Professional Documents
Culture Documents
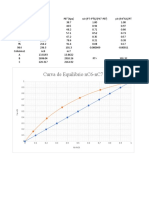
T-X-Y Diagram: Antoine's Equation Antoine's Constants Heptane Octane Log P A - B / C + T (C)
Uploaded by
Deep SinojiyaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
T-X-Y Diagram: Antoine's Equation Antoine's Constants Heptane Octane Log P A - B / C + T (C)
Uploaded by
Deep SinojiyaCopyright:
Available Formats
T-x-y Diagram
Antoine's Equation Log P = A - B / C + T(0 C)
Antoine's constants
A B C
Heptane 4.02167 1264.9 216.544
Octane 4.04358 1351.99 209.155
P 1 bar
B.P of Heptane 97.977082
B.P of Octane 125.199705
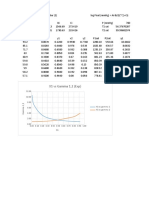
Sr. No. Temp. P1ᵒ P2ᵒ X1 Y1
1 97.977082 1 0.43812463 1 1
2 102.977082 1.15593331 0.51536201 0.7565714971 0.8745462
3 107.977082 1.33022869 0.60311985 0.5458332073 0.72608299
4 110.977082 1.44420397 0.66123284 0.4326687788 0.62486197
5 115.977082 1.65082358 0.76789302 0.262882486 0.43397261
6 120.977082 1.87954291 0.88772764 0.1131988587 0.21276211
7 123.977082 2.02800424 0.96640621 0.0316445462 0.06417527
8 125.199705 2.0910202 1 0 0
130
125
120
Temperature (°C)
115
110
105
100
95
90
0 0.2 0.4 0.6 0.8 1 1.2
x or y
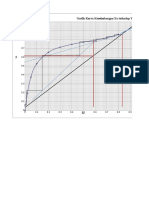
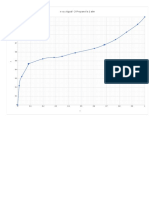
x y
x vs y
1.2
0.8
0.6
0.4
0.2
0
0 0.2 0.4 0.6 0.8 1 1.2
You might also like
- Q4 3ChEDocument6 pagesQ4 3ChEeiNo ratings yet
- Abdul Rachman Wirayudha - 17031010168Document6 pagesAbdul Rachman Wirayudha - 17031010168yudha7wira7rahmanNo ratings yet
- Sistema: Metanol (1) / Acetonidrilo (2) Modelo UNIFAC: Ganma 1Document5 pagesSistema: Metanol (1) / Acetonidrilo (2) Modelo UNIFAC: Ganma 1Carlos Coronado LezmaNo ratings yet
- T XY Heptano: Fraccion MolarDocument3 pagesT XY Heptano: Fraccion MolarJulio CaveroNo ratings yet
- Latihan RegresiDocument4 pagesLatihan RegresiIndah IrdianiNo ratings yet
- CHE 33 Report by Alyza A. SasingDocument28 pagesCHE 33 Report by Alyza A. SasingAlyza Abadies SasingNo ratings yet
- ASSIGNMENT 2 Thermodynamic ShammilDocument17 pagesASSIGNMENT 2 Thermodynamic ShammilNor Hamizah HassanNo ratings yet
- DIAGRAMA nC6-nC7Document2 pagesDIAGRAMA nC6-nC7Aylin Portillo OliveraNo ratings yet
- Batch Problema 1Document2 pagesBatch Problema 1Aylin Portillo OliveraNo ratings yet
- DIAGRAMA nC7-nC8Document2 pagesDIAGRAMA nC7-nC8Aylin Portillo OliveraNo ratings yet
- Regression StatisticsDocument9 pagesRegression StatisticsMulyadi NasrunNo ratings yet
- Diagramas Txy y PxyDocument4 pagesDiagramas Txy y PxyJose De Jesus Vega SoriaNo ratings yet
- Simple Linear Regression - Sample ProblemsDocument7 pagesSimple Linear Regression - Sample ProblemsAiden PierceNo ratings yet
- Experiment: X1 Vs Gamma 1,2 (Exp)Document5 pagesExperiment: X1 Vs Gamma 1,2 (Exp)Azizah Az ZahraNo ratings yet
- Fisicoquimica Ana y ChenteDocument7 pagesFisicoquimica Ana y ChenteelvaloyaNo ratings yet
