Professional Documents
Culture Documents
Vle For Meoh/H2O System at 1 Atm
Uploaded by
shruti JadhavOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Vle For Meoh/H2O System at 1 Atm
Uploaded by
shruti JadhavCopyright:
Available Formats
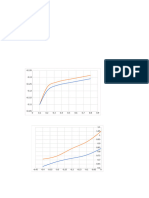
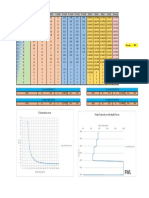
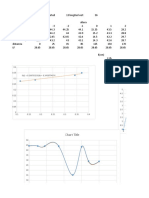
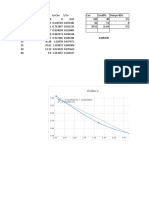
xy VLE data for methanol/water at 1 atm
Temp (oF) Temp (oC) x (mole f) y (mole f) y_calc Diagonal
212 100 0 0 0 0
209.12 98.4 0.012 0.068 0.1645219 0.012
206.42 96.9 0.02 0.121 0.2032652 0.02
204.44 95.8 0.026 0.159 0.2265856 0.026 VLE for MeOH/H2O system @ 1 atm
VLE for MeOH/H2O system @ 1 atm
203.18 95.1 0.033 0.188 0.25008944 0.033
201.38 94.1 0.036 0.215 0.2592619 0.036 220
197.96 92.2 0.053 0.275 0.30428117 0.053 1
194 90 0.074 0.356 0.34936772 0.074
191.48 88.6 0.087 0.395 0.3735767 0.087
188.42 86.9 0.108 0.44 0.4085580 0.108
200 0.75
y (mole frac MeOH)
185.72 85.4 0.129 0.488 0.4397423 0.129
182.12 83.4 0.164 0.537 0.48568712 0.164
0.5
Temperature (oF)
179.6 82 0.191 0.572 0.5173175 0.191 180
174.38 79.1 0.268 0.648 0.5951865 0.268
172.58 78.1 0.294 0.666 0.61844344 0.294
169.7 76.5 0.352 0.704 0.6663013 0.352
160
0.25
167.54 75.3 0.402 0.734 0.7039632 0.402
165.56 74.2 0.454 0.76 0.7403210 0.454
163.76 73.2 0.502 0.785 0.7717720 0.502 0
161.6 72 0.563 0.812 0.80929494 0.563 140
0
25
5
75
1
159.62 70.9 0.624 0.835 0.84450364 0.624
0.
0
25
5
75
1
156.56 69.2 0.717 0.877 0.8944959 0.717
0.
0.
0.
0.
0.
154.58 68.1 0.79 0.91 0.93112905 0.79
MeOH Content (mole fraction)
152.96 67.2 0.843 0.93 0.9564980 0.843 x (mole frac MeOH)
152.42 66.9 0.857 0.939 0.96304224 0.857
150.26 65.7 0.938 0.971 0.9997287 0.938
149 65 1 1 1.026572 1 y_calc = 1.026572 * x^0.4139722
This is a curve fit via Polymath.
(Curve above is simple connect the dots)
Experimental data from the compilation by Gmehling, J. and Onken, U. 1977. Vapor-Liquid Equilibrium Data Collection, Dechema, Frankfurt, Germany, vol. 1, p. 60.
