Professional Documents
Culture Documents
Absorption and Stripping Equipment Manual F
Uploaded by
Shoaib PathanOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Absorption and Stripping Equipment Manual F
Uploaded by
Shoaib PathanCopyright:
Available Formats
1
INDEX:
SR.
CONTENT PAGE NO.
NO.
1. INTRODUCTION 2
2. FLOWSHEET 3
3. PROCESS OPERATION 4–5
4. THEORY 6 – 12
5. TECHINICAL SPECIFICATIONS 13 – 14
6. EXPERIMENTAL MANUAL 15 – 17
7. FLOW DIAGRAM 18
8. WIRING DIAGRAM 19
ABSORPTION AND STRIPPING
2
INTRODUCTION
Solvent
Liquid applied to remove the solute from a gas stream.
Solute
Components to be removed from an entering stream.
Absorption
Unit operation where the solute of a gas is removed by being placed in contact
with a nonvolatile liquid solvent that removes the components from the gas.
Stripping
Unit operation where one or more components of a liquid stream are removed
by being placed in contact with a gas stream that is insoluble in the liquid stream.
Absorbers and strippers are often used in conjunction with each other. Absorbers are
often employed to remove trace components from gas streams.
Strippers are often applied to remove the trace components from the liquid in
a more concentrated form. Absorption and stripping operations are carried out in
vertical, cylindrical columns or towers containing plates or packing elements. The plates
and packing provide a surface area for the liquid and gas to come into contact
facilitating mass transfer between the two streams. The gas and liquid streams for both
operations are commonly counter-current for a more effective mass transfer. The
columns are simpler than those for distillation are because they commonly do not
include a condenser or a Reboiler. There are two types of absorption. The two types
are chemical and physical. In chemical absorption, the liquid solvent reacts with the gas
stream and remains in solution. In physical absorption, the solute in the gas is more
soluble in the liquid solvent and, therefore, the solute is transferred to the liquid.
Chemical is usually preferred over physical because the equilibrium for chemical
absorption is much more favorable for the separation. However, physical absorption is
important since it can be applied when chemical absorption is not possible.
ABSORPTION AND STRIPPING
3
FLOW SHEET
A diagram of an absorber-stripper system is shown in figure 0. The gas containing the
solute to be removed enters at the bottom of the absorber column. In the column, the
gas is placed in contact with the solvent that removes the solute and the purified gas
exits the top of the column. Recycled solvent enters the top of the absorber column and
exits the bottom where it is boiled and sent to the top of the stripping column. The
solute in the solvent is removed in the stripping column by a stripping gas that enters at
the bottom of the column. The solvent now exits from the bottom of the column and is
condensed before it is recycled back to the absorption column. The gas exiting the
stripping column can now be stored or processed easier.
ABSORPTION AND STRIPPING
4
PROCESS OPERATION
Changing the conditions of the absorption column can influence the effectiveness and
efficiency of absorption. Some important controllable conditions are as follows:
Pressure of the column.
Temperature of entering liquid and gas streams.
Humidity of the gas stream.
Ratio of the liquid and gas stream rates.
Raising the total pressure of the column may increase the efficiency of the separation
because increasing the pressure decreases the liquid flow rate and increases the
concentration of the gas. The temperature of entering liquid effect absorption in that it
effects the flow rate of liquid required for the separation with a given number of stages.
Increasing the temperature of the entering solvent increases the liquid flow rate
required. Inlet gases of the absorber with high humidity at a high temperature effect the
capability of the gas to consume latent heat hindering the absorption process.
Therefore, dehumidification of the inlet gas should be considered for absorbers with
large heat effects. The ratio of the liquid to gas stream rates in that if the ratio is too
low, the solute builds in the upper portion of the column causing a higher temperature
profile in the top of the column. As a result, internal cooling maybe necessary for lower
liquid to gas ratios.
LIMITATIONS
Absorption and air stripping:
For high VOC (volatile compounds) concentration above 1000 mg/l and different
VOC.
