Professional Documents
Culture Documents
Supramolecular Chemistry L2 3rd August 2020
Supramolecular Chemistry L2 3rd August 2020
Uploaded by
Saurav PaulOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Supramolecular Chemistry L2 3rd August 2020
Supramolecular Chemistry L2 3rd August 2020
Uploaded by
Saurav PaulCopyright:
Available Formats
Supramolecular Chemistry
2nd Lecture note, SkJ
03.8.2020
Chiral recognition
The most important aims of molecular recognition is chiral recognition because
it is commonly achieved in biological systems.
Receptors in our body easily distinguish between two molecules that have
same chemical composition but different structure around a chiral carbon
atom.
For example, we sense a sweet test for D-glucose, but L-glucose testes totally
different.
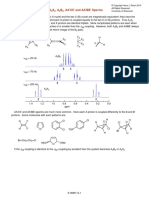
Cram demonstrated the chiral recognition of an ammonium guest using a
crown ether with axis-chiral binapthyl groups.
Chiral recognition using a crown ether
When the (s,s)-host binds to a chiral guest (s- and R --phenylethylammonium
ions), the complexes are thermodynamically different and having different
stabilities.
Binding of the R-guest to the (s,s)-host satisfies steric requirements, because
the phenyl group, the methyl group and the hydrogen can occupy the large,
medium and small sites of the crown, respectively. This complex should be
stable. In contrast, the S-guest cannot fill the sites in this desirable manner due
to its different stereochemistry. The R-guest is therefore selectively recognized
by this host.
Transformation (Function of supramolecule)
Supramolecular reactivity and catalysis
Reactivity and catalysis represent major features of the functional properties
of supramolecular system. Molecular receptors bearing appropriate reactive
groups in addition to binding sites, may complex a substrate (with given
stability, selectivity and kinetic features), react with it (with given rate,
selectivity, turnover) and release the products, thus regenerating the reagent
for a new cycle.
Supramolecular reactivity and catalysis thus involve two main steps: binding
which selects the substrate, followed by transformation of bound species into
products within the supramolecule formed.
Both steps take part in the molecular recognition of the productive substrate
and require the correct molecular information in the reactive receptor.
Compared to molecular reactivity, a binding step is involved that precedes the
reaction itself. Catalysis additionally comprises a third step, the release of the
substrate.
Catalysis by reactive cation receptor molecules
Ester cleavage processes have been most frequently investigated in enzyme
model studies. Macrocyclic polyethers fitted with side chains bearing thiol
groups cleave activated esters with marked rate enhancements and chiral
discrimination between optically active substrates.
The tetra-(L)-cysteinyl derivative of macrocycle (M) binds p-nitrophenyl esters
of aminoacids and peptides and reacts with the bound species, releasing p-
nitrophenol.
The reaction displays
i) Substrate selectivity
ii) Marked rate enhancements in favour of dipeptide ester substrates,
iii) Inhibition by complexable metal cations that displace the bound
substrate
iv) High chiral recognition between enantiomeric dipeptide esters
v) Slow but definite catalytic turnover
Catalysis by reactive anion receptor molecule.
The development of anion coordination chemistry and of anion receptor
molecules has made possible to perform molecular catalysis on anionic
substrates of chemical and biochemical interest such as adenosine
triphosphate (ATP).
ATP hydrolysis was found to be catalyzed by a number of protonated
macrocyclic polyamines.
In particular [24]N 6O2 , strongly binds ATP and markedly accelerates its
hydrolysis to ADP and inorganic phosphate over a wide pH range.
[24]N6O2
The reaction presents first-order kinetics and is catalytic with turn over.
It prceeds via initial formation of a complex between ATP and protonated
[24]N6O2, followed by an intracomplex reaction which may be involve a
combination of acid, electrostatic, and nucleophilic catalysis.
