaxis is suitable for those who are not in constant contact with wild
animals while travelling and this includes a four and five-dose regimen. Five doses are currently more common, and consists
of injections on days 0, 3, 7, 14, and 28 [1]. If the vaccine cannot be given on the first day after exposure, it should be
received as soon as possible to help prevent the risk of infection; however there is no set timeframe for how long an
individual can wait before the vaccine is no longer effective [15]. If the bite occurs in someone who has not been previously
vaccinated for rabies, a Rabies Immune Globulin (RIG) should be given within 24 hours or up to seven days after the first
dose of rabies vaccine [16]. This provides the body with immediate protection until a response to the vaccine has occurred.
The rabies vaccine provides immunity 14 to 28 days after the vaccinations are complete, and duration of protection is
usually two to three years [1].
Typhoid Fever:
Salmonella typhi is a common bacterium found predominantly in South Asia where standards of hygiene are lower.
It is transmitted by consumption of contaminated food or water; salmonella infects only humans [6]. The risk of contracting
typhoid depends on the destination, duration of travel, and precautions taken. Safe food handling practices are vital, and
travelers should avoid non-bottled water, roadside food stands, and eating raw fruits or vegetables among others [17]. If
infection occurs, symptoms of salmonella emerge one to three weeks post-infection, and present with varying degrees of
fever, headache, constipation, diarrhea, and fatigue. Untreated cases can lead to enlargement of the liver and spleen,
intestinal bleeding, or pneumonia [13].
While the infection can be treated with antibiotics, there are also two forms of vaccines: oral and intramuscular
(IM). The oral vaccine is a live attenuated mutant strain of typhi Ty21a and is administered as one capsule given on days 1,
Telephone: 1-800-667-3425 (SK); 966-6340 (Saskatoon)
Fax: (306) 966-2286 Text: (306) 260-3554
3, 5, and 7: re-vaccination, if required, is recommended after seven years. The injectable form is an inactive single dose Vi
capsular polysaccharide vaccine (ViCPS) that induces protection within a week and lasts for roughly three years. The IM
vaccine is licensed for children aged 2 and up while the oral has a minimum age requirement of 5 years [1][3].
Yellow Fever:
The virus responsible for yellow fever is flavivirus, which originates primarily in infected monkeys and is transmitted
via mosquitos. The virus is found in urban and rural areas of Africa and central South America. While most infections are
asymptomatic, acute illness can occur in two phases beginning after 3 to 6 days. The first phase is characterized by fever,
muscular pain, headache, chills, nausea and/or vomiting. Roughly 15% of all individuals will progress to the second phase
where severe fever, jaundice, abdominal pain, organ failure and hemorrhagic events may occur. Of those who reach stage
two, 50 percent will die within 10 to 14 days of onset. There is no treatment for yellow fever, so prophylactic vaccination is
extremely important [1][2][13].
Risk for contracting this virus is highest for those staying an extended period of time in rural or jungle areas at an
elevation below 2300 meters [2]. Yellow fever vaccine is a requirement for individuals entering certain high risk countries
(proven with an ‘International Certificate of Vaccination or Prophylaxis’) and is only available through a certified travel
clinic. In Saskatchewan, certified clinics are located in Saskatoon, Regina, or Prince Albert. If the patient is not eligible for the
vaccine because of a contraindication, a waiver certificate may be granted for travel to high risk countries, but an absence
of this certificate may not guarantee entry into the country [13]. An individual benefit-risk assessment is required for patient
in whom the vaccine is not recommended. Such patients include pregnant or breastfeeding women, children under nine
months of age, adults 60 years or older (primary dose), patients with an egg allergy, or immunocompromised individuals.
Yellow fever vaccine is a single dose, live attenuated virus that is administered subcutaneously (SC) [2]. It takes 10 days for
the vaccine to become effective, so the required certificate will only be valid ten days after administration [13]. A booster
dose is required after ten years if re-certification is needed [13].
