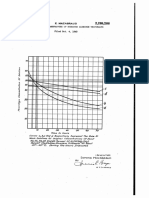
Professional Documents
Culture Documents
Us 2019415
Us 2019415
Uploaded by
Özlem Yılmaz0 ratings0% found this document useful (0 votes)
5 views2 pagespatent
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentpatent
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
5 views2 pagesUs 2019415
Us 2019415
Uploaded by
Özlem Yılmazpatent
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
Patented Oct.
29, 1935
2,019,415
UNITED STATES PATENT OFFICE
2,019,415
ALUMINIUM FORMATE AND PROCEss oF
MAKING THE SAVE
Oskar Jochem, Greiz-Dolau, and Theodor Hea
nig, Greiz, Germany, assignors to firm Zschim
mer & Schwarz Chemische Fabrik Döllau,
'Greiz-Dolau, Germany
No Drawing. Application April 2, 1933, Serial
No. 665,840. In Germany April 18, 1932
4. Claims. (C. 260-11)
The object of this invention is to make a solid
aluminium formate recrystallizable from Water fatty acids
acids with the corresponding free fatty
in excess and leaving the salt solution, thus
Solutions and the product thereof. acidified, to itself, without evaporating down, at
The aluminium salts of the lower fatty acids, the Ordinary temperature for some time, if neces
for example aluminium formate and aluminium sary with agitation.
acetate, are mainly employed for impregnation minium salts may beInobtained this way, crystalline alu- 5
from a solution
in the textile industry and for pharmaceutical Of aluminium formate of a specific gravity of
purposes: They are principally on the market 1.045 and from a solution of aluminium
0.
in the form of fairly dilute aqueous solutions, of a specific gravity of 1.015. According acetate to the
Such as, for example, aluminium acetate in the observations which have been made up to the 10
form of a 7.5 to 8% solution. Repeated attempts present, these concentrations ought not to be
have already been made to manufacture con considered as the minimum concentrations nec
centrated solutions or even solid products of the essary for the success of the proceSS. In prac
s
aluminium Salts of lower fatty acids. So far, tice, starting solutions of higher concentration
however, these attempts have failed to produce are preferably employed, for example, a solution l8
Satisfactory results, firstly because in the con of aluminium acetate of a specific gravity of
centration of the solutions turbidity and deposits 1.075 to 1.100. Complete separation of the salts:
frequently Occur, and secondly because the evap present in the starting solutions does not take
Oration and drying causes decomposition and es place, but the mother liquors may be employed
20 cape of free fatty acids. Furthermore, the Solid
end products obtained by the processes known again. The excess of free acids may be selected 20
as desired within Wide limits.
heretofore were generally either not at all Solu It has further been found that, by using suffi
ble, or Only partly soluble, in Water. ciently strong aqueous aluminium formate Solu
It has already been proposed to manufacture tions, water-soluble
25 Water-soluble crystalline aluminium formate by mate may be obtainedcrystalline aluminium for
even without the addition 28
evaporating an aluminium formate Solution in of free formic acid. Apparently, aluminium
the presence of an excess of formic acid until formate forms slightly Supersaturated Solutions,
a film appears and leaving the solution to crys which separate crystalline aluminium formate
tallize at a temperature of 25-30° C. The crys
30 talline aluminium formate So obtained cannot only when they are left to themselves for some
be recrystallized from an aqueous Solution, and time at the ordinary temperature, if desired with
agitation.
30
obviously, therefore, it is not stable in aqueous Aluminium formate Solutions. With a Specific
solutions. In this known manner of manufac gravity of 1.100 only exhibit slight crystal for
35 ture a considerable expenditure of heat is neces mation after several Weeks When no free formic
sary and loss of formic acid occurs. This process acid is added. The specific gravity of 1.100 may
has not obtained any practical importance. therefore be regarded as the minimum concen
It has furthermore been proposed to manufac tration for the manufacture of aluminium for
ture water-Soluble basic aluminium acetate and mate Without the addition of free formic acid.
