Professional Documents
Culture Documents
4350-Article Text-12361-1-10-20170903
Uploaded by
nhan phamCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
4350-Article Text-12361-1-10-20170903
Uploaded by
nhan phamCopyright:
Available Formats
Vijaya Lakshmi Marella et al / International Journal of Advances in Pharmaceutical Analysis 2017; 07(03): 24-27.
24
International Journal of Advances in Pharmaceutical Analysis
ISSN: 2277-9353 (Online)
Journal DOI: https://doi.org/10.7439/ijapa Research Article
A novel validated RP-HPLC method for the estimation of canagliflozin in
bulk and pharmaceutical dosage forms
Vijaya Lakshmi Marella*, Aisha Syed, Lakshmi Prasanna M and Buchi Naidu Nalluri
Department of Pharmaceutical Analysis, KVSR Siddhartha College of Pharmaceutical Sciences, Krishna University, India
QR Code *Correspondence Info:
Dr. Vijaya Lakshmi Marella
Department of Pharmaceutical Analysis,
KVSR Siddhartha College of Pharmaceutical Sciences,
Krishna University, India
*Article History:
Received: 22/08/2017
Revised: 29/08/2017
Accepted: 29/08/2017
DOI: https://doi.org/10.7439/ijapa.v7i3.4350
Abstract
The objective of the present study was to develop a simple, specific and accurate reverse phase high performance
liquid chromatographic method for the determination of Canagliflozin in bulk and pharmaceutical dosage forms. The
method is optimized on an inertsil ODS-3(250×4.6mm, 5µ) column with a mobile phase combination of 0.02% Formic
acid: Acetonitrile (40:60) at a flow rate 1.2ml/min and the eluents were monitored at 230nm. Under these LC conditions
Canagliflozin peak was eluted at 4.4 min. The developed method was validated as per ICH guidelines. The calibration
curve was linear over a concentration range of 10-50µg/ml (R2 =0.999) and the mean percentage assay was found to be
98.2. The statistical data proved that proposed method is accurate, precise and reproducible. The method which is LC-MS
compatible can be adopted in the routine analysis of Canagliflozin in bulk and pharmaceutical dosage forms.
Keywords: Canagliflozin, RP-HPLC Method development, Method validation.
1. Introduction
Canagliflozin is an antidiabetic drug used to
improve glycemic control in patients with type 2 diabetes.
Canagliflozin is an inhibitor of subtype 2 sodium-glucose
transport protein (SGLT2) which is responsible for at least
90% of the glucose reabsorption in the kidney (SGLT1)
being responsible for the remaining 10%). Canagliflozin is
chemically known as (2S, 3R, 4R, 5S, 6R)-2-{3-[5-[4-
Structure of canagliflozin
Fluoro-phenyl)-thiophen-2-ylmethyl]-4-methyl-phenyl}-6-
hydroxy methyl-tetrahydro-pyran-3, 4, 5-triol. Literature
review revealed that there were several analytical methods 2. Experimental Section
like HPLC [1-6], LC-MS [7], UV spectroscopy [8], HPTLC 2.1 Materials and Methods
[9] and only few RP-HPLC methods were reported for the 2.1.1 Chemicals and reagents
estimation of Canagliflozin in bulk and tablet dosage forms Canagliflozin procured from LAURUS LABS Pvt
and these methods used non-volatile buffers which reduces Ltd., Hyderabad and marketed Canagliflozin tablets
column efficiency. Hence the present work aimed at the Invokana (B-NO: FLZSN00), was bought from a local
development of a new sensitive, accurate and precise RP-
HPLC method for the estimation of Canagliflozin in bulk pharmacy with a labelled amount of 100mg. Methanol,
and tablet dosage forms and by using mobile phase that is Acetonitrile, Water, Formic acid, other chemicals and
compatible with LC-MS and to validate the same as per reagent used were of HPLC grade.
ICH guidelines [10-12].
IJAPA|VOL 07|ISSUE 03|2017 www.ssjournals.com
Vijaya Lakshmi Marella et al / A novel validated RP-HPLC method for the estimation of canagliflozin 25
2.1.2 Equipment 2.4.3 Precision
A Shimadzu prominence HPLC system provide System precision
with LC-10AT binary pump, SIL-20AHT auto sampler, and It was measured in terms of repeatability of
SPD-10A UV detector was used. Data acquisition was application of a homogenous solution by injecting six
carried out using LC solutions software. replicates of standard solution (30µg/ml) of drug substance.
