Professional Documents
Culture Documents
Isolation and Spectrophotometric Characterization o F Photosynthetic Pigments Rodney F Boyer
Uploaded by
Lucy ZuluOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Isolation and Spectrophotometric Characterization o F Photosynthetic Pigments Rodney F Boyer
Uploaded by
Lucy ZuluCopyright:
Available Formats
203
Isolation and Spectrophotometric Characterization o f hydrocarbon side chain enhances the solubility of chlorophyll in
Photosynthetic Pigments nonpolar solvents. The only difference between chlorophyll a
and b is the substituent on position 3 (ring 3): chlorophyll a has a
RODNEY F BOYER methyl group, whereas b has an aldehyde. The chlorophylls are
biosynthesized by condensation of ~-aminolevulinic acid in a
Department of Chemistry process similar to the synthesis of heme in mammals. In the
Hope College living plant cell, the chlorophylls serve as the primary photo-
Holland, MI49423-3698, USA synthetic pigments. They absorb light in the blue (450 nm) and
red regions (650-700 nm). Absorption of light, which excites an
electron in the chlorophyll molecule, provides energy for
Introduction initiation of the photosynthetic process producing NADPH and
When students in the biochemistry laboratory are introduced to ATP to be used for carbohydrate biosynthesis by the plant.
the extraction and characterization of biomolecules, the tissue of The second group of plant pigments, the carotenoids, can be
choice is most often of animal origin. Plant products are seldom divided into two different types, (1) the carotenes which contain
used; therefore, students rarely gain knowledge and appreci- only carbon and hydrogen, and (2) the xanthophylls which
ation of the unique biomolecules present in the plant kingdom. contain carbon, hydrogen and oxygen atoms in the form of
In order to fill in this gap, we have designed an experiment in hydroxyl or epoxide functional groups. The structures for several
which students isolate a mixture of pigments by solvent major carotenoids are shown in Fig 2. Note that they all contain
extraction of plant tissue, separate some of the components by 40 carbon atoms and, because of the extensive conjugation,
chromatography and identify them by visible region spectro- would be expected to be highly colored. Most carotenoids are
photometry. Students who complete this experiment gain knowl- yellow, red or orange, but some are green, pink and even black.
edge and technical skills in biomolecule extraction, chromato- Many of the bright colors found in flower petals are due to the
graphic methods, spectrophotometric techniques and plant presence of carotenoids (although some are due to antho-
pigment structure. Students will follow procedures which are cyanins). The yellow colors of fall foliage are due primarily to
typical for the general extraction and characterization of lipids. preferential destruction of the green chlorophylls, revealing the
However, unlike most lipids (especially those in animals), the carotenoid color. 6
plant pigments are highly colored and may be characterized and The percentage composition of carotenoids in plants varies
quantified by visible spectrophotometry. with growing conditions. The average weight % ranges are: 13-
carotene, 25-40%; lutein, 40-60%; violaxanthin, 10-20%; and
Theory neoxanthin, 5-13%. 2 The carotenoids are biosynthesized by
Many of the colors associated with higher plants (green leaves in condensation of acetylCoA through the mevalonic acid pathway.
the spring and summer, yellow or red leaves in the fall, the The physiological function of the carotenoids is not completely
orange color of carrots, some colors in flower petals) are due to understood; however, two probable functions have been
the presence of pigment molecules. Review articles on plant described. First, the carotenoids, particularly the xanthophylls,
pigments have been recently written by Goodwin 1 and Lichten- may participate in light adsorption for photosynthesis. It has
thaler. 2 General information on photosynthetic pigments can been shown that illuminated xanthophylls are able to transfer
also be obtained from the standard biochemistry texts by excitation energy directly to chlorophyll a. Secondly, the
Mathews and van Holde, 3 Stryer, 4 and Voet and Voet. 5 carotenes may inhibit the photo-oxidative destruction of chloro-
The major photosynthetic pigments of higher plants can be phyll, a process which consumes oxygen.
divided into two groups, the chlorophylls and the carotenoids.
Both types of pigments are present in the subcellular organelles
called chloroplasts, where they are bound to proteins in the
thylakoids, the photochemically active photosynthetic biomem-
branes. Intact pigment-protein complexes, which are held
together by weak, noncovalent bonds, have been isolated from F-Carotene
chloroplasts and characterized b~( polyacrylamide gel electro-
phoresis and isoelectric focussing/The pigments are released in
a protein-free form by grinding plant tissue in solvents such as
acetone, methanol or hexane. Since the chlorophylls and the
carotenoids are readily soluble in organic solvents, they are
classified biochemically as lipids.
The most abundant plant pigments are chlorophyll a and Lutein
chlorophyll b which occur in a ratio (a:b) of approximately 3:1.
As is shown in Fig 1, the chlorophylls possess a porphyrin ring
with a coordinated magnesium atom at its center, a fused, 5- ~ ~ ~ T ~ ~ ~ o OH
membered ring and a C20 phytyl side chain. This nonpolar,
HO
HzC=CH H C~ H2CffiCH H H-C-O
"3~c#. H~C~ceH
H H
a
Violaxanthin
o -oc.. -'Lo OH
CLN3H~I Offi~-OCH3
Chlorophyll a Chlorophyll b Neo~mnthln
Figure 1 Structures of chlorophyll a and chlorophyll b (From Figure 2 Structures of major carotenoids: B-carotene, lutein,
Moore7) violaxanthin and neoxanthin (From Lichtenthaler2)
BIOCHEMICAL EDUCATION 18(4) 1990
204
I 1
Spectral Characteristics of Plant Pigments
The intense colors of the chlorophylls and carotenoids makes
them ideal candidates for absorption spectroscopy studies. In
fact, each plant pigment studied in this experiment has a unique
visible spectrum which can provide a positive identification. As
shown in Fig 3, chlorophylls a and b have absorption maxima in
the 600-675 nm range and in the 400-475 nm range. The o*
c
absorption maxima for each peak is very dependent upon solvent
polarity. For example, the two most intense absorption peaks for g I
chlorophyll a in diethyl ether are 660 nm and 428 nm, whereas, ,~ o.50o ! 0.500
in more polar methanol, the peaks are shifted to 665 nm and
432 nm.