The above structure represents one possible binding mode of the {ATP-
protonated [24]N6O2} complex and indicates how cleavage of the terminal
phosphoryl groups might take place.
A transient intermediate identified as phosphoramidate is formed by
phosphorylation of the macrocycle by ATP and is subsequently hydrolyzed.
In this process catalyst protonated [24]N6O2 presents prototypical ATPase
activity i.e. it behaves as a proto ATpage.
ATPases are enzymes that catalyze the hydrolysis of adenosine triphosphate (ATP) into
adenosine diphosphate (ADP) and a free phosphate ion. This reaction leads to the release of
energy which is used in the cell. This process is essential for all living creatures.
Vesicular- or vacuolar-type adenosine triphosphatases (V-ATPases) are ATP-hydrolysis–driven proton
pumps.
The V-ATPases are composed of a peripheral domain (V 1 ) that carries out ATP hydrolysis and an
integral domain (V0 ) responsible for proton transport.
Co-catalysis
Schematic representation of Cocatalysis process: Group transfer and ligation reaction occurring
within the supramolecular complex formed by the binding of substrates to the two macrocyclic
subunits of a macrotricyclic coreceptor molecule.
Coreceptor molecule should be able to perform cocatalysis by bringing
together substrate(s) and cofactor(s) and mediating reaction between them
within the supramolecular structure.
Protonated [24]N6O2 macrocycle was employed as catalyst for the hydrolysis of
acetylphosphate (AcP = CH3COOPO32-), it was found to mediate the synthesis of
pyrophosphate from AcP.
Substrate consumption was accelerated and catalytic with turnover.
(PN + P)
The result obtained agrees with a catalytic cycle involving the following steps:
i) Substrate AcP binding by the protonated molecular catalyst [24]N6O2
ii) Phosphorylation of protonated [24] N 6O2 within the supramolecular
complex, giving the phosphorylation intermediate PN
iii) Binding of the substrate HPO42- (P)
iv) Phosphoryl transfer from PN to P with formation of pyrophosphate
PP
v) Release of the product and of the free catalyst for a new cycle.
The fact that [24]N 6O2 is a ditopic coreceptor containing two
diethylenetriamine subunits is of special significance for both PN and PP
formation.These subunits may cooperate in binding AcP and activating it for
phosphoryl transfer via the ammonium sites, in providing an unprotonated
nitrogen site for PN formation, as well as in mediating phosphoryl transfer
from PN to P.
Thus [24]N6O2 would combine electrostatic and nucleophilic catalysis in a
defined structural arrangement suitable for PP synthesis via two successive
phosphoryl transfers, displaying protokinase type activity.
The bond making process extends supramolecular reactivity to cocatalysis,
mediating synthetic reactions within the supramolecular entities formed by
coreceptor molecules.
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5814)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1092)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (844)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (348)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Coates 1973Document2 pagesCoates 1973Saurav PaulNo ratings yet
- Nakanishi 2004Document9 pagesNakanishi 2004Saurav PaulNo ratings yet
