Professional Documents
Culture Documents
Chloride: HZ+ H Tic144 Tic13 Hci Ti 3tic14 4tic13 Tic13 Hc1-Tic14 +HZ
Uploaded by
shruti JadhavOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chloride: HZ+ H Tic144 Tic13 Hci Ti 3tic14 4tic13 Tic13 Hc1-Tic14 +HZ
Uploaded by
shruti JadhavCopyright:
Available Formats
Inorganic Syntheses, Volume VI
Edited by Eugene G. Rochow
Copyright © 1960 by McGraw-Hill Book Company, Inc.
52 INORGANIC SYNTHESES
16. TITANIUM (111) CHLORIDE
(Titanium Trichloride)
Hz+ 2H
+
H T i c 1 4 4 Tic13 HCI +
+
Ti 3TiC14 + 4TiC13
+
TiC13 HC1- Tic14 +Hz +
SUBMITTED BY T. R. INGRAHAM,* K. W. DOWNES,*AND P. MARIER*
CHECKED B Y z. z. HUGUS,J R . , AND
~ STANLEY STEIGERt
Titanium trichloride has been prepared by reducing tita-
nium tetrachloride with metals such as aluminum,l anti-
mony, lead, sodium amalgam,2 and t i t a n i ~ mand
, ~ also by
reduction with hydrogen.4 Hydrogen reduction is pre-
ferred to metal reduction in producing a pure product
because the low volatility and the tendency to dispropor-
tionation of titanium trichloride make the clean separation
of titanium trichloride from other metal chlorides difficult.
The procedure here described utilizes a closed Pyrex-glass
apparatus into which titanium tetrachloride is fed at its
vapor pressure a t room temperature, and from which tita-
nium trichloride is recovered as a fine brownish-purple pow-
der. The hydrogen required for the arc-induced reduction
is continuously regenerated over warm metallic titanium
from the hydrogen chloride product of the arc reactione5
The apparatus is simple to construct and operates almost
continuously, unattended, to produce about 1g. of titanium
trichloride per hour.
Procedure
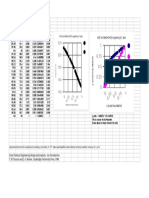
The apparatus is shown in Fig. 7. It is constructed
principally of 25-mm. Pyrex-glass tubing with packless Hoke
* Mines Branch, Department of Mines and Technical Surveys, Ottawa,
Canada; published with the permission of the director.
t University of Minnesota, Minneapolis, Minn.
T I T A N I U M ( I I I ) CHLORIDE 53
valves number 482,7 and 9, joined to the Pyrex by a brass-
to-Pyrex seal. One valve, 7, serves to control the access of
titanium tetrachloride, 5, to the system; the other, 9, seals
the system from vacuum, helium, and hydrogen sources.
8
3
10 4
FIG.7. Apparatus for the preparation of Ticla.
KEL-F gasket seals are used in the valves. When it is
necessary to open the apparatus for the discharge of prod-
uct, a Pyrex-glass pipe fitting with a Teflon gasket, 16, is
used.
The electrode section of the apparatus, 12, is its most
critical part. It consists of Pyrex tubing reduced from
25 mm. to 6.5 & 1.0 mm. i.d. over a length of 11 1 mm.
Tungsten electrodes are sealed into the Pyrex in such a way
that the space through which the arc passes is 22 5 5 mm.
in length. The top electrode is placed deeper within the
constricted zone than the bottom electrode, to aid in direct-
ing the reaction product downward through the arc. In
this way, the rapidly expanding gases from the arc force the
titanium trichloride product, 13, preferentially in the direc-
tion of the collection container, 14.
54 INORGANIC SYNTHESES
High voltages to produce the required arc within the gas
are provided by a Tesla coil leak tester, 11, connected to
one electrode. The other electrode is grounded. For long
operations, it is convenient to remove the outer casing of
the Tesla leak tester to assist in the dissipation of heat. A
titanium coupon, 3, of dimensions 1 by 15 by 120 mm. is
used for the regeneration of hydrogen and is heated by an
external tube furnace, 10, fitted with suitable controls to
maintain a temperature of 460 k 10". Hydrogen absorp-
tion by the titanium does not occur at the temperatures and
pressures of the operation.
To begin a run, the apparatus is evacuated to a pressure
of mm. of mercury and checked for leaks. When the
apparatus is found to be free from leaks and from any mois-
ture introduced during construction, helium is admitted
through 1,and an opening is made with a torch at 2 through
which the titanium coupon is placed at 3. It should be
clean and free of surface oxide coatings. The system is
then sealed with a torch at 2 and an opening made at 4.