40 aluminium formate by drying the aqueous Solu Aluminium formate Solutions of higher Concern
tions of these aluminium Salts in a finely atom tration yield large quantities of crystalline alu- 40
ized form. Even in the case of this process, it is minium formate even in a short time.
not possible to work Without considerable expen The adjustment of the aluminium. Salt Solu
diture of heat for the evaporation of the large tions to the desired specific gravity may be ef
45 quantities of water, and furthermore a compli fected by the reaction of suitable quantities of
cated and expensive apparatus is required for aluminium sulphate, calcium carbonate, and the 4:
this purpose. In this process also no crystals are
lower fatty acids in question.
obtained but only more or leSS finely-divided dry The separation of the crystals from the
residues, which still contain all the impuritiesaluminium salt solution may be assisted by add
50
of the Original Solutions. ing slight quantities of the desired crystalline
It has now been found that aluminium salts aluminium salt as inoculating Substance, if de- SO
of the lower fatty acids, more particularly alu sired after the addition of the free fatty acid.
minium acetate and aluminium formate, may be The crystal formation is likewise promoted by
manufactured without the expenditure of heat constant or occasional agitation.
and without loss of fatty acids, by mixing aque
ous solutions of the aluminium salts of lower beThe crystalline aluminium salts obtained may
readily separated from the mother liquor by 35
2 2,019,415
filtration. The aluminium formate may be line aluminium formate as inoculating substance
washed without difficulty with water, dried and tion.and is then left to itself with Occasional agita
recrystallized from Water. After 65 hours, 2580 grams of crystalline
The mother liquors remaining after the sepa aluminium formate of the same composition as
ration of the crystals may be employed again in Example 3 will have separated Out. 5
for new mixtures for the manufacture of crystal The present invention represents a consider
line aluminium salt, if desired with the free fatty able technical advance, because by means there
acid contained in the said mother liquorS. of it is p0SSible to obtain aluminium Salts of the
Eacamples lower fatty acids, such as for example aluminium
O formate and aluminium acetate, in a crystalline 10
1. 100 litres of a 7.5 to 8 per cent. Solution water-soluble form on a technically satisfactory
of basic aluminium acetate is mixed with 50 Scale from aqueous Solutions.
kilograms of glacial acetic acid, and is left to We claim:
itself for three days at the ordinary temperature 1. The method of preparing Water-soluble
With constant or occasional agitation. The fine crystalline aluminium formate which comprises lis
ly crystalline aluminium acetate which separates adding, at Ordinary temperatures, formic acid
out is filtered off. Yield: 2 kilograms. to an aqueous aluminium formate Solution of at
2. 100 litres of an aluminium formate Solution least 1.045 specific gravity, and immediately
of a specific gravity of 1.100 is mixed with 15 thereupon leaving the acidified solution to itself
kilograms of 85 per cent. formic acid and 1 kilo at Ordinary room temperatures for some time 20
gram of crystalline aluminium formate, and is with agitation, and separating the crystals from
agitated for three days at the ordinary tempera the mother liquor.
ture. The crystalline aluminium formate de 2. The method of preparing water-soluble
posited is separated by filtration, Washed with crystalline aluminium formate which comprises
25 Water and dried in the air at 50° C. Yield: 3 preparing, at ordinary temperatures, an alu-25
kilograms. minium formate solution of at least 1.100 specific
3. 10 litres of an aluminium formate Solution gravity and leaving the Solution to itself to
adjusted to a specific gravity of 1.152, the crystallize without application of heat thereto.
analysis of which gives 84.4 grams Al2O3 and 3. The method of preparing water-soluble
30 201 grams HCOOH per litre, is left to itself at the crystalline aluminium formate which comprises 30
ordinary temperature with Occasional agitation. preparing, at Ordinary temperatures, an alu
After 120 hours, 50 grams of crystalline alu minium formate Solution of at least 1.100 specific
minimum formate will have Seperated out, the gravity, adding a small quantity of crystalline
analysis of which gives 12.54 per cent. Al, 62.42 aluminium formate, agitating for SOme time at
3 5 per cent. H.COOH and the remainder 25.04 per ordinary temperatures and separating the crys-35
cent. Water, and which thus corresponds to the tals from the Solution, the entire process being
formula A1(HCOO)3.3H2O. carried out without application of heat.