Solids <2%
Absorption and steam stripping:
VOC highly soluble in water like alcohol and ketones (acetone, ethanol.).
Tb > 150 oC
Excess VOC (>10%).
Suspended solids higher than 2% tend to polymerize.
If phenols cannot be treated, use extraction.
ABSORPTION AND STRIPPING
5
Applicability
Absorption and air stripping:
Remove of VOC at low concentration (<200 mg/l) from contaminated ground water.
Absorption and steam stripping:
Remove of H2S and ammonia NH3 before biological treatment.
Best available technology for an array of organic compounds, Plastic and
synthetic\ fibers (OCPSF) category like benzene
Higher VOC concentration as with air stripping (100 mg/l and more).
Pyridines, cresols, monomers and halogenated solvents.
Elimination of flow VOC concentration from high volume flow of waste stream.
Recovery of thermally unstable materials (low boiling pt.).
ABSORPTION AND STRIPPING
6
THEORY
Design of the Gas Absorber:
Figure 1 shows the streams entering and exiting the absorption column.
General Design Decisions
The designer is required to consider and determine:
1. Entering gas (liquid) flow rate, composition, temperature, and pressure
2. Desired degree of recovery of one or more solutes
3. Choice of absorbent
4. Operating pressure and temperature
5. Minimum absorbent (stripping agent) flow rate and actual absorbent
(stripping agent) flow rate as a multiple of the minimum rate needed to make
the separation.
6. Number of equilibrium stages
7. Heat effects and need for cooling
8. Type of absorber (stripper)
9. Height and diameter of absorber (stripper)
10. Cost of vapor recovery system
ABSORPTION AND STRIPPING
7
Entering gas/liquid flow rates and composition
Entering gas composition and flow rates are generally set from the preceding
unit operation, the flash drum in our case. The pressure and temperature are also set
from this flash calculation.
Desired degree of recovery
The amount of solute recovery is generally set by the designer. It may be a
Recovery to ensure product purity requirements or to satisfy a purity requirement if the
recovered solute is a feed stream to another unit.
Selection of Solvent
The ideal absorbent should:
1. Have a high solubility for the solute(s)
2. Have a low volatility to reduce loss of absorbent
3. be noncorrosive
4. Have a low viscosity to provide a low-pressure drop
5. be nontoxic
6. be available and not expensive
ABSORPTION AND STRIPPING
8
Determination of operating pressure and temperature
a) In general, operating pressure should be high and temperature low for an
absorber, to minimize stage requirements and/or absorbent flow rate and to
lower the equipment volume required to accommodate the gas flow.
b) Operating pressure should be low and temperature high for a stripper to
minimize stage requirements or stripping agent flow rate.
Heat Effects and need for cooling
One of the most important considerations involved in designing a gas absorption
tower is to determine whether or not temperatures will vary along the length of the
tower, since the solubility of the solute gas depends strongly upon the temperature.
Heat effects that may cause temperature to vary from point to point in a gas absorber
are:
1. The heat of solution of the solute
2. The heat of vaporization or condensation of the solvent
3. The change of the sensible heat between the gas and the liquid phases
4. The loss of sensible heat form the fluids to internal and external cooling coils
or to the atmosphere via the tower walls
Types of Absorbers
Absorption and stripping are conducted in trayed towers, packed columns, spray
towers, bubble columns, and centrifugal contactors. Absorption and stripping are
frequently conducted in packed columns, particularly when (1) the required column
diameter is less than 2 ft; (2) the pressure drop must be low, as for a vacuum service; (3)
corrosion considerations favor the use of ceramic or polymeric materials; and (4) low
liquid holdup is desirable. Each type has its own benefits such as greater contact
surfaces, length of residence time required, and available packing space.
ABSORPTION AND STRIPPING
9
For the additional design considerations, one needs to specify an isothermal or non-
isothermal absorber. Each case will be dealt with separately, starting with the
isothermal, and followed by the non-isothermal with an example problem. The non-
isothermal case is of more importance as it more closely models current topics in our
design of the anhydride plant and is presented in more detail than the isothermal case.