- HW 3Document3 pagesHW 3nguyenaiquynh150801No ratings yet
- Diagrama de Equilibrio Y - XDocument4 pagesDiagrama de Equilibrio Y - XJose Elmer Santisteban SanchezNo ratings yet
- Pxy Clorobutano-ClorobencenoDocument7 pagesPxy Clorobutano-ClorobencenoDestileria POPLAR CAPITAL S.A.No ratings yet
- T Test 20antihiperglikemikDocument4 pagesT Test 20antihiperglikemik059Shafira Navra DarmawanNo ratings yet
- In Calibrating A 10Document4 pagesIn Calibrating A 10GrenlyKerehNo ratings yet
- Construction of A T-X-Y Diagram: Press To BeginDocument2 pagesConstruction of A T-X-Y Diagram: Press To BeginTÙNGNo ratings yet
- MC 3Document2 pagesMC 3Aylin Portillo OliveraNo ratings yet
- TAREA 04 Espinosa Salgado Itzel - Anacleto Sanchez OscarDocument11 pagesTAREA 04 Espinosa Salgado Itzel - Anacleto Sanchez OscarGustavo OrtizNo ratings yet
- OTKDocument5 pagesOTKBESTY LOVIANDANo ratings yet
- I A B C T /°C : Gráfico T-Xy Gráfico XyDocument6 pagesI A B C T /°C : Gráfico T-Xy Gráfico XyAntonio Martinez RamirezNo ratings yet
- Grafik Kurva Kesetimbangan Xa Terhadap YaDocument9 pagesGrafik Kurva Kesetimbangan Xa Terhadap YaMT Anzula RHNo ratings yet
- Acetone - Methanol, ChloroformDocument6 pagesAcetone - Methanol, ChloroformAlejandra InsuastyNo ratings yet
- Seminarski 2.11Document2 pagesSeminarski 2.11TarikNo ratings yet
- OTK KristiantyDocument9 pagesOTK KristiantyDewi RatnasariNo ratings yet
- LN (LN (1/ (1-f (T) ) : F (X) 2.6454117725x - 11.1626312721 R 0.8889049032Document10 pagesLN (LN (1/ (1-f (T) ) : F (X) 2.6454117725x - 11.1626312721 R 0.8889049032Jhonatan Junior Villasante RoqueNo ratings yet
- Benceno-Etilbenceno RaoultDocument5 pagesBenceno-Etilbenceno RaoultGian GiancarlosNo ratings yet
- Regression StatisticsDocument9 pagesRegression Statisticssophia del rosarioNo ratings yet
- Hometask Destilasi Biner ARDI YOHANESDocument5 pagesHometask Destilasi Biner ARDI YOHANESardiNo ratings yet
- Problema 1Document8 pagesProblema 1SALMA MAGALÍ DE LA ROSA POSADASNo ratings yet
- Regression Analysis: Source SS DF MS F P-ValueDocument4 pagesRegression Analysis: Source SS DF MS F P-ValueWahyuniNo ratings yet
- Diagram For Acetonitrile (1) Nitromethane (2) at 75 C: x1 Y1 P (Kpa)Document2 pagesDiagram For Acetonitrile (1) Nitromethane (2) at 75 C: x1 Y1 P (Kpa)izmaNo ratings yet
- Temperature Distiributions of T (X, 0.5) and T (1, Y)Document2 pagesTemperature Distiributions of T (X, 0.5) and T (1, Y)Ashish KotwalNo ratings yet
- X Vs y Agual - 2-Propanol A 1 AtmDocument3 pagesX Vs y Agual - 2-Propanol A 1 AtmvictorNo ratings yet
- Tugas 1 Termo 2 - Rabu - Putra Maulana - 5213415062Document8 pagesTugas 1 Termo 2 - Rabu - Putra Maulana - 5213415062Putra MaulanaNo ratings yet
- Universidad Nacional Del Centro Del Peru Facultad de Ingenieria Quimica Escuela Profesional de Ingenieria Quimica A/N: de La Vega de La Rosa DianaDocument16 pagesUniversidad Nacional Del Centro Del Peru Facultad de Ingenieria Quimica Escuela Profesional de Ingenieria Quimica A/N: de La Vega de La Rosa DianaafsasfNo ratings yet