Through this opening, against a gentle countercurrent of
helium, 50 ml. of purified titanium tetrachloride is admitted
to the apparatus at 5. The opening at 4 is then sealed with
a torch, the helium supply cut off, and the titanium tetra-
chloride frozen with liquid air." When freezing is com-
plete, the apparatus is thoroughly evacuated through 6,
and then valve 7 is closed. The titanium tetrachloride at
5 is then melted rapidly and brought to room temperature.
While this is taking place, hydrogen is admitted through 8
to 4 t 2 mm. of mercury with the use of a McLeod gage,
then valve 9 is closed and the furnace 10 surrounding the
titanium is brought up to 460 _+ 10". When this tempera-
ture is attained, the Tesla coil is started, and valve 7 is
opened to admit titanium tetrachloride vapor to the sys-
tem. This results immediately in the production of finely
powdered titanium trichloride which collects on the inside
of orifice 12 and is blown down the tube by the circulating
* Because of its lower freezing temperature, helium is preferred to argon.
TITANIUM(IIII) CHLORIDE 55
gas stream. The powdered titanium trichloride, 13, settles
in the graduated container, 14. Because the powder is elec-
trostatically charged, some of it is attracted to the glass
walls of the vertical tubing. It may be continuously dis-
lodged by a mechanical vibrator, 15.
At the end of a run, when reservoir 14 is filled with tita-
nium trichloride, valve 7 is closed and the gaseous atmos-
phere of the apparatus is removed by pumping through 6.
Helium is then admitted through 1, and clamp 16 loosened.
Against a gentle countercurrent of helium, container 14 is
removed and replaced by a duplicate container, previously
flushed with helium. After the clamps are tightened at 16,
the system is again evacuated to mm. of mercury and
cylinder hydrogen admitted through 8 to 4 2 2 mm. of
mercury. Valve 9 is closed and valve 7 opened. When
the arc is struck again, the reaction is resumed. The run
may be continued without further interruption or attention
until another container has been filled with titanium tri-
chloride, or the titanium or the titanium tetrachloride sup-
ply is exhausted. Occasionally it may be necessary to
replenish the hydrogen in the system when a large amount
of titanium trichloride has been formed, owing presumably
to the adsorption of hydrogen on the finely divided titanium
trichloride.
As an alternative to the injection of hydrogen into the
system at the beginning of an experiment, a solution of
hydrogen chloride in titanium tetrachloride may be substi-
tuted for the titanium tetrachloride. This can be prepared
by the addition of a small amount of water to titanium tet-
rachloride. The amount of the solution used is chosen so
that when the hydrogen is liberated from it during the run,
the pressure of hydrogen in the system will be approxi-
mately the same as that obtained by hydrogen injec-
tions. A convenient method of determining the amount of
hydrogen chloride in the titanium tetrachloride has been
published.'
The yield of titanium trichloride from the arc, based on
56 INORGANIC SYNTHESES
the consumption of metallic titanium, is over 90%. The
titanium trichloride, with the exception of adsorbed hydro-
gen, hydrogen chloride, or titanium tetrachloride, which
may be removed by a vacuum treatment, is pure and free
from titanium dichloride and other metallic chlorides.
Properties
Titanium trichloride, as prepared by this method, is a
brownish-purple powder with a bulk density varying from
0.07 to 0.25 g./cc. The particles vary in size from a few
microns to aggregates of several hundred microns. The
material is very reactive, being immediately hydrolyzed by
moisture and pyrophoric in air. Any transfer or handling
of the powder must be done in a dry box under nitrogen or
an inert gas, if contamination is to be prevented.
Titanium trichloride is stable up to temperatures of about
500". Above this temperature, under vacuum, it dispro-
portionates quantitatively to titanium dichloride and tita-
nium tetrachloride. Some of the dichloride disproportion-
ates to metal under these conditions.
References
1. W. C . SCHUMB and R. J. SUNDSTROM: J . Am. Chem. Soc., 66,596 (1933).
2. 0. PFORDTEN: Liebigs Ann. Chem., 237, 217 (1887).
3. J. D. FAST:2. anorg. u. allgem. Chem., 241, 42 (1939).
4. N. BAEZINGER and R. E. RUNDLE: Acta Cryst., 1, 274 (1948).
5. T. R. INGRAHAM, K. W. DOWNES, and P. MAFXER: Can. J . Chem., 36,
850 (1957).