from Chemical Engineering Design and Analysis: An Introduction
T. M. Duncan and J. A. Reimer, Cambridge University Press, 1998.
You might also like
- Global Procurement Shared ServiceDocument4 pagesGlobal Procurement Shared ServiceBeroe Inc.100% (2)
- HJ125 8 Manual de RepuestosDocument72 pagesHJ125 8 Manual de RepuestosFelix Rivera100% (1)
- Lab 3Document2 pagesLab 3ridicheNo ratings yet
- Batch Distillation DataDocument2 pagesBatch Distillation DataEmmanuel PlazaNo ratings yet
- 1:24,000 Utm Grid Each Mark Is 100 Meters: ©2001 MaptoolsDocument1 page1:24,000 Utm Grid Each Mark Is 100 Meters: ©2001 MaptoolsAdilson Leite ProençaNo ratings yet
- Methanol Water Xy DataDocument1 pageMethanol Water Xy DataAjay TulpuleNo ratings yet
- Vle For Meoh/H2O System at 1 Atm Vle For Meoh/H2O System at 1 AtmDocument1 pageVle For Meoh/H2O System at 1 Atm Vle For Meoh/H2O System at 1 AtmClemenNo ratings yet
- Pg068 - T11 Conductor ResistanceDocument1 pagePg068 - T11 Conductor ResistanceDolyNo ratings yet
- Sepe HW 1Document5 pagesSepe HW 1Amen ChughtaiNo ratings yet
- Table 4.2ADocument1 pageTable 4.2ALaura SlaNo ratings yet
- Tarea 1.2 Ambos ProblemasDocument12 pagesTarea 1.2 Ambos ProblemasOlman VargasNo ratings yet
- Actuarial EsDocument2 pagesActuarial EsCarlos A-cNo ratings yet
- Chart Title Chart TitleDocument4 pagesChart Title Chart TitleDeyner Ayala RamosNo ratings yet
- 3 Peluqueros FormulasDocument8 pages3 Peluqueros FormulasAraceli PerezNo ratings yet
- MeOH H2O TxyDocument1 pageMeOH H2O TxyDwiki RamadhanNo ratings yet
- Distillation Report - Rotation 1Document1 pageDistillation Report - Rotation 1jlcheefei9258No ratings yet
- MeOH H2O TxyDocument1 pageMeOH H2O TxyYrjell ObsiomaNo ratings yet
- Volumen de Corte Vias de AccesoDocument4 pagesVolumen de Corte Vias de AccesoCru Z JuanNo ratings yet
- Espectro De Diseño Sismico Gbds: Espectro Zona 3-Suelo S4 (1.5 Kg/cm2>σadm ≥ 0.5Kg/cm2)Document4 pagesEspectro De Diseño Sismico Gbds: Espectro Zona 3-Suelo S4 (1.5 Kg/cm2>σadm ≥ 0.5Kg/cm2)OscarQuirogaNo ratings yet
- Enzyme Kinetics AssignementDocument1 pageEnzyme Kinetics AssignementLEXTER SOLISNo ratings yet
- Bao Cao Thi NghiemDocument2 pagesBao Cao Thi Nghiemnam tranNo ratings yet
- B R TF - (kf1) TF - (kf2) TF - (kf3) TF - (kf4) Kf1 Kf2: Temperatura A Diferentes KFDocument2 pagesB R TF - (kf1) TF - (kf2) TF - (kf3) TF - (kf4) Kf1 Kf2: Temperatura A Diferentes KFDiego HernándezNo ratings yet
- GRAFICASDocument19 pagesGRAFICASAlfredo TlapaleNo ratings yet
- Lixiviacion Extraccion L L 2013 2Document22 pagesLixiviacion Extraccion L L 2013 2Alexis Yairs Romero MunarizNo ratings yet
- Low Speed 1Document16 pagesLow Speed 1dimasNo ratings yet
- Steam Qulity Out Versus Pressure Thermal Efficiency Versus PressureDocument2 pagesSteam Qulity Out Versus Pressure Thermal Efficiency Versus PressureebrahimNo ratings yet
- Total 55 3395 97.57: For Group Data Class Fi Xi Fixi Fi Log XiDocument14 pagesTotal 55 3395 97.57: For Group Data Class Fi Xi Fixi Fi Log XiFakhrul JonyNo ratings yet
- Diatom 24Document191 pagesDiatom 24handikajati kusumaNo ratings yet
- Smith Chart For Excel - RFMDDocument6 pagesSmith Chart For Excel - RFMDAstro MikeNo ratings yet
- Grafik Percobaan 2Document2 pagesGrafik Percobaan 2Koplok GamingNo ratings yet
- Histogram: UB UA TI F FR FK FRK F.TDocument4 pagesHistogram: UB UA TI F FR FK FRK F.Tramadhan susanprayogoNo ratings yet
- UntitledDocument4 pagesUntitledMiguel angel Córdoba rojasNo ratings yet
- J-Saturation Curve Water Saturation With Depth CurveDocument1 pageJ-Saturation Curve Water Saturation With Depth Curveel hadiNo ratings yet
- Manzana EDocument1 pageManzana ECristiaN luna itoNo ratings yet
- Absheron Cdfw-Feb06-2017Document13 pagesAbsheron Cdfw-Feb06-2017Seymur AkbarovNo ratings yet