- Dielectric Materials: Dielectric Materials Are and Used Principally in andDocument7 pagesDielectric Materials: Dielectric Materials Are and Used Principally in andSaurav PaulNo ratings yet
- Voltagedependent Optical Activity of A Twisted Nematic Liquid CrystalDocument3 pagesVoltagedependent Optical Activity of A Twisted Nematic Liquid CrystalSaurav PaulNo ratings yet
- Structure Problem Solving Using H and C NMR Spectral Data Tutorial SessionDocument12 pagesStructure Problem Solving Using H and C NMR Spectral Data Tutorial SessionSaurav PaulNo ratings yet
- Basic Concept of C NMR: Subject ChemistryDocument15 pagesBasic Concept of C NMR: Subject ChemistrySaurav PaulNo ratings yet
- Funahashi 1997Document7 pagesFunahashi 1997Saurav PaulNo ratings yet
- 22 and Applications of C NMR: Subject ChemistryDocument13 pages22 and Applications of C NMR: Subject ChemistrySaurav PaulNo ratings yet
- 1455787138CHE P12 M29 EtextDocument7 pages1455787138CHE P12 M29 EtextSaurav PaulNo ratings yet
- 1455787242CHE P12 M30 EtextDocument8 pages1455787242CHE P12 M30 EtextSaurav PaulNo ratings yet
- A Highly Selective and Sensitive Fluorescent Chemosensor For Fe in Physiological Aqueous SolutionDocument2 pagesA Highly Selective and Sensitive Fluorescent Chemosensor For Fe in Physiological Aqueous SolutionSaurav PaulNo ratings yet
- 1455786764CHE P12 M24 E-TextDocument10 pages1455786764CHE P12 M24 E-TextSaurav PaulNo ratings yet
- 1455877785CHE P12 M35 EtextDocument8 pages1455877785CHE P12 M35 EtextSaurav PaulNo ratings yet
- Towards Complex Matter: Supramolecular Chemistry and Self-OrganizationDocument19 pagesTowards Complex Matter: Supramolecular Chemistry and Self-OrganizationSaurav PaulNo ratings yet
- 1455787432CHE P12 M33 E-TextDocument12 pages1455787432CHE P12 M33 E-TextSaurav PaulNo ratings yet
- Account Statement From 1 Sep 2019 To 11 Aug 2020: TXN Date Value Date Description Ref No./Cheque No. Debit Credit BalanceDocument2 pagesAccount Statement From 1 Sep 2019 To 11 Aug 2020: TXN Date Value Date Description Ref No./Cheque No. Debit Credit BalanceSaurav PaulNo ratings yet
- The Relative NMR Sensitivity of Nucleus at Constant Magnetic FieldDocument4 pagesThe Relative NMR Sensitivity of Nucleus at Constant Magnetic FieldSaurav PaulNo ratings yet
- AA'BB' SpectraDocument11 pagesAA'BB' SpectraSaurav PaulNo ratings yet
- Account Statement From 1 Oct 2020 To 6 Oct 2020Document1 pageAccount Statement From 1 Oct 2020 To 6 Oct 2020Saurav PaulNo ratings yet
- Chapter 6 Introduction To Thermodynamics PDFDocument17 pagesChapter 6 Introduction To Thermodynamics PDFSaurav PaulNo ratings yet
- 41 Topics in Organometallic ChemistryDocument11 pages41 Topics in Organometallic ChemistrySaurav PaulNo ratings yet
- Novel Bunyavirus in Domestic and Captive Farmed Animals, Minnesota, USADocument4 pagesNovel Bunyavirus in Domestic and Captive Farmed Animals, Minnesota, USASaurav PaulNo ratings yet
- The Structure of The Atom: Randima Piyumalie GalhenageDocument5 pagesThe Structure of The Atom: Randima Piyumalie GalhenageSaurav PaulNo ratings yet
- Antioxidants 10 01315Document24 pagesAntioxidants 10 01315carloshgmedeirosNo ratings yet
- Gen Bio CN # 9Document2 pagesGen Bio CN # 9Fabula , Lawrence Joe B. Student , AU - Jose RizalNo ratings yet
- List of Food Additives Permitted Under Food RegulationsDocument46 pagesList of Food Additives Permitted Under Food RegulationsHappy GuyNo ratings yet