Prepared by: Kalen MacLeod, Pharmacy Intern
Reviewed by: Carmen Bell, BSP; Terry Damm, BSP; Karen Jensen, BSP, MSc
Telephone: 1-800-667-3425 (SK); 966-6340 (Saskatoon)
Fax: (306) 966-2286 Text: (306) 260-3554
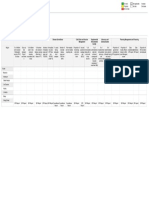
Vaccine Consider Dose schedule Time to onset Duration of Contraindications Adverse Notes
for & Efficacy protection reactions
Cholera/ETEC Emergency/ 2 doses 1-6 wks 2 weeks after Cholera – 2 Hypersensitivity to Mild GI Avoid food/
PO relief apart last dose years previous dose disturbances water/ meds 1
(Killed) workers 25-86% ETEC – 3 hr before &
DUKORAL months after
Hep A/B Close (Hep A only) – 2 2-4 weeks after Hep A– 20+ Decreased efficacy Local Junior
IM contact with doses 0, 6-12 first dose years with: injection site vaccines of
(Killed) locals in months Smoking, older, reactions HAVRIX and
A: (AVAXIM high risk (Hep B only) – 3 Hep A – 80-90% Hep B – 25+ obesity, alcoholism, TWINRIX
HAVRIX) area doses 0, 1, 6 years immunocompromised available for
B: (ENGERIX-B months Hep B – 95-
1
, celiac dx, ages 1-18
RECOMBIVAX Hep A/Hep B – 3 100% hemodialysis
& Infanrix) doses at
TWINRIX 0,1,6months or
(A&B) 0,7,21 days +
VIVAXIM (A & 1year
Typhoid)
Japanese High risk 2 doses 0,28 Variable 1 year Hypersensitivity to Local Not approved
Encephalitis during days 10-28 days after previous dose. injection site for use <18 yrs
IM transmission last dose reactions
(Killed) season Caution in pregnancy
IXIARO (summer 95% have
and fall) antibodies 28
nd
days after 2
dose
Rabies Close Prophylactic – 3 14-28 days 2-3 years Hypersensitivity to Malaise, If contact
IM contact with doses 0,7,21-28 previous dose generalized occurs with
(Killed) saliva of days IM - aches, previous
IMOVAX- unknown Treatment – 5 95-98% headaches prophylaxis,
Rabies animal doses seroconversion give 2 booster
RabAvert 0,3,7,14,28 days doses on 0&3
days
Rabies Immune RIG - 24 hours - RIG - Immediate
Globulin (RIG) 7 days after first protection
IM dose
Typhoid Those Oral - One cap Typh-O – 7 days Oral – 7 years Hypersensitivity to Local Oral use only
PO (Live) visiting poor given on days previous dose injection site for ≥5yrs of
VIVOTIF sanitation 1,3,5,7 Typh-I – 14 days IM – 3 years reactions age
areas IM- Oral vaccine is
IM (Killed) Single dose contraindicated in Oral: Ab IM use only
TYPHERIX injection 50% pregnancy, IBD, and pain, N/V/D, for ≥2yrs of
TYPHIM Vi immunocompromised Rash, age
1
Headache
Yellow Fever Extended Single dose 10 days 10 years Pregnant or Rare Also use
SQ period in breastfeeding, <9 neurological mosquito
(Live) rural/jungle 85% @ 10 days months, >60 yrs encephalitis protection
YF-VAX <2300m or 99% @ 30 days (primary dose), or multi-
3
(nets, spray ,
high risk Immunocompromised organ etc.)