2.2 Chromatographic conditions Method precision
Inertsil ODS-3 column (250×4.6mm, 5µ) and a It was measured in terms of repeatability of
mobile phase consisting of 0.02% Formic acid and application by giving six replicates of sample solution of
acetonitrile in the ratio of 40:60 (v/v) at a flow rate of drug product, 30µg/ml. In each case, standard deviation and
1.2ml/min were employed. For quantitative analytical percentage relative standard deviation were evaluated to
purpose, the eluents were monitored by the UV detector at a ascertain the repeatability of the method.
wavelength of 230nm with an injection volume of 10µl and 2.4.4 Accuracy
maintaining ambient temperature conditions. Accuracy of the developed method was confirmed
2.3 Preparation of stock and standards by performing standard addition and recovery studies. In
10mg of canagliflozin was accurately weighed and this method, the sample solution was spiked with known
transferred to a clean and dry 10ml volumetric flask concentrations of standard solutions within the linearity
containing 5mlof methanol, sonicated for 1minute and the range at three different levels, 80, 100 and 120% of test
volume is made up to the mark with methanol to obtain a concentration each in triplicate and analysed. The
final concentration of 1mg/ml. Calibration standards in the percentage recovery and percentage relative standard
concentration range of 10-50µg/ml were prepared by deviation were tabulated at each level.
adequate dilution of the stock solution with 0.02% Formic 2.4.5 Limit of detection (LOD), limit of quantification
(LOQ)
acid as diluent.
The LOD and LOQ were determined from the
2.4 Method validation
calibration curve by standard deviation of the response and
2.4.1 Specificity
slope method using the formula LOD= 3.3 σ/s and LOQ=
The specificity of the method was established by
10 σ/s, where, σ=standard deviation of the intercepts and S=
comparing the chromatograms of drug substance and drug
mean of slopes obtained in the calibration plots.
product with that of blank and placebo. Absence of peaks
2.4.6 Robustness
other than the drug peak such as those of impurities or
Robustness of the proposed method was
excipients ascertains the specificity of the method.
determined by making small deliberate changes in flow rate
2.4.2 Linearity
(±0.2ml/min) and mobile phase (±2%) of the optimized
Calibration curves were plotted to establish
method. The deviation in parameters such as tailing factor,
linearity of the method by employing five standard
theoretical plates at each condition was tabulated.
solutions in the concentration range of 10-50µg/ml each in
2.4.7 System suitability
triplicate using an injection volume of 10µl. Statistical
System suitability of the method was established
analysis of the resultant data was performed and parameters
by injecting increasing volumes (10-50µL) of the same
such as slope, intercept, and regression equation were
homogenous standard solution, 30µg/ml. The percentage
calculated.
relative standard deviation of retention time, tailing factor
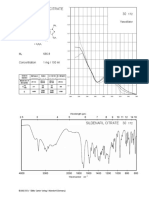
Figure 2: Calibration curve of Canagliflozin
and theoretical plate number with change in volume was
evaluated.
2.4.8 Stability of solution
Stability of stock solution was determined at a
temperature of 2-8oC by analysing the stock solution at
regular time intervals over a period of 24hours.
mV
35 Detector A Ch1:230nm
4.423
30
25
20
15
10
0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 min
IJAPA|VOL 07|ISSUE 03|2017 www.ssjournals.com
Vijaya Lakshmi Marella et al / A novel validated RP-HPLC method for the estimation of canagliflozin 26
2.5 Assay or any other impurities was observed at the elution time of
Canagliflozin tablets (Invokana-100mg) were Canagliflozin implying the specificity of the method for
taken to estimate the suitability of proposed method to detection of Canagliflozin.
estimate Canagliflozin in tablet formulation. For this 20 3.2.2 Linearity
tablets were taken, coating was removed, tablets were Linearity was established over a concentration
weighed individually to determine the average tablet range of 10-50µg/mL by plotting a graph of concentration
weight. Tablets are then powdered using mortor and pestle, versus respective peak areas. Regression analysis of the
the powder equivalent to 10mg of Canagliflozin was data thus obtained showed a good regression coefficient of
accurately weighed and transferred into a 10ml volumetric 0.999 with a regression equation y=6292.x-311.6. The
flask containing 5ml methanol. The final volume was made standard deviation and percentage relative standard
up to the mark with methanol. The solution was sonicated deviation values were calculated at each concentration
for 1min to ensure solubility of drug. The solution was which were found to be within limits i.e., less than 2. The
centrifuged for 2min and supernatant was collected, data obtained was shown in table 1.
adequate dilution was done using 0.02% formic acid obtain 3.2.3 Precision
a sample solution with a final concentration of 30µg/ml. System and method precision studies were carried
The drug content in the formulation was quantified by out by giving six replicate injections of 30µg/mL standard
comparing the peak area obtained with that of the standard solution of drug and sample solution of drug product
solution. respectively. The data obtained was shown in table 1. The
percentage relative standard deviation obtained was found
3. Results and discussion to be less than 2% for peak areas as per specifications
Literature survey revealed that there were several 3.2.4 Accuracy
analytical methods like Spectrophotometry, HPLC, LC-MS, Accuracy of the method was determined by
LC, GCMS and Only few RP-HPLC methods were reported employing standard addition method. The amount of
for the estimation of Canagliflozin in bulk and tablet dosage recovery and percentage relative standard deviation at each
forms and these methods used non-volatile buffers which level i.e., 80, 100 and 120% of test concentration was
reduces column efficiency. Hence the present work aimed calculated and it was found to be less than 2 percent. The
at the development of a new sensitive, accurate and precise accuracy data was represented in table 1.