- Perpan UpilDocument78 pagesPerpan UpilRomy Muchamad RizkyNo ratings yet
- Sistema: Agua (1) / 1,4 Dioxano (2) Modelo UNIFACDocument5 pagesSistema: Agua (1) / 1,4 Dioxano (2) Modelo UNIFACEstrella GonzálesNo ratings yet
- Part 1B: SQRT (T)Document2 pagesPart 1B: SQRT (T)Naeem GulNo ratings yet
- F1G113078 UasDocument11 pagesF1G113078 UasSabanNo ratings yet
- Relajacion CorregidoDocument10 pagesRelajacion CorregidoLaura Benitez LozanoNo ratings yet
- Component-A (MVC)Document16 pagesComponent-A (MVC)westewrNo ratings yet
- 10.2 EjercicioDocument6 pages10.2 EjercicioRichard ParkerNo ratings yet
- Ge/Rtx1X2 Versus X1 Graph: 0.6 0.7 F (X) - 0.1566247204X + 0.6668560901 R 0.7438724523Document11 pagesGe/Rtx1X2 Versus X1 Graph: 0.6 0.7 F (X) - 0.1566247204X + 0.6668560901 R 0.7438724523Radhi AbdullahNo ratings yet
- Exel Modul 3Document4 pagesExel Modul 3Marsela KurniaNo ratings yet
- Exercicios PIIDocument7 pagesExercicios PIIDaniloMaiaNo ratings yet
- Tarea 3Document5 pagesTarea 3Okay?No ratings yet
- Ejercicio 09-10Document2 pagesEjercicio 09-10Diana Gabriela Roman PerezNo ratings yet
- Inf BombasDocument10 pagesInf BombasWara Martha Pacheco HuancaNo ratings yet
- T-X-Y Diagram For Benzene (1) /ethylbenzene (2) at 90kpaDocument3 pagesT-X-Y Diagram For Benzene (1) /ethylbenzene (2) at 90kpasiti azilaNo ratings yet
- Ejemplo Integracion NumericaDocument22 pagesEjemplo Integracion NumericaSebastian Castro OchoaNo ratings yet
- Benceno-Etilbenceno Raoult (Autoguardado)Document12 pagesBenceno-Etilbenceno Raoult (Autoguardado)renzo6tello6cribilleNo ratings yet
- Curva de Equilibrio: Etanol A P°1 P°2 x1 Y1 B C Agua A B C Temp (°C)Document2 pagesCurva de Equilibrio: Etanol A P°1 P°2 x1 Y1 B C Agua A B C Temp (°C)Carlos FloresNo ratings yet
- Balance Global Balance Por Componete 0.00315 Calculo de Reflureflujo Linea de Alimentacion Pendiente (m1) X yDocument3 pagesBalance Global Balance Por Componete 0.00315 Calculo de Reflureflujo Linea de Alimentacion Pendiente (m1) X yTanitDayanaPerezNo ratings yet
- Temperatura n-hexano n-octano Xa1 Ya1 α kpa kpa: Chart TitleDocument4 pagesTemperatura n-hexano n-octano Xa1 Ya1 α kpa kpa: Chart TitleMauricio MartinezNo ratings yet
- Lecture 7Document12 pagesLecture 7Hansen NagariaNo ratings yet
- Purification of Industrial Wastewater With Vetiver Grasses Vetiveria ZizaniDocument7 pagesPurification of Industrial Wastewater With Vetiver Grasses Vetiveria Zizanino_orabsharinaNo ratings yet
- Chapt 12 Summpt 2Document34 pagesChapt 12 Summpt 2CuriousNo ratings yet
- Pmce MergedDocument506 pagesPmce MergedDeep SinojiyaNo ratings yet
- Module 5 Condensation and BoilingDocument80 pagesModule 5 Condensation and BoilingDeep SinojiyaNo ratings yet