1 2
country , failure
Severe egg allergy
DOSING for ADULTS ONLY
1
Immunocompromised: Active HIV infection, immune modulators (Remicade, etc), Steroids (≥20mg/day
prednisone for 14 days or more), cancer, etc
2
Viscerotropic disease (multi organ failure) with fatality rate in excess of 60% - 2.4/100,000 vaccine doses [18]
Neurotropic disease seen in patients under 6 months or over 60 years – 0.13-0.8/100,000 vaccines doses [18]
3
Insect repellents: Adults 12 & up: 20-30% DEET (lasts 6.5-8 hours) or 20% icaridin (lasts 8 hours) / Kids 6 months-12 years: 20% icaridin or 10% DEET TID [19]
PO = Oral; IM = Intramuscular; SQ = Subcutaneous
Extra:
Combination Typhoid & Hep A vaccine available under ViVAXIM as IM injection for persons >15 years of age
Visit http://www.phac-aspc.gc.ca/publicat/cig-gci/p04-eng.php for complete information on each vaccine
Telephone: 1-800-667-3425 (SK); 966-6340 (Saskatoon)
Fax: (306) 966-2286 Text: (306) 260-3554
References:
1. Phac-aspc.gc.ca. Canadian Immunization Guide - Public Health Agency of Canada. [Online] Available from: http://www.phac-
aspc.gc.ca/publicat/cig-gci/index-eng.php#toc [Accessed Mar 2014].
2. Who.int. WHO | Vaccines. [Online] Available from: http://www.who.int/ith/vaccines/en/ [Accessed Mar 2014].
3. Saskatoonhealthregion.ca. Programs and Services - Public Health - About the International Travel Clinic. [Online] Available from:
http://www.saskatoonhealthregion.ca/your_health/ps_itc_about_us.htm
4. Iamat.org. International Association for Medical Assistance to Travellers. [Online] Available from: http://www.iamat.org/ [Accessed
Mar 2014].
5. Phac-aspc.gc.ca. Where are you travelling? - Travel Health - Public Health Agency of Canada. [Online] Available from:
http://www.phac-aspc.gc.ca/tmp-pmv/countries-pays/index-eng.php [Accessed Mar 2014].
6. Leggat P. Pre-Travel Health Consultation. [e-book] Witwatersrand: University of the Witwatersrand.; 2014. Available from:
http://www.google.ca/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&ved=0CC4QFjAB&url=http%3A%2F%2Fwww.pitt.edu%2
F~super7%2F19011-20001%2F19321.ppt&ei=3kEfU_c4kImiBITCgGg&usg=AFQjCNEtHidgBJFCaNteleut-
xTOlXTeyA&bvm=bv.62788935,d.cGU&cad=rja.
7. Cdc.gov. CDC - Vaccine Safety. [Online] Available from: http://www.cdc.gov/vaccinesafety/index.html [Accessed 20 Mar 2014].
8. Vaccineinformation.org. Vaccine Information You Need from the Immunization Action Coalition. [Online] Available from:
http://www.vaccineinformation.org/ [Accessed Mar 2014].
9. Online.lexi.com. Lexicomp Online Login. [Online] Available from: http://online.lexi.com/lco/action/interact [Accessed 20 Mar 2014].
10. Wwwnc.cdc.gov. General Recommendations for Vaccination & Immunoprophylaxis - Chapter 2 - 2014 Yellow Book | Travelers' Health
| CDC. [Online] Available from: http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-2-the-pre-travel-consultation/general-
recommendations-for-vaccination-and-immunoprophylaxis [Accessed 20 Mar 2014].
11. Phac-aspc.gc.ca. Principles of Vaccine Interchangeability - Part 1 - General Guidelines - Canadian Immunization Guide. [Online]
Available from: http://www.phac-aspc.gc.ca/publicat/cig-gci/p01-06-eng.php [Accessed 20 Mar 2014].
12. Cdc.gov. CDC DVH - HBV FAQs for Health Professionals. [Online] Available from:
http://www.cdc.gov/hepatitis/HBV/HBVfaq.htm#recctbl [Accessed 20 Mar 2014].
13. Travel.gc.ca. Vaccination - Travel.gc.ca. [Online] Available from: http://travel.gc.ca/travelling/health-safety/vaccines [Accessed Mar
2014].