4. 10 litres of an aluminium formate solution 4. Solid aluminium formate of the formula,
adjusted to a specific gravity of 1.220, the Al(HCOO)3.3H2O, exhibiting the property of re
40 analysis of which gives 125.5 grams Al2O3, and Crystallizing from aqueous solutions. 40
336 grams HCOOH per litre, is mixed at the OSKAR JOCHEMI.
ordinary temperature with 50 grams of crystal THEODOR HENNIG.
You might also like
- Ammonium Sulfate PDFDocument6 pagesAmmonium Sulfate PDFSabhaya Chirag100% (1)
- Acid Etch For Aluminum ExtrusionDocument10 pagesAcid Etch For Aluminum ExtrusionLeonel Velasquez100% (1)
- OpenPitOpt PDFDocument150 pagesOpenPitOpt PDFJose JoelNo ratings yet
- Deformation of SolidsDocument31 pagesDeformation of SolidsWadood Ahmed100% (4)
- Maint - Check List - Oil Filled TransforsDocument2 pagesMaint - Check List - Oil Filled TransforsramNo ratings yet
- AluminaDocument5 pagesAluminaehsannasiri100% (1)
- Low Temp. EvaporationDocument40 pagesLow Temp. EvaporationManvi Sharma100% (1)
- The Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresFrom EverandThe Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresRating: 5 out of 5 stars5/5 (1)
- Eni - Iraq Zubair Oil Field Development Project: Alstom Middle East, FZE, Dubai, UAEDocument7 pagesEni - Iraq Zubair Oil Field Development Project: Alstom Middle East, FZE, Dubai, UAEmohammad.a.d94No ratings yet
- Preparation of Alum CBSE 12Document12 pagesPreparation of Alum CBSE 12Aditya ChopraNo ratings yet
- Android Application DevelopmentDocument32 pagesAndroid Application DevelopmentAmi VermaNo ratings yet
- Chemistry Investigatory ProjectDocument11 pagesChemistry Investigatory ProjectSidharth Sarma67% (3)
- Alumina ProcessDocument16 pagesAlumina Processashwini_kumar1984No ratings yet
- Potash Alum Chemistry Invesigatory Project Class 12Document14 pagesPotash Alum Chemistry Invesigatory Project Class 12Niklesh SelvaNo ratings yet
- To Prepare Potash Alum From Aluminium ScrapDocument21 pagesTo Prepare Potash Alum From Aluminium ScrapRitik Mittal50% (4)
- Class 12th Chemistry Project Preparation of Potash AluminiumDocument15 pagesClass 12th Chemistry Project Preparation of Potash AluminiumKritika SharmaNo ratings yet
- Brochure PolymegDocument27 pagesBrochure PolymegÖzlem YılmazNo ratings yet
- Steel Making2Document147 pagesSteel Making2satish_trivediNo ratings yet
- Aluminium CompoundDocument17 pagesAluminium Compoundmouse1201100% (1)
- Chemistry Investigatory Project On PrepaDocument12 pagesChemistry Investigatory Project On PrepaSarvesh BoseNo ratings yet
- Logs:: ConcentratorDocument9 pagesLogs:: ConcentratorEniNo ratings yet
- The Preparation of Common Alum From Scrap AluminiumDocument13 pagesThe Preparation of Common Alum From Scrap AluminiumJoe Calvin RossarioNo ratings yet
- A01 527 PDFDocument17 pagesA01 527 PDFjaimeNo ratings yet
- United States Patent (191: Mccallister (45) June 28, 1977Document3 pagesUnited States Patent (191: Mccallister (45) June 28, 1977Calvin VinNo ratings yet
- Potash Alum 12Document16 pagesPotash Alum 12Xiao ZhanNo ratings yet
- (Patent) US1618105Document2 pages(Patent) US1618105Pavita SalsabilaNo ratings yet
- Aluminum Compounds, Inorganic: 1. Aluminum Sulfate and AlumsDocument16 pagesAluminum Compounds, Inorganic: 1. Aluminum Sulfate and AlumspradipNo ratings yet