Absorber Design for the isothermal case
Isothermal design will not be covered in great detail because it is of little relevance to
our process design problem. However, for some applications it may be necessary to use
inter-stage cooling and force isothermal condition if the temperature change in the
absorption column is great enough to cause large solvent losses.
Determination of absorbent (stripping agent) flow rates
Material balances written around one end of the tower in figure 1 and an arbitrary
intermediate stage n, yield:
YN+1=Xn (L'/G') +Y1-Xo (L'/G') (1)
ABSORPTION AND STRIPPING
10
Yn=Xn+1(L'/G') +Yo-X1(L'/G') (2)
These equations are called operating line equations and when plotted represent the
conditions at the top and bottom of the towers. The slope of the line yields the liquid to
gas ratio (L'/G'). Given the gas flow rate, the minimum solvent flow rate can be
calculated from the relation:
L'min=G'm (3)
Once the minimum solvent flow rate is found, the actual solvent can be found by
multiplying the minimum flow rate by a factor of 1.4.
To obtain the equilibrium line for the plot, one would use the following derivation. A
Balance around a section of the tower will give:
Yacet = (moles acetone/moles inerts) / (moles acetone + inerts / moles inerts)
Yacet = Y / 1 + Y
And similarly, for the liquid component:
Xacet = X / 1 – X
Applying the equilibrium constraint on the system:
Yacet = K (Xacet)
Giving:
(Y / 1 + Y) = K(X / 1 – X)
Solving for Y:
Y = KX / 1 + X – KX (4)
Equation 4 is the equation that determines the equilibrium line in the plot of the
McCabe- Thiele diagram. The McCabe-Thiele diagram will be discussed following, as a
graphical method of determining the theoretical number of stages or plates in the
absorber. Also, this equation can be used for the non-isothermal case due to the
dependence of the K values on the temperature of each stage.
ABSORPTION AND STRIPPING
11
Number of Equilibrium stages
The number of equilibrium stages can be calculated either graphically or
algebraically. When the graphical method is chosen, a McCabe-Thiele diagram is
constructed by plotting the equilibrium line and the equilibrium curve. The number of
stages can be found to reach a specified degree of purity by stepping off between the
equilibrium curve and the operating line. This method is illustrated in the figure 4.
The algebraic method for determining the number of equilibrium stages is more precise
than the graphical method. The Colburn equation (Perry's 7th edition) for the number of
equilibrium stages is:
Nt= [ln [(1-A-1) (y1-y2)/ (y2-y2) +A-1]] / ln (A) (5)
Where
A absorption factor = L'/mG'
y1 mole fraction of solute in entering gas
y2 mole fraction of solute in exiting gas
y2mole fraction of solute in equilibrium with incoming solvent
Once the theoretical number of equilibrium stages has been determined, the actual
number of equilibrium stages can be calculated from the relation:
Eo=Nt/Na (6)
Where
ABSORPTION AND STRIPPING
12
Eo stage efficiency
Na actual number of stages
Nt theoretical number stages
The stage efficiency can be found to be a complex function of the flow rates of
vapor and liquid streams, the geometry and design of the contacting trays, and the
properties of the vapor and liquid streams. The stage efficiency can be found multiple
ways such as from performance data, semi-theoretical models, scaled up laboratory
data, and the O'Connell co-relation for plate efficiency. The O'Connell correlation can be
used given that the operating conditions fall within those covered by the correlation.
Once the efficiency has been estimated, the number of actual stages can be determined.
Absorber Design for the non-isothermal case
Since non-isothermal operation is of more value given our current process design
considerations, a methodology will be given to find all needed values for the design. The
methodology is illustrated with an example problem. The equations for each step are in
bold, the answers to these equations for our example are presented below the
equations in normal faced text.