14. Jensen B, Regier L. RxFiles. 9th ed. Saskatoon, SK: RxFiles; 2011.
15. Ndhealth.gov. Rabies Questions and Answers Page. [Online] Available from: http://www.ndhealth.gov/disease/rabies/qanda.htm
[Accessed 20 Mar 2014].
16. Uptodate.com. Rabies. [Online] Available from: http://www.uptodate.com/contents/rabies-beyond-the-basics [Accessed 20 Mar
2014].
17. Vaccineinformation.org. Vaccine Information You Need from the Immunization Action Coalition. [Online] Available from:
http://www.vaccineinformation.org/ [Accessed Mar 2014].
18. Phac-aspc.gc.ca. Adverse Events Following Immunization Reporting Form - Immunization & Vaccines - Public Health Agency of
Canada. [Online] Available from: http://www.phac-aspc.gc.ca/im/aefi-essi-form-eng.php [Accessed Mar 2014].
19. Canadianpharmacistsletter.therapeuticresearch.com. The latest Canadian guidelines for preventing insect bites. [Online] Available
from:
http://canadianpharmacistsletter.therapeuticresearch.com/pl/ArticleDD.aspx?nidchk=1&cs=CEPDA&s=PLC&pt=6&fpt=31&dd=
290727&pb=PLC&searchid=45526592 [Accessed Mar 2014]
Telephone: 1-800-667-3425 (SK); 966-6340 (Saskatoon)
Fax: (306) 966-2286 Text: (306) 260-3554
You might also like
- USMLE Step 3 Lecture Notes 2021-2022: Internal Medicine, Psychiatry, EthicsFrom EverandUSMLE Step 3 Lecture Notes 2021-2022: Internal Medicine, Psychiatry, EthicsRating: 5 out of 5 stars5/5 (9)
- Clinical Management Review 2023-2024: Volume 1: USMLE Step 3 and COMLEX-USA Level 3From EverandClinical Management Review 2023-2024: Volume 1: USMLE Step 3 and COMLEX-USA Level 3Rating: 5 out of 5 stars5/5 (1)
- BAMS Students MnemonicsDocument8 pagesBAMS Students MnemonicsMSKCNo ratings yet
- Expanded Program On Immunization (Philippines)Document14 pagesExpanded Program On Immunization (Philippines)Erick AbarientosNo ratings yet
- Right To Refuse Flu ShotsDocument4 pagesRight To Refuse Flu ShotsBZ Riger100% (2)
- Epi PowerpointDocument29 pagesEpi PowerpointFelisa Lacsamana Gregorio100% (3)
- Expanded Program On Immunization (Philippines)Document14 pagesExpanded Program On Immunization (Philippines)Erick AbarientosNo ratings yet
- Adult ScheduleDocument3 pagesAdult ScheduledrmanojvimalNo ratings yet
- Adult Immunization ScheduleDocument3 pagesAdult Immunization ScheduleBryan Mae H. DegorioNo ratings yet
- Typhoid Fever Follow-Up PDFDocument16 pagesTyphoid Fever Follow-Up PDFPlot BUnniesNo ratings yet
- Guidelines For Vaccination in Normal Adults in India - PMCDocument14 pagesGuidelines For Vaccination in Normal Adults in India - PMCSKMH INSURANCENo ratings yet
- Figure 1. Recommended Immunization Schedule For Adults Aged 19 Years or Older, by Vaccine and Age GroupDocument2 pagesFigure 1. Recommended Immunization Schedule For Adults Aged 19 Years or Older, by Vaccine and Age GroupmsarasNo ratings yet
- Antibiotik For DearrheaDocument11 pagesAntibiotik For DearrheaFerinaTarizaIINo ratings yet
- Research Article: Efforts To Improve Immunization Coverage During Pregnancy Among Ob-GynsDocument9 pagesResearch Article: Efforts To Improve Immunization Coverage During Pregnancy Among Ob-GynsTantiaDeviNo ratings yet