Proc. Roy. SOC.(London), 204A, 309 (1950).
6. A. D. MCQUILLAN:
Can. J . Chem., 33, 1731 (1955).
7. T. R. TNGRAHAM:
You might also like
- Cryogenics Safety Manual: A Guide to Good PracticeFrom EverandCryogenics Safety Manual: A Guide to Good PracticeNo ratings yet
- ' United States - Patent Office: Patented Nov. 15, 1949Document7 pages' United States - Patent Office: Patented Nov. 15, 1949nazanin timasiNo ratings yet
- A System of Instruction in the Practical Use of the BlowpipeFrom EverandA System of Instruction in the Practical Use of the BlowpipeNo ratings yet
- TiH2 by Ball Milling 1997Document7 pagesTiH2 by Ball Milling 1997CrystalNo ratings yet
- Kroll ProcessDocument5 pagesKroll ProcessNavarro SalgadoNo ratings yet
- Case Study: Testing of PetroGuard For Use As Hazardous Chemical Spill ControlDocument2 pagesCase Study: Testing of PetroGuard For Use As Hazardous Chemical Spill ControlGuardian Environmental TechnologiesNo ratings yet
- HAZMAT Articles - Titanium TetrachlorideDocument2 pagesHAZMAT Articles - Titanium TetrachlorideRadko BankrasNo ratings yet
- Titanium Complete ReportDocument26 pagesTitanium Complete ReportRohan Stephen Luke67% (3)
- 10 PDFDocument2 pages10 PDFAlodia VaniaNo ratings yet
- Jurnal KristalisasiDocument7 pagesJurnal KristalisasiIrvan Key RizkyNo ratings yet
- Powerpoint Presentation On Extraction of TitaniumDocument31 pagesPowerpoint Presentation On Extraction of TitaniumLuis Sahoo90% (10)
- Kinetics of Titanium (LV) Chloride Oxidation: Sotiris Pratsinis, Hebi Bai, and Pratim BiswastDocument5 pagesKinetics of Titanium (LV) Chloride Oxidation: Sotiris Pratsinis, Hebi Bai, and Pratim Biswastghinasaleh97No ratings yet
- PP 34-35 Conceptual Design of Tritium Extraction System MIRALDocument2 pagesPP 34-35 Conceptual Design of Tritium Extraction System MIRALEditorijset IjsetNo ratings yet
- Zinc Electrowinning & Zinc CathodesDocument19 pagesZinc Electrowinning & Zinc CathodesfarhadNo ratings yet
- Hydrolysis of TiCl4 Initial Steps in The Production of TiO2 PDFDocument10 pagesHydrolysis of TiCl4 Initial Steps in The Production of TiO2 PDFganeshdhageNo ratings yet
- INTRODUCTION TO CHEMICAL ENGINEERING Assignment 1Document4 pagesINTRODUCTION TO CHEMICAL ENGINEERING Assignment 1vibbhavdchandan17No ratings yet
- Recent Progress in Titanium Extraction and RecyclingDocument14 pagesRecent Progress in Titanium Extraction and Recyclingraihan dzakyNo ratings yet
- Titanium : Karan Saxena Class Xi-A ROLL NO.-22Document18 pagesTitanium : Karan Saxena Class Xi-A ROLL NO.-22Karan SaxenaNo ratings yet
- Processes For Recycling: 4.4.1.2.1 Conventional Kroll ProcessDocument14 pagesProcesses For Recycling: 4.4.1.2.1 Conventional Kroll Processelma watNo ratings yet
- Lab Rep VD Draft 2Document12 pagesLab Rep VD Draft 2John Mar OrnaNo ratings yet
- Review Extractive Metallurgy Titanium - Daffa HandyanDocument6 pagesReview Extractive Metallurgy Titanium - Daffa HandyanDaffa HandyanNo ratings yet
- Nano Titania PowerPoint Presentationد رضويDocument40 pagesNano Titania PowerPoint Presentationد رضويArilu2010No ratings yet
- Thorium-Fueled Underground Power PlantDocument7 pagesThorium-Fueled Underground Power Plantalfonico100% (1)
- Electrochemical Process of Titanium ExtractionDocument5 pagesElectrochemical Process of Titanium ExtractionHooman BaghbanNo ratings yet
- Tici,: Production and Purification ofDocument5 pagesTici,: Production and Purification ofkannanjuNo ratings yet
- Get FileDocument14 pagesGet FilealiosarusNo ratings yet
- Laboratory Extraction of Copper From Chalcocite by Roasting Reduction and SmeltingDocument16 pagesLaboratory Extraction of Copper From Chalcocite by Roasting Reduction and SmeltingRodrigo GarcíaNo ratings yet