- New Microsoft Office Word DocumentDocument26 pagesNew Microsoft Office Word DocumentAnitha SathishNo ratings yet
- ProjectDocument7 pagesProjectsivaNo ratings yet
- Potashalum Chem ProjectDocument16 pagesPotashalum Chem ProjectJanani KaNo ratings yet
- Chemistry Project On Potash AlumDocument9 pagesChemistry Project On Potash AlumQwerty UiopNo ratings yet
- Investigatory Project ChemDocument12 pagesInvestigatory Project Cheminfinity GOD GamersNo ratings yet
- Chemistry ProjectDocument18 pagesChemistry ProjectMd AzmalNo ratings yet
- Us2094573-Production of Potassumsulphate Ammonium Sulphate Double SaltDocument2 pagesUs2094573-Production of Potassumsulphate Ammonium Sulphate Double Saltkvsj2001No ratings yet
- Chemistry Project 2010-11Document14 pagesChemistry Project 2010-11Sejal PandeyNo ratings yet
- Process: The BayerDocument2 pagesProcess: The BayerKurtWatleyNo ratings yet
- Chemistry ProjectDocument15 pagesChemistry Projectsubhashchandraj70No ratings yet
- CHEMISTRY PROJECT Class XIIDocument15 pagesCHEMISTRY PROJECT Class XIIKapil KumarNo ratings yet
- SukanyaDocument2 pagesSukanyaSrijaNo ratings yet
- Chemistry Investigatory Project On Preparationn-Of-Potassh-AlumDocument14 pagesChemistry Investigatory Project On Preparationn-Of-Potassh-AlumsumitshashimaNo ratings yet
- Us2554459Document4 pagesUs2554459Farah Aulia Prihasti 1707122999No ratings yet
- United States Patent Office: Patented Oct. 17, 1950Document4 pagesUnited States Patent Office: Patented Oct. 17, 1950varadjoshi41No ratings yet
- Chemistry ProjectDocument19 pagesChemistry ProjectNimisha DhakadNo ratings yet
- Potash Alum FinalDocument13 pagesPotash Alum FinalAnonymous yX4s4KHeEuNo ratings yet
- Descripsi Process Direct Neutralization Oe Synthetic ManufactureDocument9 pagesDescripsi Process Direct Neutralization Oe Synthetic Manufacturerifqi fatmalaNo ratings yet
- Patent Office.: United StatesDocument2 pagesPatent Office.: United StatesAlan PradanaNo ratings yet
- Laeticia Rodrigues - P21008 - MetullurgyDocument2 pagesLaeticia Rodrigues - P21008 - MetullurgyLAETICIA RODRIGUESNo ratings yet
- Chemistry Investigatory AaravDocument11 pagesChemistry Investigatory AaravaaravNo ratings yet
- Dokumen - Tips - Chemistry Project On Preparation of Potash AlumDocument13 pagesDokumen - Tips - Chemistry Project On Preparation of Potash AlumSarvesh BoseNo ratings yet
- Aiiii: July 7, 1942. E. Mazabraud 2,289,286Document3 pagesAiiii: July 7, 1942. E. Mazabraud 2,289,286Özlem YılmazNo ratings yet
- Aluminium As Building MaterialDocument10 pagesAluminium As Building MaterialKŕishňã AgârwaĺNo ratings yet
- Chemistry Project On Preparation of Potash Alum PDFDocument3 pagesChemistry Project On Preparation of Potash Alum PDFSreeja SatheeshNo ratings yet
- Shaurya Chem Project - Potash Alum From Scrap AluminiumDocument8 pagesShaurya Chem Project - Potash Alum From Scrap AluminiumShaurya KapoorNo ratings yet
- Recovery of Fine Aluminum Hydroxide With High Whiteness Index From Low Quality Bauxite Using Caustic Roasting and Water LeachingDocument10 pagesRecovery of Fine Aluminum Hydroxide With High Whiteness Index From Low Quality Bauxite Using Caustic Roasting and Water LeachingSyifaNo ratings yet