- 6.3.1 PSNZ Influenza Vaccine Reclassification Application Jan 18Document9 pages6.3.1 PSNZ Influenza Vaccine Reclassification Application Jan 18Kresna CahyadiNo ratings yet
- Peace Corps MTG FOIA Volunteer Health SupportDocument199 pagesPeace Corps MTG FOIA Volunteer Health SupportAccessible Journal Media: Peace Corps DocumentsNo ratings yet
- Adult Pocafeket SizeDocument2 pagesAdult Pocafeket SizedadfNo ratings yet
- Health Advice and Immunizations for TravelersFrom EverandHealth Advice and Immunizations for TravelersNo ratings yet
- Ofad 022Document7 pagesOfad 022An iNo ratings yet
- What Everyone Should Know About VaccinesDocument3 pagesWhat Everyone Should Know About VaccinesNicholay AtanassovNo ratings yet
- General Recommendation On ImmunizationDocument19 pagesGeneral Recommendation On ImmunizationRickyNoviantoNo ratings yet
- The Need For Another Typhoid Fever Vaccine: EditorialcommentaryDocument3 pagesThe Need For Another Typhoid Fever Vaccine: Editorialcommentaryshelly_shellyNo ratings yet
- Rotavirus Vaccines: Targeting The Developing World: SupplementarticleDocument7 pagesRotavirus Vaccines: Targeting The Developing World: SupplementarticleAbigailNo ratings yet
- Table 2: Summary of WHO Position Papers - Recommended Routine Immunizations For ChildrenDocument8 pagesTable 2: Summary of WHO Position Papers - Recommended Routine Immunizations For Childrenfadityo1No ratings yet
- Immunization Programs For Infants, Children, Adolescents, and Adults: Clinical Practice Guidelines by The Infectious Diseases Society of AmericaDocument24 pagesImmunization Programs For Infants, Children, Adolescents, and Adults: Clinical Practice Guidelines by The Infectious Diseases Society of AmericaEugene YeboahNo ratings yet
- Cholera ImmunisationDocument2 pagesCholera ImmunisationShynne RPhNo ratings yet
- Seasonal InfluenzaDocument4 pagesSeasonal InfluenzaRachitNo ratings yet
- Art 19661Document4 pagesArt 19661toribio1898No ratings yet
- EPIDocument10 pagesEPIBo ChoiNo ratings yet
- H1N1 Clinician Guidance Final Version1Document2 pagesH1N1 Clinician Guidance Final Version1cha_cruz_6No ratings yet
- VaccineDocument3 pagesVaccinePapitas FritasNo ratings yet
- Diabetes: Primary PreventionDocument7 pagesDiabetes: Primary PreventionVidal IzayNo ratings yet
- Practice Changing UpDates - UpToDateDocument13 pagesPractice Changing UpDates - UpToDatemayteveronica1000No ratings yet
- Adult Combined ScheduleDocument5 pagesAdult Combined SchedulelcmurilloNo ratings yet
- Anaerobe: Lynne V. McfarlandDocument7 pagesAnaerobe: Lynne V. Mcfarlanderna sulistiyawatiNo ratings yet
- Epi NotesDocument5 pagesEpi NoteshoneykrizelNo ratings yet
- Good Health in the Tropics: Advice to Travellers and SettlersFrom EverandGood Health in the Tropics: Advice to Travellers and SettlersNo ratings yet
- Guia de Vacunacion FelinaDocument5 pagesGuia de Vacunacion FelinaRaul KimNo ratings yet
- WSAVA Vaccination Guidelines 2024Document40 pagesWSAVA Vaccination Guidelines 2024Final ShineNo ratings yet