- TiO2 Titanium Dioxide Extraction Production Project PresentationDocument48 pagesTiO2 Titanium Dioxide Extraction Production Project Presentationkaranved780% (5)
- Thionyl Chloride ReactionsDocument7 pagesThionyl Chloride ReactionsMaxim MaximovNo ratings yet
- Uses of Titanium DioxideDocument5 pagesUses of Titanium DioxideAbdul RazzaqueNo ratings yet
- Mills 2002Document9 pagesMills 2002Herald MatiusNo ratings yet
- Advance in Titanium ProductionDocument9 pagesAdvance in Titanium ProductionAde SatriaNo ratings yet
- Synthesis of Sulfuric by The Contact Process: Student Laboratory ExperimentDocument2 pagesSynthesis of Sulfuric by The Contact Process: Student Laboratory ExperimentAnonymous spna8hNo ratings yet
- Production of Titanium DioxideDocument16 pagesProduction of Titanium Dioxidehaisamdo100% (1)
- Nitric Acid: Chemical Process IndustriesDocument13 pagesNitric Acid: Chemical Process Industries78623No ratings yet
- Cadmium Removal From Water Using Thiolactic Acid-Modified Titanium Dioxide NanoparticlesDocument5 pagesCadmium Removal From Water Using Thiolactic Acid-Modified Titanium Dioxide NanoparticlesSabiho GinoNo ratings yet
- Irnano Ti O2Document8 pagesIrnano Ti O2Sutha SenthilNo ratings yet
- A Novelmethod To Detect The Water Leakage From Tuyere Nose Cooling Circuit in BFDocument10 pagesA Novelmethod To Detect The Water Leakage From Tuyere Nose Cooling Circuit in BFAnuj SinghNo ratings yet
- IPT HCLDocument35 pagesIPT HCLParv pandyaNo ratings yet
- Some Significant Solids: For ofDocument6 pagesSome Significant Solids: For ofAhsanatul GhinaNo ratings yet
- IJNTR02050006 بحثي دكتوراهDocument7 pagesIJNTR02050006 بحثي دكتوراهHaidar AlmayahyNo ratings yet
- Andrussow HCN Process With Ammonia RecycleDocument5 pagesAndrussow HCN Process With Ammonia RecycleKarolina Wieszczycka100% (1)
- 3 Watkinson Fouling-ConceptDocument8 pages3 Watkinson Fouling-ConceptAnonymous AtAGVssJNo ratings yet
- Mechanism of Titanium Sponge Formation in The Kroll Reduction ReactorDocument11 pagesMechanism of Titanium Sponge Formation in The Kroll Reduction ReactorSrinivasulu PuduNo ratings yet
- HCLDocument8 pagesHCLRahul MathurNo ratings yet
- Chapter 57 - Titanium - 2015 - Handbook On The Toxicology of MetalsDocument10 pagesChapter 57 - Titanium - 2015 - Handbook On The Toxicology of MetalsChanWingSanNo ratings yet
- Evaporation of TitaniumDocument11 pagesEvaporation of Titaniumnandza99No ratings yet
- Lesson 26Document4 pagesLesson 26Alfredo MagtaanNo ratings yet
- Paper Cloracion y Reduccion Con MagnesioDocument3 pagesPaper Cloracion y Reduccion Con MagnesioBryan Roncal LlajarunaNo ratings yet
- SHEn 2015Document6 pagesSHEn 2015Wágner B SilvaNo ratings yet
- HCLDocument13 pagesHCLHussein AlkafajiNo ratings yet
- New Microsoft Office Word DocumentDocument37 pagesNew Microsoft Office Word DocumentmirzariponNo ratings yet
- United States Patent (19) : Piccolo Et Al. 56 References CitedDocument8 pagesUnited States Patent (19) : Piccolo Et Al. 56 References Citednazanin timasiNo ratings yet
- Toluenediamine PDFDocument15 pagesToluenediamine PDFAmalia RizkaNo ratings yet
- United States Patent (19) : Correia Et AlDocument3 pagesUnited States Patent (19) : Correia Et AlVatsal KardaniNo ratings yet
- The Growth Kinetics of Tio Nanoparticles From Titanium (Iv) Alkoxide at High Water/ Titanium RatioDocument5 pagesThe Growth Kinetics of Tio Nanoparticles From Titanium (Iv) Alkoxide at High Water/ Titanium RatioVarisa RahmawatiNo ratings yet
- TGA-FTIR Study of The Vapors Released by Triethylamine-Acetic Acid Mixtures 2012Document7 pagesTGA-FTIR Study of The Vapors Released by Triethylamine-Acetic Acid Mixtures 2012Gilson Furtado SouzaNo ratings yet