RP-HPLC method for the estimation of Canagliflozin in Table 1: Linearity, Precision and Accuracy data
Validation data of Canagliflozin
bulk and tablet dosage forms and by using mobile phase
Range
that is compatible with LC-MS. 10-50µg/ml
Regression equation
Linearity Y= 6292.4±311.6
3.1 Method development Regression
0.999
Different trials were performed with various coefficient (R2)
Accuracy Level of Addition Mean Recovery
columns, mobile phase combination and diluents for
(n=3) (percent) (RSD)
optimum elution of Canagliflozin. Intial trial was carried 80 98.44(0.087)
with INERTSIL ODS-3 column (250×4.6mm, 5µ) using a 100 99.21(0.040)
mobile phase of 0.02% formic acid and acetonitrile in the 120 100.31(0.017)
Precision (n=6)
ratio of 50:50(v/v) at a flow rate of 1ml/min. With these
Average Peak area of
LC conditions, Canagliflozin peak was eluted preceding the System
the standard sample 189694(0.7622)
Precision
solvent front with a tailing factor (2.45). The mobile phase (RSD)
combination was further changed to 40:60(v/v) of 0.02% Average peak area of
Method
the Assay sample 188928.33(0.709)
formic acid and acetonitrile at a flow rate of 1ml/min. With Precision
(RSD)
these LC conditions, the Canagliflozin was eluted as sharp Percent assay
Mean ± SD 99.57 (0.705)
peak at 4.43min which showed ideal peak properties. The (n=3)
standard chromatogram of Canagliflozin was shown in 3.2.5 LOD and LOD
figure.1. Limit of detection and limit of quantification
The developed method was validated as per ICH values were calculated employing the average slope and
guidelines. standard deviation ratio method. The LOD and LOQ values
3.2 Method validation obtained were 0.00136µg/mL and 0.00414µg/mL
3.2.1 Specificity respectively.
Specificity of the method was as certained from 3.2.6 Robustness
the overlay of the standard, sample, blank, placebo Robustness studies were carried out by making
chromatograms. No interference by the matrix, excipients small deliberate changes in the flow rate, (±0.2mL/min) and
IJAPA|VOL 07|ISSUE 03|2017 www.ssjournals.com
Vijaya Lakshmi Marella et al / A novel validated RP-HPLC method for the estimation of canagliflozin 27
mobile phase composition (±2%) and %RSD was Acknowledgements
calculated for parameters such as number of theoretical The authors are thankful to K.V.S.R. Siddhartha
plates, tailing factor with respect to the changes were College of pharmacy for providing chemicals and
tabulated. The results obtained were satisfactory and shown instruments and Laurus Labs Pvt. Ltd, Hyderabad, India for
in table 2. providing samples for research.
Table 2: Robustness data
Chromatographic Retention Theoretical Tailing
parameters time(min) plates (N) factor References
Mobile phase composition ratio [1]. Maddu Suma, et al., RP-HPLC Method development
(0.02% Formic acid: Acetonitrile) and validation for the estimation of Canagliflozin
42:58 4.749min 7494.864 1.013 tablet dosage form.
40:60 4.423min 6938.178 1.041 [2]. Nareddy Preethi Reddy, et al., RP-HPLC Method
38:62 4.12min 6932.838 1.063 Development and validation for the simultaneous
Flow rate (mL/min) Estimation of Metformic and Canagliflozin in Tablet
1 5.246min 8290.151 1.041 Dosage Form.