- Vaccine-Preventable Diseases and Vaccines: 6.1 General ConsiderationsDocument63 pagesVaccine-Preventable Diseases and Vaccines: 6.1 General ConsiderationsjuliaNo ratings yet
- Vaccines: Frequency of Adverse Events Following Q Fever Immunisation in Young AdultsDocument13 pagesVaccines: Frequency of Adverse Events Following Q Fever Immunisation in Young AdultslilahgreenyNo ratings yet
- Annex 48 Clinical Guidelines For Covid in Malaysia 4th Edition 19102021 FinaleDocument175 pagesAnnex 48 Clinical Guidelines For Covid in Malaysia 4th Edition 19102021 FinaleEfendi AnuarNo ratings yet
- Rotaviruses Diarrhea: What Are The Benefits of The Rotavirus Vaccine?Document5 pagesRotaviruses Diarrhea: What Are The Benefits of The Rotavirus Vaccine?Mikko ArriesgoddessNo ratings yet
- Table 2: Summary of WHO Position Papers - Recommended Routine Immunizations For ChildrenDocument7 pagesTable 2: Summary of WHO Position Papers - Recommended Routine Immunizations For ChildrenKrishnendu PramanikNo ratings yet
- Annals of Medicine - HPV VaccineDocument12 pagesAnnals of Medicine - HPV VaccineJudicial Watch, Inc.100% (3)
- Nutrients: The Use of Enteral Nutrition in The Management of StrokeDocument6 pagesNutrients: The Use of Enteral Nutrition in The Management of StrokeMiranda MetriaNo ratings yet
- Nutrients: The Use of Enteral Nutrition in The Management of StrokeDocument6 pagesNutrients: The Use of Enteral Nutrition in The Management of StrokeMita SeptoryNo ratings yet
- Expanded Program On ImmunizationDocument4 pagesExpanded Program On ImmunizationMac Lester DumagNo ratings yet
- Types of ImmunizationsDocument21 pagesTypes of Immunizationsmacmactongos100% (6)
- Varicella-Zoster Virus (Chickenpox) : Click The Icon To See An X-Ray of Pneumonia Following Exposure To ChickenpoxDocument4 pagesVaricella-Zoster Virus (Chickenpox) : Click The Icon To See An X-Ray of Pneumonia Following Exposure To ChickenpoxerfinaNo ratings yet
- 1 - CEA - UAS Juni 2020Document9 pages1 - CEA - UAS Juni 2020astri rahayuNo ratings yet
- Akin Et Al Diabetes Vaccination Cost EffectivenessDocument14 pagesAkin Et Al Diabetes Vaccination Cost Effectivenessreza_adrian_2No ratings yet
- Immunization Routine Table1Document9 pagesImmunization Routine Table1MeeKo VideñaNo ratings yet
- Squires 2017Document10 pagesSquires 2017Emile CardNo ratings yet
- Summary of The WHO Position On Rubella Vaccine-July 2020Document3 pagesSummary of The WHO Position On Rubella Vaccine-July 2020Enchantia R. NightshadeNo ratings yet
- Tentang PCVDocument12 pagesTentang PCVJanuwar LukitaNo ratings yet
- National Vaccination ProgramDocument10 pagesNational Vaccination ProgramMysheb SSNo ratings yet
- Adult Combined ScheduleDocument5 pagesAdult Combined ScheduleAwal Safar MNo ratings yet
- SF Vaccination Leaflet - WebDocument12 pagesSF Vaccination Leaflet - Webaakash11No ratings yet
- Stay Healthy, Stay On Top of Vaccinations: CDC Click Here For The CDC's Full Vaccination ChartDocument4 pagesStay Healthy, Stay On Top of Vaccinations: CDC Click Here For The CDC's Full Vaccination ChartcircularreasoningNo ratings yet