- VertederoDocument4 pagesVertederoIván VargasNo ratings yet
- Datos y Cálculos de La Sección TransversalDocument5 pagesDatos y Cálculos de La Sección TransversalCRISTIANNo ratings yet
- Deltah E K0 R Dtadiabático CP C1 0 C2 0 PDocument2 pagesDeltah E K0 R Dtadiabático CP C1 0 C2 0 PJhonNo ratings yet
- Kaiser TDocument1 pageKaiser Tvineetghule17No ratings yet
- Auxiliar 347Document5 pagesAuxiliar 347Juan Carlos CruzNo ratings yet
- Volume Products (Volume)Document8 pagesVolume Products (Volume)Rajendra ShettyNo ratings yet
- 1 Usa $ BDT 80 PSC DZN Price Usa $ BDT Rate Total Usa$ Total BDTDocument2 pages1 Usa $ BDT 80 PSC DZN Price Usa $ BDT Rate Total Usa$ Total BDTMomen SarkarNo ratings yet
- Book 1Document6 pagesBook 1Jocker GreickNo ratings yet
- القوى الدافعة 121Document2 pagesالقوى الدافعة 121Aya MokatrenNo ratings yet
- Date - Randament - LP 3Document5 pagesDate - Randament - LP 3Ecaterina MoruzNo ratings yet
- Chart TitleDocument11 pagesChart TitleEsperanza Ramirez MercadoNo ratings yet
- Cinetica Batch LevDocument11 pagesCinetica Batch LevEric GabrielNo ratings yet
- C.S.Tecozautla Propuesta 710 M2-ModelDocument1 pageC.S.Tecozautla Propuesta 710 M2-ModelSebastian CifuentesNo ratings yet
- Pa WK14 50382170Document3 pagesPa WK14 50382170sushmaNo ratings yet
- Tarea E L-LDocument4 pagesTarea E L-LRoco neluNo ratings yet
- Anggraini Lenry Rahman (1910247973) - TUGAS 1 - Sistem Pengambilan KeputusanDocument8 pagesAnggraini Lenry Rahman (1910247973) - TUGAS 1 - Sistem Pengambilan Keputusananggraini.lenryrahmaNo ratings yet
- Our Works Can Be Followed Here, We Are Looking Forward To Receiving Your OpinionsDocument26 pagesOur Works Can Be Followed Here, We Are Looking Forward To Receiving Your Opinionsjust meNo ratings yet
- Εργασια 1ηDocument10 pagesΕργασια 1ηpamari17870No ratings yet
- Smith Chart For Excel v2p1Document17 pagesSmith Chart For Excel v2p1ankabangNo ratings yet
- Diagram Respirasi Serangga: Waktu (Detik)Document7 pagesDiagram Respirasi Serangga: Waktu (Detik)Haripo'o SuyatmoNo ratings yet
- Activity 3 ExperimentDocument5 pagesActivity 3 ExperimentMichael StanwayNo ratings yet
- Analisis Acumulativo Analisis Deiferencial: Dpi, MM Dpi, MMDocument2 pagesAnalisis Acumulativo Analisis Deiferencial: Dpi, MM Dpi, MMVALENTINA VANEGAS ARBOLEDANo ratings yet
- CALIBRATION COLUMN 150 & 500 ML-ModelDocument1 pageCALIBRATION COLUMN 150 & 500 ML-ModelsindoroprintNo ratings yet
- 2019-Me-111 Lab Report 2 (Quick Return Mechanism)Document8 pages2019-Me-111 Lab Report 2 (Quick Return Mechanism)touqeerahmad 9058No ratings yet
- ICT Lab No 4 GraphsDocument1 pageICT Lab No 4 Graphstahaarshad799No ratings yet
- CBR Results For Collected Soil Sample From Filling Area (Base Course) at NRO of IOCL at Munavalli Village of Shiggaon, Haveri DistDocument5 pagesCBR Results For Collected Soil Sample From Filling Area (Base Course) at NRO of IOCL at Munavalli Village of Shiggaon, Haveri DistDevaraj H.ANo ratings yet
- Math Practice Simplified: Decimals & Percents (Book H): Practicing the Concepts of Decimals and PercentagesFrom EverandMath Practice Simplified: Decimals & Percents (Book H): Practicing the Concepts of Decimals and PercentagesRating: 5 out of 5 stars5/5 (3)
- Chloride: HZ+ H Tic144 Tic13 Hci Ti 3tic14 4tic13 Tic13 Hc1-Tic14 +HZDocument5 pagesChloride: HZ+ H Tic144 Tic13 Hci Ti 3tic14 4tic13 Tic13 Hc1-Tic14 +HZshruti JadhavNo ratings yet
- Terminal Velocity of Falling Particle: Inputs From The Problem Suitable Drag CoefficientDocument2 pagesTerminal Velocity of Falling Particle: Inputs From The Problem Suitable Drag Coefficientshruti JadhavNo ratings yet
- Process Equiment DesignDocument4 pagesProcess Equiment Designshruti JadhavNo ratings yet