1.2 4.423min 6938.178 1.041 [3]. Deepak Gaware, et al., A Validation Stability
1.4 3.818min 6141.29 1.033
indicating RP-HPLC method for Simultaneous
3.2.7 System suitability determination of metformin and Canagliflozin in
System suitability testing is an integral part of the Pharmaceutical formulation.
analytical procedure. System suitability studies were carried [4]. Ishpreet Kaur, et at., Development and Validation of a
out by injecting five times a 1µg/mL standard concentration Stability-Indicating Reverse Phase HPLC-PDA
of Canagliflozin at different injection volumes ranging from Method for Determination of Canagliflozin in Bulk
10-50µL. The %RSD for system suitability test parameters and Pharmaceutical Dosage Form
[5]. Uttam Prasad Panigrahy, et al., A novel validated RP-
like tailing factor and theoretical plate number were less
HPLC-DAD method for the simultaneous estimation
than 2 indicating the present conditions were suitable for of metformin Hydrochloride and Canagliflozin in bulk
the analysis of Canagliflozin. and pharmaceutical tablet dosage form with forced
3.3 Assay degradation studies.
The percentage assay of the drug in the [6]. Sonia D’souza, et al., Stability indicating assay
formulation was obtained by comparing the peak areas of method development and validation to simultaneously
the sample with that of the standard and they were found to estimate metformin hydrochloride and Canagliflozin
by RP-HPLC.
be 98.25 respectively.
[7]. Kobuchi S, et al., A validated LC-MS/MS method for
3.4 Stability of the analytical solution the determination of Canagliflozin, a sodium-glucose
The stability of the stock and standard solution co-transporter 2 (SGLT-2) inhibitor, in a lower
were determined by analysis the samples under volume of rat plasma: application to pharmacokinetic
refrigeration (8±1o C) at different time intervals up to studies in rats.
24hours. The percent variation in assay values at different [8]. Sharad Wakode, et al., Develop and validation of UV
time interval were found to be less than 2 when compared spectroscopic method for the determination of
Canagliflozin bulk and pharmaceutical dosage form.
with the initial zero time interval solution , thus indicating [9]. Kaur, et al., Development and validation of stability-
that the solution were stable for a period of 24hours when indicating HPTLC method for the estimation of
stored at 8o C. Canagliflozin in bulk and pharmaceutical dosage
form.
4. Conclusion [10]. Validation of analytical procedures, ICH Harmonized
Canagliflozin is an antidiabetic drug used to Therapeutic Guidelines, Q2B, 1997.
improve glycemic control in patients with type 2 diabetes. [11]. International conference on harmonization (ICH),
A sensitive RP-HPLC-PDA method was developed for the Validation of Analytical Procedures: text and
methodology Q2 (R1), Geneva, 2005.
determination of Canagliflozin in bulk and pharmaceutical
[12]. ICH Harmonized-Tripartite Guidelines. Validation of
dosage form with LC-MS compatible mobile phase Analytical Procedure.
composition in a short run time. The developed method was
validated as per ICH guidelines (Q2R1)
IJAPA|VOL 07|ISSUE 03|2017 www.ssjournals.com
You might also like
- Stability-indicating RP-HPLC method for racecadotril quantificationDocument8 pagesStability-indicating RP-HPLC method for racecadotril quantificationAnjay MalikNo ratings yet
- Jurnal HPLCDocument3 pagesJurnal HPLCRiche Dewata S.No ratings yet
- A Validated RP-HPLC Method For The Determination of Bendamustine Hydrochloride in Tablet Dosage Form Using Gemcitabine Hydrochloride As Internal StandardDocument8 pagesA Validated RP-HPLC Method For The Determination of Bendamustine Hydrochloride in Tablet Dosage Form Using Gemcitabine Hydrochloride As Internal StandardHeidi HughesNo ratings yet
- Development and Validation of New RP HPLC Method For Analysis of Capecitabine in Pharmaceutical Dosage Form - Ijsit - 2.1.3Document10 pagesDevelopment and Validation of New RP HPLC Method For Analysis of Capecitabine in Pharmaceutical Dosage Form - Ijsit - 2.1.3International Journal of Science Inventions TodayNo ratings yet