- Adult ScheduleDocument3 pagesAdult ScheduleerilarchiNo ratings yet
- Psmid CoAdministration of COVID nonCOVID VaxDocument3 pagesPsmid CoAdministration of COVID nonCOVID VaxarchtgozarNo ratings yet
- 10 JU Detail Fill Up FormDocument2 pages10 JU Detail Fill Up FormMSKCNo ratings yet
- Coconut Oil and Virgin Coconut Oil An Insight IntoDocument3 pagesCoconut Oil and Virgin Coconut Oil An Insight IntoMSKCNo ratings yet
- Pcog Profile of Ageratum Conyzoides PL & Simplicia.Document5 pagesPcog Profile of Ageratum Conyzoides PL & Simplicia.MSKCNo ratings yet
- Korea 6.monograph Part IIDocument239 pagesKorea 6.monograph Part IIMSKCNo ratings yet
- Media EjournalofdenistryDocument3 pagesMedia EjournalofdenistryMSKCNo ratings yet
- ABS Ay Panaceae For Oral HealthDocument1 pageABS Ay Panaceae For Oral HealthMSKCNo ratings yet
- Rasasindura Chandradoya Rasa Pharm AnalDocument3 pagesRasasindura Chandradoya Rasa Pharm AnalMSKCNo ratings yet
- Chapter 03 Respiratory Nafisa Dilshan 102 2221203 - 102Document6 pagesChapter 03 Respiratory Nafisa Dilshan 102 2221203 - 102MSKCNo ratings yet
- SIFCRACT English BrochureDocument6 pagesSIFCRACT English BrochureMSKCNo ratings yet
- Jioh 02 04 010Document4 pagesJioh 02 04 010MSKCNo ratings yet
- Polymorphism Prostate CancerDocument5 pagesPolymorphism Prostate CancerMSKCNo ratings yet
- Oil Pulling Therapy 2008Document2 pagesOil Pulling Therapy 2008MSKCNo ratings yet
- Sunthyadi Yoga Kavala and Pratisarana in TonsillitisDocument2 pagesSunthyadi Yoga Kavala and Pratisarana in TonsillitisMSKCNo ratings yet
- 060 Chemical Properties 10sDocument10 pages060 Chemical Properties 10sMSKCNo ratings yet
- Objectives: The Nature of MatterDocument40 pagesObjectives: The Nature of MatterMSKCNo ratings yet
- 010 Matter and EnergyDocument25 pages010 Matter and EnergyMSKCNo ratings yet
- 050 Chemical and Physical Properties of Matter 13sDocument13 pages050 Chemical and Physical Properties of Matter 13sMSKCNo ratings yet
- 040 Physical & Chemical Properties 13sDocument13 pages040 Physical & Chemical Properties 13sMSKCNo ratings yet
- Electronic Tongue Plant Rasa AIIMS NMRDocument4 pagesElectronic Tongue Plant Rasa AIIMS NMRMSKCNo ratings yet
- Rev Ahara PakaDocument5 pagesRev Ahara PakaMSKCNo ratings yet
- CTRI RA Ay Med Guru Snigdha Guna of Kapha - Clin Higwadi Curna - Sunthyadi Kvath in Ama VataDocument3 pagesCTRI RA Ay Med Guru Snigdha Guna of Kapha - Clin Higwadi Curna - Sunthyadi Kvath in Ama VataMSKCNo ratings yet
- Yuvan Pidika Acne EtiologyDocument8 pagesYuvan Pidika Acne EtiologyMSKCNo ratings yet
- 1 o Tanwar Sthanya KshayaDocument7 pages1 o Tanwar Sthanya KshayaMSKCNo ratings yet
- Ayurveda Perspective of Pediatric Disease (Bala Roga)Document3 pagesAyurveda Perspective of Pediatric Disease (Bala Roga)MSKCNo ratings yet
- 10 Reporting Formats HP Oct2017Document65 pages10 Reporting Formats HP Oct2017Devendra Singh Tomar100% (1)