- Tech Specs: Temperature ControllerDocument2 pagesTech Specs: Temperature Controllershruti JadhavNo ratings yet
- eLTE5.0 DBS3900 Product Description (3GPP)Document37 pageseLTE5.0 DBS3900 Product Description (3GPP)Wisut MorthaiNo ratings yet
- Trigen Húmero + ShureshotDocument56 pagesTrigen Húmero + Shureshotgpe nola jpNo ratings yet
- CR-IR346RU Service ManualDocument70 pagesCR-IR346RU Service ManualEver Soto LopezNo ratings yet
- L5586Document1 pageL5586Gastón PomarNo ratings yet
- Weir Ball ValveDocument20 pagesWeir Ball ValveAmr ZayanNo ratings yet
- CH 05 - Discrete Probability Distributions: Page 1Document50 pagesCH 05 - Discrete Probability Distributions: Page 1Hoang HaNo ratings yet
- ETP48200-C5E2 Embedded Power User Manual (SMU02C)Document105 pagesETP48200-C5E2 Embedded Power User Manual (SMU02C)julio villaNo ratings yet
- FATEHGARH-II 765-400-220KV SLD REv 01-ModelDocument1 pageFATEHGARH-II 765-400-220KV SLD REv 01-ModelANUP KAMBOJNo ratings yet
- Why The Circumvention of Geoblocks SHD Be IllegalDocument26 pagesWhy The Circumvention of Geoblocks SHD Be IllegalArjun YadavNo ratings yet
- 2023 Global State of Business Analysis ReportDocument16 pages2023 Global State of Business Analysis ReportTam PhanNo ratings yet
- Kids Playing Music PowerPoint TemplatesDocument48 pagesKids Playing Music PowerPoint TemplatesKyou KazuneNo ratings yet
- Fuse Types, Short-Circuit, Load Variation and Current LimiterDocument5 pagesFuse Types, Short-Circuit, Load Variation and Current LimiterM Zeeshan Nawaz M Zeeshan NawazNo ratings yet
- 100870-623 System Configuration Doc, ARCDocument14 pages100870-623 System Configuration Doc, ARCAhmad Maaz AliNo ratings yet
- Veeam Iq: Propartner Training GuideDocument6 pagesVeeam Iq: Propartner Training GuideSakthi Murugan SNo ratings yet
- Automatic Battery ChargerDocument4 pagesAutomatic Battery ChargerInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Week 6 HomeworkDocument12 pagesWeek 6 HomeworkYinwu ZhaoNo ratings yet
- Deep Learning Based Trajectory Optimization For UAVDocument23 pagesDeep Learning Based Trajectory Optimization For UAVSalil SharmaNo ratings yet
- Product Information: Toshiba X-Ray Tube D-0712 / D-0712S / D-0712SB Stationary Anode X-Ray TubeDocument8 pagesProduct Information: Toshiba X-Ray Tube D-0712 / D-0712S / D-0712SB Stationary Anode X-Ray TubeKamilNo ratings yet
- Identity Theft and Your Social Security Number: SSA - GovDocument8 pagesIdentity Theft and Your Social Security Number: SSA - GovJade Allen100% (1)
- Summative Test On EMPOWERMENT TECHNOLOGY Week 7Document1 pageSummative Test On EMPOWERMENT TECHNOLOGY Week 7rufino delacruz100% (1)
- On MovingconeDocument7 pagesOn MovingconeSukhamMichaelNo ratings yet
- Mysqldayroma Mysqlshellandvisualstudiocodeextensionv3 220928142035 488af3f0Document126 pagesMysqldayroma Mysqlshellandvisualstudiocodeextensionv3 220928142035 488af3f0Junior NogueiraNo ratings yet
- Engineering For Sustainable Development: United Nations Educational, Scientific and Cultural OrganizationDocument183 pagesEngineering For Sustainable Development: United Nations Educational, Scientific and Cultural OrganizationdrhipoNo ratings yet
- Bank Customer With Loan Database SampleDocument1 pageBank Customer With Loan Database SampleRajib KumarNo ratings yet
- Stands Out From The Crowd in Mobile X-Ray Imaging: MOBILETT Elara MaxDocument20 pagesStands Out From The Crowd in Mobile X-Ray Imaging: MOBILETT Elara MaxShadi MasriNo ratings yet
- 009 Institutional HistoryDocument21 pages009 Institutional HistoryKelvin SouzaNo ratings yet
- Redway Power DeatailsDocument16 pagesRedway Power Deatailscleaningservice423No ratings yet
- XT3 Cable SpecDocument65 pagesXT3 Cable Specdưỡng PhanNo ratings yet