ABSORPTION AND STRIPPING
13
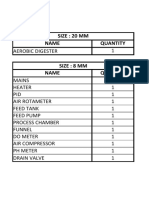
SPECIFICATIONS
Deoxygenating Column
Height : 1570mm
Internal diameter : 26mm
Material : Borosilicate Glass
Absorption Column
Height : 900mm
Internal diameter : 32mm
Material : Borosilicate Glass
Packing Material : Borosilicate glass balls of 4-5 mm diameter
Stripping Column
Height : 900mm
Internal diameter : 32mm
Material : Borosilicate Glass
Packing Material : Borosilicate glass balls of 4-5 mm diameter
Air Flow Meter (Rotameter)
Range : 0.5 to 5.0 l/min
Nitrogen Flow Meters (Rotameter)
Range : 0.5 to 5.0 l/min (one each for deoxygenating column and
stripping column)
Water Flow Meters (Rotameter)
No. : 2 (one each for absorption column and stripping column)
Range : 0.2 to 2.0 l/min
Stripping Column Water Feed Pump
10 l/min @ 1 m head
Absorption Column Water Feed Pump
10 l/min @ 1 m head
ABSORPTION AND STRIPPING
14
Deoxygenating Column Water Feed Pump
20 l/min @ 2 m head
Absorption Column Air Feed Pump/ Air Compressor
24 l/min @ 1kg/cm2
Water Sump Tank
Nos.: 2 (one each for absorption column and stripping column)
MOC: SS 304
Capacity: approx. 40 liters
Dissolved Oxygen Meter
Nos. :1
Saturation:
Range : 0 to 199.9%
Accuracy : ± 0.5%
Resolution : 0.1%
Temperature compensation : Automatic
Concentration:
Range : 0 to 19.9ppm
Accuracy : ± 0.2ppm
Resolution : 0.1ppm
Temperature compensation : Automatic
Air Compressor:
MOC of Tank : MS
Discharge Pressure : 2 Kg/cm2
Capacity of Tank : 45 Liter
Motor : 0.5 HP Singla Phase
Two cylinders of compressed Nitrogen with pressure regulator should also be included
in the scope of supply.
ABSORPTION AND STRIPPING
15
EXPERIMENTAL PROCEDURE
1. Fill the water in the first sump tank and leave empty the other two sump tanks.
2. Now measure D.O by means of D.O meter of First sump tank’s water.
3. Pump this water through water rotameter at desired flow rate and feed it to 1 st
column (Deoxygenating column) from top. At the same time supply the N 2 from
the bottom of the column through nitrogen rotameter at desired flow rate.
4. Collect the water (Deoxygenated water) in the second sump tank and measure
its D.O.
5. Start the second pump and supply this water to the second column (Absorption
Column) from top and supply the air from the bottom of the column through air
rotameter by means of air compressor.
6. Collect the water (Absorption Column) in the Third sump tank and measure its
D.O.
7. Start the Third pump and supply this water to the Third column (Stripping
Column) from top and supply the N 2 from the bottom of the column through
Nitrogen rotameter at desired flowrate.
8. Measure the final D.O of water outlet and find the concentration changes in
Deoxygenating Column, Absorption Column and Stripping Column.