- 265-270_JPTCP+March+0016Document6 pages265-270_JPTCP+March+0016lifehaxus8426No ratings yet
- March April2011 Article6Document5 pagesMarch April2011 Article6Dita SusantiNo ratings yet
- Jps R 07091513Document5 pagesJps R 07091513Ahmed SuhailNo ratings yet
- Estabilidad de La IndometacinaDocument7 pagesEstabilidad de La IndometacinaJosé RojasNo ratings yet
- HPLC Method for Zinc Carnosine AnalysisDocument5 pagesHPLC Method for Zinc Carnosine AnalysisSouheila MniNo ratings yet
- HPLC METHOD VALIDATIONDocument5 pagesHPLC METHOD VALIDATIONbavirisettikiranNo ratings yet
- A Validated RP-HPLC Method For The Determination of Celecoxib in Bulk and Pharmaceutical Dosage FormDocument5 pagesA Validated RP-HPLC Method For The Determination of Celecoxib in Bulk and Pharmaceutical Dosage FormOskar LazaroNo ratings yet
- Research Articel (ESC)Document8 pagesResearch Articel (ESC)artaNo ratings yet
- Development and Validation of Reversed-Phase HPLC Method For Simultaneous Estimation of Rosuvastatin and Fenofibrate in Tablet Dosage FormDocument6 pagesDevelopment and Validation of Reversed-Phase HPLC Method For Simultaneous Estimation of Rosuvastatin and Fenofibrate in Tablet Dosage FormshraddhaJPNo ratings yet
- DiacereinDocument6 pagesDiacereinRikin ShahNo ratings yet
- Stability Indicating RP-HPLC Method for Drug EstimationDocument15 pagesStability Indicating RP-HPLC Method for Drug EstimationAfonso RobertoNo ratings yet
- Rapid RP-HPLC Method For The Quantification of Glabridin in Crude Drug and in Polyherbal FormulationDocument6 pagesRapid RP-HPLC Method For The Quantification of Glabridin in Crude Drug and in Polyherbal Formulationahmetgezer34No ratings yet
- Development and Validation of A HPTLC Method For Rivaroxaban in Human Plasma For A Pharmacokinetic StudyDocument6 pagesDevelopment and Validation of A HPTLC Method For Rivaroxaban in Human Plasma For A Pharmacokinetic Studypramod aloorNo ratings yet
- Validation of UV Spectrophotometric Method For Determination of AtenololDocument4 pagesValidation of UV Spectrophotometric Method For Determination of AtenololElfiaNeswitaNo ratings yet
- Development_and_validation_of_a_reversed (1)Document14 pagesDevelopment_and_validation_of_a_reversed (1)Karina Guadarrama HernándezNo ratings yet
- Development and Validation of HPLC Method For The Estimation of Nicergoline in Marketed FormulationsDocument5 pagesDevelopment and Validation of HPLC Method For The Estimation of Nicergoline in Marketed FormulationsRatnakaram Venkata NadhNo ratings yet
- Validated RP-HPLC Method For Analysis of Aripiprazole in A FormulationDocument6 pagesValidated RP-HPLC Method For Analysis of Aripiprazole in A Formulationblashyrkh_79No ratings yet
- Estimation of Satranidazole in Bulk and Tablet Dosage Form by RP-HPLCDocument3 pagesEstimation of Satranidazole in Bulk and Tablet Dosage Form by RP-HPLCGautam GurjarNo ratings yet
- 20211226124933a5 64 JCM 2108 2174Document11 pages20211226124933a5 64 JCM 2108 2174Venkat PalaganiNo ratings yet
- Nadifloxacin - HPTLC Stability Indicating PDFDocument8 pagesNadifloxacin - HPTLC Stability Indicating PDFNájla KassabNo ratings yet
- PoloDocument3 pagesPoloRaja AbhilashNo ratings yet
- Determination and Validation of Uv Spectrophotometric Method For Estimation of Bicalutamide TabletDocument5 pagesDetermination and Validation of Uv Spectrophotometric Method For Estimation of Bicalutamide TabletGembong Van BeethovenNo ratings yet
- 26537-Article Text-147230-1-10-20190103 PDFDocument4 pages26537-Article Text-147230-1-10-20190103 PDFSoheil JafariNo ratings yet
- 26537-Article Text-147230-1-10-20190103 PDFDocument4 pages26537-Article Text-147230-1-10-20190103 PDFSoheil JafariNo ratings yet
- Mitijps PaperDocument7 pagesMitijps PaperBrijeshkunvar MishraNo ratings yet
- 02.literature RiviewDocument8 pages02.literature RiviewPhariNo ratings yet
- 10 Ac18Document14 pages10 Ac18Oskar LazaroNo ratings yet
- Templete Research PaperDocument23 pagesTemplete Research Paperlalit4u78No ratings yet