- Reaction TypesDocument10 pagesReaction TypesaqibazizkhanNo ratings yet
- The Bayer ProcessDocument1 pageThe Bayer ProcessAqsa BanoNo ratings yet
- ChemDocument21 pagesChemNaitik TiwariNo ratings yet
- CHEMDocument14 pagesCHEMBimal KumariNo ratings yet
- Synthesis of Common Alum: Chrome Alum KCR (So) - 12H ODocument11 pagesSynthesis of Common Alum: Chrome Alum KCR (So) - 12H OidaayudwitasariNo ratings yet
- Chemistry Investigatory ProjectDocument14 pagesChemistry Investigatory ProjectDinesh Kanna KingNo ratings yet
- Chemistry ProjectDocument13 pagesChemistry ProjectSrijaNo ratings yet
- Cupery Sulfamic Acid A New Industrial ChemicalDocument5 pagesCupery Sulfamic Acid A New Industrial ChemicalAaron Troy SmallNo ratings yet
- Chem Investigatory ProjectDocument9 pagesChem Investigatory Projectsaai gopanNo ratings yet
- Investigatory Project On Preparation of Potash Alum From Scrap AluminiumDocument20 pagesInvestigatory Project On Preparation of Potash Alum From Scrap AluminiumRam Swaroop0% (1)
- 2Document10 pages2soumya.sj09No ratings yet
- The Effect of HMMM Crosslinker On The Coating Properties of Chemoenzymatically Synthesized Aliphatic Urethane Oil - PCI MagazineDocument10 pagesThe Effect of HMMM Crosslinker On The Coating Properties of Chemoenzymatically Synthesized Aliphatic Urethane Oil - PCI MagazineÖzlem YılmazNo ratings yet
- Werner Blank Reaction MechanismDocument19 pagesWerner Blank Reaction MechanismÖzlem YılmazNo ratings yet
- KahlWax 7304 - 2020-06-04Document1 pageKahlWax 7304 - 2020-06-04Özlem YılmazNo ratings yet
- 135 PDFDocument9 pages135 PDFÖzlem YılmazNo ratings yet
- SDS - Dispex® AA 4144Document9 pagesSDS - Dispex® AA 4144Özlem YılmazNo ratings yet
- Determination of The Intrinsic Viscosity and Molecular Weight of Poly (Methyl Methacrylate) Blends (#648289) - 903143Document6 pagesDetermination of The Intrinsic Viscosity and Molecular Weight of Poly (Methyl Methacrylate) Blends (#648289) - 903143Özlem YılmazNo ratings yet
- Texapon N 70 TDDocument5 pagesTexapon N 70 TDÖzlem YılmazNo ratings yet
- Safety Data Sheet: According To 1907/2006/EC, Article 31Document6 pagesSafety Data Sheet: According To 1907/2006/EC, Article 31Özlem YılmazNo ratings yet
- Safety Data Sheet: According To 1907/2006/EC, Article 31Document6 pagesSafety Data Sheet: According To 1907/2006/EC, Article 31Özlem Yılmaz0% (1)
- Tara Gums DsDocument8 pagesTara Gums DsÖzlem YılmazNo ratings yet
- United States Patent: (10) Patent No.: US 9.234,068 B2Document21 pagesUnited States Patent: (10) Patent No.: US 9.234,068 B2Özlem YılmazNo ratings yet
- Aiiii: July 7, 1942. E. Mazabraud 2,289,286Document3 pagesAiiii: July 7, 1942. E. Mazabraud 2,289,286Özlem YılmazNo ratings yet
- The Castor Oil Based Water Borne Polyurethane Dispersion Effect of - NCO/OH Content: Synthesis, Characterization and PropertiesDocument11 pagesThe Castor Oil Based Water Borne Polyurethane Dispersion Effect of - NCO/OH Content: Synthesis, Characterization and PropertiesÖzlem YılmazNo ratings yet
- Aluminum Formate (AF) : Synthesis, Characterization and Application in Dye Wastewater TreatmentDocument12 pagesAluminum Formate (AF) : Synthesis, Characterization and Application in Dye Wastewater TreatmentÖzlem YılmazNo ratings yet