9. Observe all the data and note down.
OBSERVATIONS
1. Flow rate of water : ______ LPM
2. Flow rate of air : ______ LPM
3. Flow rate of N2 : ______ LPM
4. D.O. of entering stream to deoxygenating tower: ______
5. D.O. of existing stream from deoxygenating tower/entering stream to absorption
tower: ______
ABSORPTION AND STRIPPING
16
6. D.O. of existing stream from absorption tower/entering stream to stripping
tower:
7. D.O. of existing stream from stripping tower :
ABSORPTION AND STRIPPING
17
SAMPLE OBSERVATION
1. Flow rate of water : 1 LPM
2. Flow rate of air : 2 LPM
3. Flow rate of N2 : 2 LPM
4. D.O. of entering stream to deoxygenating tower: 7.1
5. D.O. of existing stream from deoxygenating tower/entering stream to absorption
tower: 3.5
6. D.O. existing stream from absorption tower/entering stream to stripping tower:
5.6
7. D.O. of existing stream from stripping tower : 4.5
SAMPLE OBSERVATION
1. Flow rate of water : 1.5 LPM
2. Flow rate of air : 2 LPM
3. Flow rate of N2 : 2 LPM
4. D.O. of entering stream to deoxygenating tower: 7.1
5. D.O. of existing stream from deoxygenating tower/entering stream to absorption
tower: 4
6. D.O. existing stream from absorption tower/entering stream to stripping tower:
5.7
7. D.O. of existing stream from stripping tower : 6.1
ABSORPTION AND STRIPPING
18
ABSORPTION AND STRIPPING
19
ABSORPTION AND STRIPPING
You might also like
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (589)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (842)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5807)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- ES1745 Installation Instructions Rev - CDocument12 pagesES1745 Installation Instructions Rev - CIfran Sierra100% (1)
- Hydraulics ProblemsDocument1 pageHydraulics ProblemsAmanda Smith0% (1)
- Ball Mill Manual FDocument11 pagesBall Mill Manual FShoaib Pathan100% (3)
- Section3 - Downhole SAND CONTROL ComponentsDocument58 pagesSection3 - Downhole SAND CONTROL ComponentsSorin100% (2)
- Ammonia Production From Natural Gas-Haldor Topsoe ProcessDocument22 pagesAmmonia Production From Natural Gas-Haldor Topsoe ProcessYash BhimaniNo ratings yet
- Leak Off Test GuidelineDocument4 pagesLeak Off Test Guidelineruler3382No ratings yet
- Curso Ipims CompletionDocument951 pagesCurso Ipims CompletionpietrokiNo ratings yet
- Atmospheric Air Ejectors PDFDocument2 pagesAtmospheric Air Ejectors PDFVirendra KumarNo ratings yet
- Panasonic R134a DG Series AAA2000CE8Document4 pagesPanasonic R134a DG Series AAA2000CE8Crystal VenomNo ratings yet
- Cyclone Seperator Experimental ManualDocument12 pagesCyclone Seperator Experimental ManualShoaib PathanNo ratings yet
- Design of Heat ExchangerDocument26 pagesDesign of Heat ExchangerSatish YadavNo ratings yet
- Leaf Filter Experimental ManualDocument12 pagesLeaf Filter Experimental ManualShoaib Pathan67% (3)
- Vibrating Screen Experimental ManualDocument16 pagesVibrating Screen Experimental ManualShoaib Pathan50% (2)
- Heat Exchanger Group 17Document22 pagesHeat Exchanger Group 17ja ma0% (1)
- Apparatus For Friction Losses in Pipe Fittings ManualDocument17 pagesApparatus For Friction Losses in Pipe Fittings ManualShoaib PathanNo ratings yet
- Apparatus For Measurement of Thermal Conductivity of Good and Bad Conductors Manual FDocument13 pagesApparatus For Measurement of Thermal Conductivity of Good and Bad Conductors Manual FShoaib PathanNo ratings yet
- Leaching Apparatus: Technical Specifications: The Equipment Consists Mainly of The Following: 1) Percolation LeacherDocument1 pageLeaching Apparatus: Technical Specifications: The Equipment Consists Mainly of The Following: 1) Percolation LeacherShoaib PathanNo ratings yet