- Indian Journal of Research in Pharmacy and BiotechnologyDocument144 pagesIndian Journal of Research in Pharmacy and BiotechnologyDebjit Bhowmik0% (1)
- Stability Indicating RP-HPLC Method For Simultaneous Determination of Perindopril and Indapamide in Pharmaceutical Dosage FormDocument9 pagesStability Indicating RP-HPLC Method For Simultaneous Determination of Perindopril and Indapamide in Pharmaceutical Dosage FormBoovizhikannan ThangabalanNo ratings yet
- Development and Validation of Stability Indicating HPTLC Method For Estimation of Swertiamarin in Bulk and Dosage FormDocument5 pagesDevelopment and Validation of Stability Indicating HPTLC Method For Estimation of Swertiamarin in Bulk and Dosage Formshraddha5jNo ratings yet
- 3845-Article Text-10897-2-10-20200114Document5 pages3845-Article Text-10897-2-10-20200114nhan phamNo ratings yet
- Article WJPR 1408528531Document9 pagesArticle WJPR 1408528531sripathy84No ratings yet
- A Validated RP-HPLC Method For The Estimation of Lapatinib in Tablet Dosage Form Using Gemcitabine Hydrochloride As An Internal StandardDocument5 pagesA Validated RP-HPLC Method For The Estimation of Lapatinib in Tablet Dosage Form Using Gemcitabine Hydrochloride As An Internal StandardRatnakaram Venkata NadhNo ratings yet
- Development and Validation of RP-HPLC Method For Simultaneous Estimation of Ivermectin and Clorsulon in Ivercam InjectionDocument9 pagesDevelopment and Validation of RP-HPLC Method For Simultaneous Estimation of Ivermectin and Clorsulon in Ivercam InjectionFaelFernandesNo ratings yet
- Assay of Anidulafungin by HPLC - 1Document10 pagesAssay of Anidulafungin by HPLC - 1Nur SamsiyahNo ratings yet
- Jaoac 0311Document11 pagesJaoac 0311adolfo olmosNo ratings yet
- Spectrochimica Acta Part A: Molecular and Biomolecular SpectrosDocument8 pagesSpectrochimica Acta Part A: Molecular and Biomolecular Spectrosadolfo olmosNo ratings yet
- To Develop HPLC Method For The Assay of Memantine Hydrochloride Tablets Using Refractive Index (Ri) DetectorDocument7 pagesTo Develop HPLC Method For The Assay of Memantine Hydrochloride Tablets Using Refractive Index (Ri) DetectorBaru Chandrasekhar RaoNo ratings yet
- A Comparative Study For The Quantitative Determination of Paracetamol in Tablets Using UVDocument7 pagesA Comparative Study For The Quantitative Determination of Paracetamol in Tablets Using UVRizqita Atikah SNo ratings yet
- Development and Validation of HPLC Method For The Estimation of Lapatinib in Bulk Drugs and Pharmaceutical FormulationsDocument8 pagesDevelopment and Validation of HPLC Method For The Estimation of Lapatinib in Bulk Drugs and Pharmaceutical FormulationspalkybdNo ratings yet
- A Validated Gradient Stability-Indicating LC MethoDocument7 pagesA Validated Gradient Stability-Indicating LC MethoHammam HafidzurahmanNo ratings yet
- Research Article: Received: 23 July 2016, Revised and Accepted: 30 September 2016Document7 pagesResearch Article: Received: 23 July 2016, Revised and Accepted: 30 September 2016AndreyNo ratings yet
- STABILITY INDICATING ASSAY METHOD DEVELOPMENT AND VALIDATION OF PREGABALIN IN PHARMACEUTICAL DOSAGE FORMS BY RP-HPLC P.Sneha, Prathima SrinivasDocument10 pagesSTABILITY INDICATING ASSAY METHOD DEVELOPMENT AND VALIDATION OF PREGABALIN IN PHARMACEUTICAL DOSAGE FORMS BY RP-HPLC P.Sneha, Prathima SrinivasiajpsNo ratings yet
- Lorno HPLCDocument5 pagesLorno HPLCmostafaNo ratings yet
- RP-HPLC Method Validation Closantel TabletsDocument6 pagesRP-HPLC Method Validation Closantel TabletsLaOde AdinNo ratings yet
- HPLC PDFDocument7 pagesHPLC PDFJorge AlisteNo ratings yet
- Ijppr 2021Document16 pagesIjppr 2021Smita NayakNo ratings yet
- Method validation for simultaneous estimation of Ofloxacin and Ornidazole by RP-HPLCDocument7 pagesMethod validation for simultaneous estimation of Ofloxacin and Ornidazole by RP-HPLCHugo Snchez muñozNo ratings yet
- Analytical Method Development and Validation of Teneligliptin by Using RP HPLC With ICH GuidelinesDocument5 pagesAnalytical Method Development and Validation of Teneligliptin by Using RP HPLC With ICH GuidelinesEditor IJTSRDNo ratings yet