- Elenco Prodotti: Products ListDocument31 pagesElenco Prodotti: Products ListÖzlem YılmazNo ratings yet
- Dhairya D. Mehta, M. Cem Akatay, Eric A. Stach, W. Nicholas Delgass, Fabio H. RibeiroDocument1 pageDhairya D. Mehta, M. Cem Akatay, Eric A. Stach, W. Nicholas Delgass, Fabio H. RibeiroÖzlem YılmazNo ratings yet
- Hydrogen and Syngas Production From Gasification of Lignocellulosic Biomass in Supercritical Water MediaDocument5 pagesHydrogen and Syngas Production From Gasification of Lignocellulosic Biomass in Supercritical Water MediaÖzlem YılmazNo ratings yet
- AME 20231 SolutionsDocument78 pagesAME 20231 SolutionsÖzlem YılmazNo ratings yet
- Castor OilDocument6 pagesCastor OilÖzlem YılmazNo ratings yet
- Peikko HPKM Column Shoe Technical ManualDocument24 pagesPeikko HPKM Column Shoe Technical ManualstrakdesmeNo ratings yet
- Vmeca, Generador de Vacio Serie VQDocument6 pagesVmeca, Generador de Vacio Serie VQjoselin salasNo ratings yet
- BSmith 356registry JetSettingDocument3 pagesBSmith 356registry JetSettingAlvaro MontesinosNo ratings yet
- Manual de Generador de VaporDocument12 pagesManual de Generador de Vaporgacosta008No ratings yet
- 2011-03 Selection of Pumps PDFDocument47 pages2011-03 Selection of Pumps PDFKwabena OtchereNo ratings yet
- Toshiba 26W330D LCDTVDocument66 pagesToshiba 26W330D LCDTVvideosonNo ratings yet
- Tabela de Transistores Saida HorizontalDocument6 pagesTabela de Transistores Saida HorizontalRogério CunhaNo ratings yet
- Configuration of UMT-3 TribometerDocument2 pagesConfiguration of UMT-3 TribometerEmpowered GanwarNo ratings yet
- Fire Protection System: For IKEA TebrauDocument35 pagesFire Protection System: For IKEA TebrauLee Sien100% (1)
- Advan EX 850 1300 1700 2250: Competence in Woven BagsDocument4 pagesAdvan EX 850 1300 1700 2250: Competence in Woven BagsPhuong VyNo ratings yet
- Project Fair Report FormatDocument5 pagesProject Fair Report FormatBhavik PrajapatiNo ratings yet
- MakeX - Team Automaton - Final NotebookDocument23 pagesMakeX - Team Automaton - Final NotebookRishi KatariaNo ratings yet
- Malaysia Asean Science Olympiads: Rules and RegulationsDocument12 pagesMalaysia Asean Science Olympiads: Rules and RegulationsKarren Cacabelos SurNo ratings yet
- SimpleGISBasedMethodforDesigningFiber NetworkDocument10 pagesSimpleGISBasedMethodforDesigningFiber NetworkDaniel Franco SantanaNo ratings yet
- Cost ManagementDocument4 pagesCost ManagementGhosh DipankarNo ratings yet
- SS Landing Gear - SampleDocument28 pagesSS Landing Gear - Samplecyberstroke100% (1)
- Niagara 381 381 L UkDocument8 pagesNiagara 381 381 L UkFABIAN ALEXANDER ROA LAGUNANo ratings yet
- NSI 02 and Guidance Issue 8Document31 pagesNSI 02 and Guidance Issue 8stefan998877No ratings yet
- AI Q1. Attempt Any FourDocument7 pagesAI Q1. Attempt Any FourziddirazanNo ratings yet
- Furtune Silicon AdDocument81 pagesFurtune Silicon AdBogdanNo ratings yet
- 97F 53motorhomeDocument224 pages97F 53motorhomerukford1No ratings yet
- Free Content Delivered With Solid Edge: Class Standard DescriptionDocument29 pagesFree Content Delivered With Solid Edge: Class Standard DescriptionSergio Leite SilvaNo ratings yet
- DAFTAR ISI Hal 7 - 9Document3 pagesDAFTAR ISI Hal 7 - 9Khima AlbiNo ratings yet