- Cre Lab EquipmentDocument1 pageCre Lab EquipmentShoaib PathanNo ratings yet
- Forced Draft Tray DryerDocument1 pageForced Draft Tray DryerShoaib PathanNo ratings yet
- Vibrating Screen: Technical SpecificationDocument1 pageVibrating Screen: Technical SpecificationShoaib PathanNo ratings yet
- Cocurrent and Counter Current Liquid-Liquid ExtractorDocument2 pagesCocurrent and Counter Current Liquid-Liquid ExtractorShoaib PathanNo ratings yet
- Basket CentrifugeDocument1 pageBasket CentrifugeShoaib PathanNo ratings yet
- Sedimentation Equipment: Technical SpecificationDocument1 pageSedimentation Equipment: Technical SpecificationShoaib PathanNo ratings yet
- Cyclone Separator and Water Scrubber: Technical SpecificationsDocument1 pageCyclone Separator and Water Scrubber: Technical SpecificationsShoaib PathanNo ratings yet
- Wetted Wall Column: Technical SpecificationsDocument1 pageWetted Wall Column: Technical SpecificationsShoaib PathanNo ratings yet
- Leaching Apparatus Experimental ManualDocument12 pagesLeaching Apparatus Experimental ManualShoaib PathanNo ratings yet
- Force Draft Tray Dryer Experimental ManualDocument21 pagesForce Draft Tray Dryer Experimental ManualShoaib Pathan100% (2)
- Wetted Wall Column Experimental ManualDocument17 pagesWetted Wall Column Experimental ManualShoaib PathanNo ratings yet
- Sedimentation Unit Experimental ManualDocument17 pagesSedimentation Unit Experimental ManualShoaib PathanNo ratings yet
- Heat Pipe DemonstratorDocument10 pagesHeat Pipe DemonstratorShoaib PathanNo ratings yet
- Heat Pipe DemonstratorDocument10 pagesHeat Pipe DemonstratorShoaib PathanNo ratings yet
- Proses Pembuatan Membran: I Gede Wenten Aryanti P.T.PDocument2 pagesProses Pembuatan Membran: I Gede Wenten Aryanti P.T.Pazizia harmesNo ratings yet
- HT QB A2Document27 pagesHT QB A2Shakil MaddilaNo ratings yet
- Ocf WBDocument16 pagesOcf WBTE B 07 Bhamare DigvijayNo ratings yet
- Ee Mod 3 and 4Document3 pagesEe Mod 3 and 4irshadNo ratings yet
- Chapter 08: TORSION of Shafts: Beam4Document7 pagesChapter 08: TORSION of Shafts: Beam4sandeshlikesNo ratings yet
- Wiljam Flight Training: HydraulicsDocument60 pagesWiljam Flight Training: Hydraulicszanazan11No ratings yet
- Engineering Hydrology: CE - 354 Lecture No. 03Document29 pagesEngineering Hydrology: CE - 354 Lecture No. 03rtthjtyNo ratings yet
- What Is A Wear Pad Functions of Pipe Wear PadsDocument3 pagesWhat Is A Wear Pad Functions of Pipe Wear PadsPrabhakar KumarNo ratings yet
- Casting DefectsDocument2 pagesCasting DefectskoushikraoNo ratings yet
- University of OttawaDocument7 pagesUniversity of Ottawasanvel123No ratings yet
- Books: Hydraulics EngineeringDocument20 pagesBooks: Hydraulics EngineeringWasif RiazNo ratings yet
- Climate - Thermal ComfortDocument16 pagesClimate - Thermal ComfortKeshav AnandNo ratings yet
- Membrane LG CW 4040 SFDocument1 pageMembrane LG CW 4040 SFPT Deltapuro IndonesiaNo ratings yet
- Rotamat Sludge Acceptance Plant Ro 3: The Quality Company - WorldwideDocument4 pagesRotamat Sludge Acceptance Plant Ro 3: The Quality Company - WorldwideUhwan SubhanNo ratings yet
- Test ReportDocument4 pagesTest ReportshivendrakumarNo ratings yet
- P2 Boiler ElectricDocument2 pagesP2 Boiler ElectricIlham HarisNo ratings yet
- Simulation of G R A N U L A R Layer Inversion in Liquid Fluidized BedsDocument9 pagesSimulation of G R A N U L A R Layer Inversion in Liquid Fluidized BedsAlmir Guilherme RittaNo ratings yet
- Fds DispersionDocument13 pagesFds Dispersionparry1701No ratings yet
- DB 01.01.26.04 Pump S200 SSDocument2 pagesDB 01.01.26.04 Pump S200 SSPRAMOD KUMARNo ratings yet
- Chennai Memtech2009jan2013Document19 pagesChennai Memtech2009jan2013Purush JayaramanNo ratings yet