- The Diffusion of Hydrogen and Helium ThroughDocument8 pagesThe Diffusion of Hydrogen and Helium ThroughElenaNo ratings yet
- Chemical Processing of CeramicsDocument737 pagesChemical Processing of CeramicsArindam DeyNo ratings yet
- Production of Lithium Oxide by Decomposition Lithium Carbonate in The Flow of A Heat CarrierDocument6 pagesProduction of Lithium Oxide by Decomposition Lithium Carbonate in The Flow of A Heat CarrierArdu StuffNo ratings yet
- Air Velocity Thermal ResistanceDocument2 pagesAir Velocity Thermal ResistanceRabindraUpretiNo ratings yet
- Terminal Velocity of Falling Particle: Inputs From The Problem Suitable Drag CoefficientDocument2 pagesTerminal Velocity of Falling Particle: Inputs From The Problem Suitable Drag Coefficientshruti JadhavNo ratings yet
- Vle For Meoh/H2O System at 1 AtmDocument1 pageVle For Meoh/H2O System at 1 Atmshruti JadhavNo ratings yet
- CHDocument2 pagesCHDebottamSarkarNo ratings yet
- Curve Fitting TechniquesDocument14 pagesCurve Fitting TechniquesAveenNo ratings yet
- Quick Start Guide: New To Microsoft Teams? Use This Guide To Learn The BasicsDocument5 pagesQuick Start Guide: New To Microsoft Teams? Use This Guide To Learn The BasicsDaniel LeeNo ratings yet
- Utc 421PDocument4 pagesUtc 421PDeep PrajapatiNo ratings yet
- Tech Specs: Temperature ControllerDocument2 pagesTech Specs: Temperature Controllershruti JadhavNo ratings yet
- Process Equiment DesignDocument4 pagesProcess Equiment Designshruti JadhavNo ratings yet
- Tesla WirelessDocument6 pagesTesla WirelessMarko KoraćNo ratings yet
- Patent Application Publication (10) Pub. No.: US 2011/0156494 A1Document16 pagesPatent Application Publication (10) Pub. No.: US 2011/0156494 A1Tony GaryNo ratings yet
- Microwave and Millimeter Wave Power BeamingDocument31 pagesMicrowave and Millimeter Wave Power BeamingMonkeyFromMarsNo ratings yet
- Radiant Energy and Overunity 3Document9 pagesRadiant Energy and Overunity 3Zdenko PopNo ratings yet
- Investigative ProjectDocument37 pagesInvestigative ProjectAndrea Solas100% (4)
- The Manual of Free Energy Devices and Systems (1991)Document128 pagesThe Manual of Free Energy Devices and Systems (1991)kury93% (184)
- New QBhve3yearDocument29 pagesNew QBhve3yearArun SelvarajNo ratings yet
- Nikola Teslas Free Electricity Electronic CircuitDocument7 pagesNikola Teslas Free Electricity Electronic CircuitTudor ApostuNo ratings yet
- The Strange Life of Nikola TeslaDocument8 pagesThe Strange Life of Nikola TeslaMohammad MetlejNo ratings yet
- Tesla Coil Driver Using SG3525 1 PDFDocument1 pageTesla Coil Driver Using SG3525 1 PDFNguyen Phuoc HoNo ratings yet
- Exploring The Heart and Aether in Energy Medicine: AddressDocument44 pagesExploring The Heart and Aether in Energy Medicine: AddressWalter Schmitt100% (1)
- Magnetic Resonant AmplifierDocument31 pagesMagnetic Resonant Amplifierlittledigger100% (7)
- Easy Tesla CoilDocument17 pagesEasy Tesla CoilBeto53100% (2)
- Flying Russian EnglishDocument17 pagesFlying Russian EnglishRodrigo LozanoNo ratings yet
- SR - Part Test-2 Q.paperDocument12 pagesSR - Part Test-2 Q.paperM JEEVARATHNAM NAIDUNo ratings yet
- Understanding Nikola Tesla's Work - by Kon PappasDocument65 pagesUnderstanding Nikola Tesla's Work - by Kon Pappaskonpappas100% (4)
- Nikola TeslaDocument5 pagesNikola TeslaNinaNo ratings yet
- Tesla Switch Solar Charger To Debut at Bedini ConferenceDocument8 pagesTesla Switch Solar Charger To Debut at Bedini Conferenceutorrent411No ratings yet
- Chapter 3-Lossy CapacitorsDocument24 pagesChapter 3-Lossy Capacitorsmarceloassilva7992No ratings yet
- TeslaDocument3 pagesTeslaMike AtencioNo ratings yet