- Role of Atp In-Wps OfficeDocument14 pagesRole of Atp In-Wps OfficeElsa Barrientos BatasNo ratings yet
- Starch-Composition, Fine Structure and Architecture: Richard F. Tester, John Karkalas, Xin QiDocument15 pagesStarch-Composition, Fine Structure and Architecture: Richard F. Tester, John Karkalas, Xin Qierlita indah astariNo ratings yet
- Free Energy of A ReactionDocument38 pagesFree Energy of A ReactionjdNo ratings yet
- PyrosequencingDocument4 pagesPyrosequencingFarzini100% (3)
- Anexo 1. Botham, K Mayes, P. (2022) - Chapter 11 - Bioenergetics - The Role of ATP. McGraw Hill.Document9 pagesAnexo 1. Botham, K Mayes, P. (2022) - Chapter 11 - Bioenergetics - The Role of ATP. McGraw Hill.Liliana LNo ratings yet
- Tetrapotassium PyrophosphateDocument2 pagesTetrapotassium PyrophosphateAftab S. MirzaNo ratings yet
- Bio EnergeticsDocument8 pagesBio EnergeticsSamuel RookieNo ratings yet
- 23C 112201 Kikk Lpa3pre Ver4Document12 pages23C 112201 Kikk Lpa3pre Ver4annisa nur ainiNo ratings yet
- Use of Phosphates in Meat ProductsDocument9 pagesUse of Phosphates in Meat ProductsNabil SouissiNo ratings yet
- Standard For Quick Frozen Fish Fillets Codex Stan 190 - 1995Document7 pagesStandard For Quick Frozen Fish Fillets Codex Stan 190 - 1995remyNo ratings yet
- Tetrabenzyl Pyrophosphate Synthesis. I. Khorana. J. Chem. Soc., 2257, (1953)Document4 pagesTetrabenzyl Pyrophosphate Synthesis. I. Khorana. J. Chem. Soc., 2257, (1953)Evan EsceNo ratings yet
- JR 9430000043Document3 pagesJR 9430000043Anonymous FigYuONxuuNo ratings yet
- BIOLOGICAL OXIDATION & Principle of Energy MetabolismDocument84 pagesBIOLOGICAL OXIDATION & Principle of Energy MetabolismOdi YuventiusNo ratings yet
- A Blend of Skimmed Milk and Vegetable Fat in Powdered FormDocument8 pagesA Blend of Skimmed Milk and Vegetable Fat in Powdered FormAnuradha MarapanaNo ratings yet
- JECFA Evaluations-TETRASODIUM PYROPHOSPHATEDocument1 pageJECFA Evaluations-TETRASODIUM PYROPHOSPHATETim MoserNo ratings yet
- Why Nature Chose Phosphates - F H Westheimer - 1987Document7 pagesWhy Nature Chose Phosphates - F H Westheimer - 1987Antonio Vázquez MotaNo ratings yet
- Rettberg Biendl Garbe 2018Document21 pagesRettberg Biendl Garbe 2018AmandaAlmeidaNo ratings yet
- C-Bacterial Metabolism-Springer-Verlag New York (1986)Document370 pagesC-Bacterial Metabolism-Springer-Verlag New York (1986)xuantra100% (1)
- Cooper PlatingDocument12 pagesCooper Platingmiguelin9169100% (1)
- Frozen Fish Fillets - Gso 1406-2005Document9 pagesFrozen Fish Fillets - Gso 1406-2005mmammerNo ratings yet
- Phản ỨngDocument1 pagePhản ỨngThanh TuanNo ratings yet
- Ingredients DBDocument336 pagesIngredients DBhpatel0014407No ratings yet
- BFSA (Food Additives)Document48 pagesBFSA (Food Additives)shahriar.codexNo ratings yet
- Of Three Acid: Isolation Phosphatases From Wheat GermDocument6 pagesOf Three Acid: Isolation Phosphatases From Wheat GermBarry WhiteNo ratings yet
- Organic Chemistry PDFDocument468 pagesOrganic Chemistry PDFIrina Stefania0% (1)
- TT21 28112019BNN (E)Document40 pagesTT21 28112019BNN (E)Thanh Tâm TrầnNo ratings yet
- General Biology 1: Quarter 2 - Week 1 - Module 1Document11 pagesGeneral Biology 1: Quarter 2 - Week 1 - Module 1Regine Ann ViloriaNo ratings yet