- 1 s2.0 S0165022X05001119 MainDocument14 pages1 s2.0 S0165022X05001119 MainBivin EbenezerNo ratings yet
- OutDocument11 pagesOutAngel EivinoNo ratings yet
- 10.1515 - Revac 2022 0039Document12 pages10.1515 - Revac 2022 0039yordanosezerihun07No ratings yet
- Experimental approaches to Biopharmaceutics and PharmacokineticsFrom EverandExperimental approaches to Biopharmaceutics and PharmacokineticsNo ratings yet
- A Laboratory Manual of Physical PharmaceuticsFrom EverandA Laboratory Manual of Physical PharmaceuticsRating: 2.5 out of 5 stars2.5/5 (2)
- Voltammetric Oxidation of Ambroxol and ADocument5 pagesVoltammetric Oxidation of Ambroxol and Anhan phamNo ratings yet
- 12 Spectroscopic Method For Determination of Desloratadine PDFDocument4 pages12 Spectroscopic Method For Determination of Desloratadine PDFnhan phamNo ratings yet
- Fulltext - Ajapc v3 Id1062Document6 pagesFulltext - Ajapc v3 Id1062nhan phamNo ratings yet
- Farmacia Materias Activos en 161Document1 pageFarmacia Materias Activos en 161nhan phamNo ratings yet
- Multifunctional Oligoetherols and Thermally Resistant PU FoamsDocument7 pagesMultifunctional Oligoetherols and Thermally Resistant PU Foamsnhan phamNo ratings yet
- Formulation and in Vitro Evaluation of Fast Disintegrating Levocetirizine Dihydrochloride Tablets PDFDocument6 pagesFormulation and in Vitro Evaluation of Fast Disintegrating Levocetirizine Dihydrochloride Tablets PDFnhan phamNo ratings yet
- 0002 - Complete Monograph Methods - Esomeprazole - MMDocument29 pages0002 - Complete Monograph Methods - Esomeprazole - MMnhan phamNo ratings yet
- Additive 458 PDFDocument2 pagesAdditive 458 PDFdeziri mohamedNo ratings yet
- Effects of Omega-3 Co-Administered With Therapeutic Dose of Lornoxicam On Male Rats' LiverDocument6 pagesEffects of Omega-3 Co-Administered With Therapeutic Dose of Lornoxicam On Male Rats' Livernhan phamNo ratings yet
- Rifampicin, Isoniazid and Ethambutol TabletsDocument3 pagesRifampicin, Isoniazid and Ethambutol Tabletsnhan phamNo ratings yet
- ICH Guideline Q3C (R6) On Impurities: Guideline For Residual SolventsDocument39 pagesICH Guideline Q3C (R6) On Impurities: Guideline For Residual Solventsnhan phamNo ratings yet
- Pharmaceutical - Technology ExcipientDocument166 pagesPharmaceutical - Technology ExcipientlovehopeNo ratings yet
- Estimation of Levocetirizine in Bulk and Formulation by First Order Derivative Area Under Curve UV-Spectrophotometric MethodsDocument7 pagesEstimation of Levocetirizine in Bulk and Formulation by First Order Derivative Area Under Curve UV-Spectrophotometric Methodsnhan phamNo ratings yet
- Development and Validation of New HPLC MDocument6 pagesDevelopment and Validation of New HPLC Mnhan phamNo ratings yet
- 3845-Article Text-10897-2-10-20200114Document5 pages3845-Article Text-10897-2-10-20200114nhan phamNo ratings yet
- UV and IR Spectra Pharmaceutical Substances (UV and IR) and Pharmaceutical and Cosmetic Excipients (IR)Document1 pageUV and IR Spectra Pharmaceutical Substances (UV and IR) and Pharmaceutical and Cosmetic Excipients (IR)nhan phamNo ratings yet
- Influence of Some Starch Binders On The Brittle FRDocument5 pagesInfluence of Some Starch Binders On The Brittle FRnhan phamNo ratings yet
- Spectro Uorimetric Determination of Sildenafil: A New Analytical Alternative For Its AnalysisDocument8 pagesSpectro Uorimetric Determination of Sildenafil: A New Analytical Alternative For Its Analysisnhan phamNo ratings yet
- IP 2010 Sildenafil TabletsDocument2 pagesIP 2010 Sildenafil Tabletsnhan phamNo ratings yet
- Effects of Omega-3 Co-Administered With Therapeutic Dose of Lornoxicam On Male Rats' LiverDocument6 pagesEffects of Omega-3 Co-Administered With Therapeutic Dose of Lornoxicam On Male Rats' Livernhan phamNo ratings yet
- Moisture and volatile matter analysisDocument3 pagesMoisture and volatile matter analysisZeref SaintNo ratings yet
- Simultaneous Estimation of Related Compounds in Esomeprazole and Naproxen Tablets by Using Ion Pair Reverse Phase HPLCDocument5 pagesSimultaneous Estimation of Related Compounds in Esomeprazole and Naproxen Tablets by Using Ion Pair Reverse Phase HPLCnhan phamNo ratings yet