- TeslaDocument9 pagesTeslakrishnaNo ratings yet
- Mini Tesla Coil New deDocument28 pagesMini Tesla Coil New deHemang I VanzaraNo ratings yet
- Wireless ElectricityDocument26 pagesWireless ElectricityJames jill100% (1)
- PreviewpdfDocument41 pagesPreviewpdfJulio Cesar Tapia ReyesNo ratings yet
- Nikola Tesla Discovering The FutureDocument46 pagesNikola Tesla Discovering The Futureraul delgado100% (2)
- Math 1 Final Exam Math 1 Final ExamDocument2 pagesMath 1 Final Exam Math 1 Final ExamNelson LaurdenNo ratings yet
- Tesla Coil EquationsDocument10 pagesTesla Coil EquationsdmenonNo ratings yet
- What Exactly Is A Tesla CoilDocument88 pagesWhat Exactly Is A Tesla CoilKWojtekNo ratings yet
- FREE ENERGY Tesla Secrets For EverybodyDocument58 pagesFREE ENERGY Tesla Secrets For Everybodyxavier102772100% (1)
- Soal FisikaDocument30 pagesSoal FisikaKevin SahalaNo ratings yet
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactFrom EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactRating: 5 out of 5 stars5/5 (5)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 5 out of 5 stars5/5 (4)
- AP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeFrom EverandAP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeRating: 5 out of 5 stars5/5 (1)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideFrom EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNo ratings yet
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- Sodium Bicarbonate: Nature's Unique First Aid RemedyFrom EverandSodium Bicarbonate: Nature's Unique First Aid RemedyRating: 5 out of 5 stars5/5 (21)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- Guidelines for Chemical Process Quantitative Risk AnalysisFrom EverandGuidelines for Chemical Process Quantitative Risk AnalysisRating: 5 out of 5 stars5/5 (1)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (90)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsFrom EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsNo ratings yet
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeRating: 4 out of 5 stars4/5 (1)
- Process Plant Equipment: Operation, Control, and ReliabilityFrom EverandProcess Plant Equipment: Operation, Control, and ReliabilityRating: 5 out of 5 stars5/5 (1)
- AP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeFrom EverandAP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeNo ratings yet
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- The Periodic Table: A Very Short IntroductionFrom EverandThe Periodic Table: A Very Short IntroductionRating: 4.5 out of 5 stars4.5/5 (3)
- Tribology: Friction and Wear of Engineering MaterialsFrom EverandTribology: Friction and Wear of Engineering MaterialsRating: 5 out of 5 stars5/5 (1)
- Functional Safety from Scratch: A Practical Guide to Process Industry ApplicationsFrom EverandFunctional Safety from Scratch: A Practical Guide to Process Industry ApplicationsNo ratings yet
- High School Chemistry: Comprehensive Content for High School ChemistryFrom EverandHigh School Chemistry: Comprehensive Content for High School ChemistryNo ratings yet
- Taste: Surprising Stories and Science About Why Food Tastes GoodFrom EverandTaste: Surprising Stories and Science About Why Food Tastes GoodRating: 3 out of 5 stars3/5 (20)
- Bioplastics: A Home Inventors HandbookFrom EverandBioplastics: A Home Inventors HandbookRating: 4 out of 5 stars4/5 (2)
- Water-Based Paint Formulations, Vol. 3From EverandWater-Based Paint Formulations, Vol. 3Rating: 4.5 out of 5 stars4.5/5 (6)
- Nuclear Energy in the 21st Century: World Nuclear University PressFrom EverandNuclear Energy in the 21st Century: World Nuclear University PressRating: 4.5 out of 5 stars4.5/5 (3)