- SYBTech PO221 PC 01b Polymer Classification PPT 2020-07-28Document29 pagesSYBTech PO221 PC 01b Polymer Classification PPT 2020-07-28Deep SinojiyaNo ratings yet
- Chapter 3Document20 pagesChapter 3Nathalia DelgadoNo ratings yet
- Polymer Waste Management: Prof. Amar ArakhDocument42 pagesPolymer Waste Management: Prof. Amar ArakhDeep SinojiyaNo ratings yet
- An Analysis of Fuel Cell Technology For Sustainable Transport in AsiaDocument13 pagesAn Analysis of Fuel Cell Technology For Sustainable Transport in AsiaDeep SinojiyaNo ratings yet
- Acid Base ChemistryDocument12 pagesAcid Base ChemistryDeep SinojiyaNo ratings yet
- Applications of Biosurfactants in The Petroleum Industry and The Remediation of Oil SpillsDocument21 pagesApplications of Biosurfactants in The Petroleum Industry and The Remediation of Oil SpillsDeep SinojiyaNo ratings yet
- Inventions 04 00028Document10 pagesInventions 04 00028Ravish ChouhanNo ratings yet
- Alternative Fuels For Internal Combustion EnginesDocument23 pagesAlternative Fuels For Internal Combustion EnginesNoør ShåikNo ratings yet
- State Report-Rajasthan WebDocument124 pagesState Report-Rajasthan WebDeep SinojiyaNo ratings yet
- Ideal Handbook of MathDocument19 pagesIdeal Handbook of MathDurgaNo ratings yet
- Static Mixer Bulletin 13Document12 pagesStatic Mixer Bulletin 13Adolfo Perez MonteroNo ratings yet
- Chart Title: Antoine Equation: Log P (Bar) A - B / (C + T (C) )Document2 pagesChart Title: Antoine Equation: Log P (Bar) A - B / (C + T (C) )Deep SinojiyaNo ratings yet
- Movie ReviewDocument3 pagesMovie ReviewDeep SinojiyaNo ratings yet
- $r117hue PDFDocument51 pages$r117hue PDFDeep SinojiyaNo ratings yet
- State Report-Rajasthan WebDocument124 pagesState Report-Rajasthan WebDeep SinojiyaNo ratings yet
- Water Technology Lecture 11 Sludge TreatmentDocument46 pagesWater Technology Lecture 11 Sludge TreatmentDeep SinojiyaNo ratings yet
- Jaipur Dravyavati ProjectDocument57 pagesJaipur Dravyavati ProjectLakshit JoshiNo ratings yet
- Biodiesel Turmeric Leaves Are The Waste Product TurmericDocument2 pagesBiodiesel Turmeric Leaves Are The Waste Product TurmericDeep Sinojiya0% (1)
- Unit 1 Refinery Products PDFDocument23 pagesUnit 1 Refinery Products PDFDeep SinojiyaNo ratings yet
- Conversion Reactor PDFDocument3 pagesConversion Reactor PDFDeep SinojiyaNo ratings yet
- Unit 4 Bitumen - Blowing PDFDocument8 pagesUnit 4 Bitumen - Blowing PDFDeep SinojiyaNo ratings yet
- Gate Mathematics: MAI) 4SDocument258 pagesGate Mathematics: MAI) 4SManivannan VadiveluNo ratings yet
- The Role of Biosurfactants in The Continued Drive For Environmental SustainabilityDocument12 pagesThe Role of Biosurfactants in The Continued Drive For Environmental SustainabilityDeep SinojiyaNo ratings yet
- Unit 4 Bitumen - Blowing PDFDocument8 pagesUnit 4 Bitumen - Blowing PDFDeep SinojiyaNo ratings yet