- eZH Ealth: Dosage Seq. Date (Mm/dd/yyyy) Vaccine Brand Name of Vaccinator Batch No. Lot NoDocument1 pageeZH Ealth: Dosage Seq. Date (Mm/dd/yyyy) Vaccine Brand Name of Vaccinator Batch No. Lot NoRvBombetaNo ratings yet
- Vaccines & AutismDocument5 pagesVaccines & AutismDennis FerrellNo ratings yet
- 2022 To 2024 Workplan National Advisory Committee On Immunization (NACI) - Canada - CaDocument9 pages2022 To 2024 Workplan National Advisory Committee On Immunization (NACI) - Canada - CakelvinkinergyNo ratings yet
- Immunization ReportDocument38 pagesImmunization ReportAlyssa GwenNo ratings yet
- Maklumat Vaksinasi: Vaccination DetailsDocument1 pageMaklumat Vaksinasi: Vaccination DetailsZulkarnain KamalNo ratings yet
- What About Autism?: How Do We Know?Document2 pagesWhat About Autism?: How Do We Know?jenysazedaNo ratings yet
- Quotes On VaccinesDocument25 pagesQuotes On VaccinesilluzoneDOTnet100% (2)
- Toronto Public Health - Immunization of ChildrenDocument8 pagesToronto Public Health - Immunization of ChildrenCityNewsTorontoNo ratings yet
- Novel COVID-19 VaccineDocument25 pagesNovel COVID-19 VaccineJuan VidrioNo ratings yet
- Session 10 KaddarDocument38 pagesSession 10 KaddarsuybaNo ratings yet
- VaccinesDocument29 pagesVaccinesCassandra EnriquezNo ratings yet
- Assignment VDocument2 pagesAssignment VShagun VermaNo ratings yet
- Certificate NayanDocument1 pageCertificate NayanNayan Netaji KumbharNo ratings yet
- Grade 10 Summative TestDocument4 pagesGrade 10 Summative TestJaymar B. MagtibayNo ratings yet
- Complete Vaccine ListingDocument5 pagesComplete Vaccine ListingSyed Esa MushranNo ratings yet
- Diphtheria and MeaslesDocument35 pagesDiphtheria and MeaslesMurugesanNo ratings yet
- Jurnal Difteri English PDFDocument9 pagesJurnal Difteri English PDFLisbet Anita SimamoraNo ratings yet
- 11 - VPD Surveillance Performance Analysis 2021 November 2021 (29122021)Document57 pages11 - VPD Surveillance Performance Analysis 2021 November 2021 (29122021)Kiky RizkyNo ratings yet
- DPT VaccinesDocument2 pagesDPT VaccinesSowmiyaNo ratings yet
- Immunization PPT - EditedDocument22 pagesImmunization PPT - EditedAlfred Mutua MutungaNo ratings yet
- COVID - 19 Treatment and VaccinesDocument2 pagesCOVID - 19 Treatment and Vaccinesridwan_olawale_1No ratings yet
- Certificate - 2022-12-23T121112.931Document1 pageCertificate - 2022-12-23T121112.931Hasan JamalNo ratings yet
- Covid VaccinesDocument41 pagesCovid Vaccinesashima mehtaNo ratings yet
- Certificate For COVID-19 Vaccination: Beneficiary DetailsDocument1 pageCertificate For COVID-19 Vaccination: Beneficiary DetailsK1K5KNo ratings yet
- EPI Scorecard - Sudan - Q1 - 2018Document1 pageEPI Scorecard - Sudan - Q1 - 2018محمد أبو القاسم عليNo ratings yet
- BSN 1a Micro para ReportDocument2 pagesBSN 1a Micro para ReportJohn Steven Bringuelo FloroNo ratings yet
- ImmunisationDocument29 pagesImmunisationOjambo FlaviaNo ratings yet
- Freedom To Choose COVID-19 Vaccine: The Dengvaxia ScareDocument3 pagesFreedom To Choose COVID-19 Vaccine: The Dengvaxia ScareAlyanna Ysabelle VistanNo ratings yet