- Articulo 4.Document11 pagesArticulo 4.Yajaira MenesesNo ratings yet
- Dr. G Praveen KumarDocument36 pagesDr. G Praveen KumarMuhammad Riaz BhattiNo ratings yet
- Excipients Improve Dissolution of Compounded CapsulesDocument9 pagesExcipients Improve Dissolution of Compounded CapsulesJuan PerezNo ratings yet
- Antimalarial Drug Resistance: Linking To The Clinic: Plasmodium Falciparum Parasite BiologyDocument12 pagesAntimalarial Drug Resistance: Linking To The Clinic: Plasmodium Falciparum Parasite BiologyNava AlisiaNo ratings yet
- EMS Drug DilutionDocument21 pagesEMS Drug Dilutionthompson godfreyNo ratings yet
- Status EpilepticusDocument28 pagesStatus EpilepticusDaniel AlfredNo ratings yet
- Nursing Pharmacology COMPLETEDocument40 pagesNursing Pharmacology COMPLETEMonique Leonardo100% (8)
- Drug Study 1Document4 pagesDrug Study 1Anj MinguitoNo ratings yet
- J Neurol 2023 Jul 270 (7) 3654-3666Document13 pagesJ Neurol 2023 Jul 270 (7) 3654-3666lucas javier CobraNo ratings yet
- Somand David June 24 Hypertensive UrgencyDocument20 pagesSomand David June 24 Hypertensive UrgencyafifahNo ratings yet
- Nanotechnology and Its Applications in Medicine 2161 0444 1000247Document9 pagesNanotechnology and Its Applications in Medicine 2161 0444 1000247nur trismawatiNo ratings yet
- Initial Full-Dose Heparin For Adult: 1-Venous Thromboembolism (DVT/PE), TreatmentDocument3 pagesInitial Full-Dose Heparin For Adult: 1-Venous Thromboembolism (DVT/PE), TreatmentLamNo ratings yet
- Drug Study of EpinephrineDocument3 pagesDrug Study of EpinephrineChristina mikaela CabusaoNo ratings yet
- Fenofibratenicotinamide Cocrystals Molecular Docking Studies and Evaluation in Tablet Dosage FormDocument9 pagesFenofibratenicotinamide Cocrystals Molecular Docking Studies and Evaluation in Tablet Dosage FormvinayNo ratings yet
- Drugs Used in Pediatric DentistryDocument33 pagesDrugs Used in Pediatric Dentistryaamee9690% (10)
- Register For Medicines 2019 Human UseDocument320 pagesRegister For Medicines 2019 Human UseLeroy LupiyaNo ratings yet
- Malaria: MicrobiologyDocument9 pagesMalaria: MicrobiologyAbdul-rhman AlmarshoodNo ratings yet
- Literature Review Parkia BiglobosaDocument7 pagesLiterature Review Parkia Biglobosaeldcahvkg100% (1)
- Cefuroxime Drug StudyDocument1 pageCefuroxime Drug StudyACOB, Jamil C.No ratings yet
- Discontinuing An IV InfusionDocument2 pagesDiscontinuing An IV InfusionNoel83% (6)
- 08-Cholinergic AntagonistsDocument18 pages08-Cholinergic AntagonistsمشاعرمبعثرةNo ratings yet
- Cisplatin dosage, side effects, and nursing responsibilitiesDocument1 pageCisplatin dosage, side effects, and nursing responsibilitieskyawNo ratings yet
- State of The Evidence Traffic Lights 2019: Systematic Review of Interventions For Preventing and Treating Children With Cerebral PalsyDocument22 pagesState of The Evidence Traffic Lights 2019: Systematic Review of Interventions For Preventing and Treating Children With Cerebral PalsyCéline FrédérickNo ratings yet
- Structure-Activity Relationship of Antithrombotic Polysaccharide DerivativesDocument4 pagesStructure-Activity Relationship of Antithrombotic Polysaccharide DerivativesCamelia AlexandraNo ratings yet
- Vascular Pharmacology Smooth Muscle (Issn Book 78) 1st EditionDocument430 pagesVascular Pharmacology Smooth Muscle (Issn Book 78) 1st EditionΑΘΑΝΑΣΙΟΣ ΚΟΥΤΟΥΚΤΣΗΣNo ratings yet
- Anggi Retno Wardani - 40121064Document91 pagesAnggi Retno Wardani - 40121064febyNo ratings yet
- Sedative HypnoticsDocument41 pagesSedative HypnoticsPatrick Juacalla100% (2)
- Dutta - Bioavailability of Divalproex Extended-Release Formulation - 2004Document8 pagesDutta - Bioavailability of Divalproex Extended-Release Formulation - 2004Thaís Nunes dos AnjosNo ratings yet
- Assignment - Headache DisordersDocument2 pagesAssignment - Headache DisordersAyessa SalazarNo ratings yet
- A Review On Phytochemical and Pharmacological Properties of Holy 2018 PDFDocument16 pagesA Review On Phytochemical and Pharmacological Properties of Holy 2018 PDFCDB 1st Semester 2077No ratings yet