l.(a:
[~.9"
B.B~
u :
~_~.~. -~_~Z:- \ ~" o
11
aoo.o ~o.o 4so.o sso.o
~Q. 4 Wavelength (nm)
Q.Z Figure 4 Visible absorption spectra for four carotenoids: ~-
carotene (--), lutein (--), violaxanthin (.-.), and neoxanthin ( . . . )
(From Lichtenthaler 2)
4i~Q ~BO Bgl~l 7BO
N z v m l mr, o c h ( h n )
measurement at wavelength, z. These equations are designed for
the use when 100% acetone is the solvent.
Practical Aspects of the Experiment
0.5
Plant pigments are isolated by careful extraction of plant tissue
(green or dried leaves) with 100% acetone. 7 The extraction may
w
be carried out with either a mortar and pestle or with a glass
a
u homogenizer. Plant cells have a tough wall and the addition of an
NgtLB
.¢1 abrasive agent such as quartz sand enhances the extraction
L
a
Nt~.2
process. Both types of plant pigments, chlorophylls and caro-
tenoids, are extremely light-sensitive and are readily destroyed
B.
by photo-bleaching; therefore, the extraction process should be
performed rapidly and in subdued lighting. If quantitative
determinations are to be made for each pigment, the absorption
,aa ~';=, sa'e 7e~
HIval mnl~thCnm:l data must be obtained immediately after extraction.
A wide range of chromatographic techniques, including
Figure 3 Visible absorption spectra for chlorophyll a (a) and column, paper, thin-layer and high-pressure liquid chromatog-
chlorophyll b (b) raphy, have been applied to the separation of photosynthetic
pigments. 2 There is great historical interest here, because
In contrast to the chlorophylls, which absorb light in two Tswett, in the first demonstration of chromatography in 1906,
regions of the visible spectrum, the carotenoids exhibit intense separated a plant pigment extract on CaCO3 and MgO columns. 8
absorption in just one, 350-500 nm. Fig 4 compares the In the present experiment, either paper or thin-layer chromatog-
absorption spectra of four common carotenoids. As with the raphy are used to separate the pigments. Both techniques
chlorophylls, the absorption maxima of the carotenoids vary provide an excellent resolution of the two chlorophylls and of the
with polarity of the solvent. 13-carotene in diethyl ether has a major carotenoids. After solvent elution of the pigments from
kma~ of 449.8 nm, but in the more polar acetone, the hmax is the paper or silica gel, the visible absorption spectrum of each is
454 nm. determined. Several types of plant tissue may be used. Some
In addition to its use in pigment identification, spectrophoto- recommendations include, fresh leaves (tree, plant, grass,
metric data may be used to measure the concentrations of spinach), green algae or mosses. For variety, students may be
pigments in a plant extract. Data from spectral analysis were asked to bring their own samples for analysis.
used to calculate absorption coefficients for each pigment in The approximate time requirements for each part of the
order to derive the following equations. 2 experiment are:
Ca = 11.24A661.6 - 2.04-4644.8 A Extraction of the Pigments - - 30 minutes,
Cb = 20.13A644.S - 4.19A6m.6 B Determination of Pigment Concentration-- 15 minutes,
Ca+b = 7.05A661.6 + 18.09A64a.s C Chromatography and Elution of Pigments - - 1 hour, and
Cx+c = (1000A470 - 1.90Ca - 63.14Cb)/214 D Measurement of Visible Absorption Spectra-- 1 hour.
Since the pigment extract rapidly deteriorates as a result of
where: Ca = concentration of chlorophyll a in micrograms per photo-oxidation, it is critical that students work rapidly, but
milliliter of plant extract solution (l~g/ml), Cb = concentration of safely and efficiently. Part B must be completed immediately
chlorophyll b in ixg/ml, Ca+b = concentration of total chloro- after the extraction. Parts C and D may be completed at a later
phyll in p,g/ml, Cx+c = concentration of total carotenoids time, but, in the meantime, the plant extracts must be stored
(xanthophylls and carotenes) in p,g/ml, and Az = absorbance refrigerated in a concentrated form and under nitrogen gas.
BIOCHEMICAL EDUCATION 18(4) 1990
205
Materials and Supplies appropriate solvent, petroleum ether:acetone (9:1). A chamber
Plant tissues (leaves, moss, algae, etc); acetone, 100%; mortar may be prepared by pouring 7 ml of solvent into a 250 ml
and pestle or glass homogenizer; quartz sand; small funnel and beaker. Cover with a watch glass. If a thin-layer plate is used,
qualitative grade filter paper; test tubes, 100 mm x 12 ram; place directly in a chamber containing petroleum ether:di-
microcapillaries or tapered capillaries; anhydrous sodium sulf- oxane:2-propanol (70:30:10) and cover. When the solvent has
ate, Na2SO4; UV-VIS spectrophotometer; glass cuvets, 3 or risen to within 0.5 cm from the top of the paper or TLC plate,
1 ml; metal spatula or clean razor blade; materials for paper remove from the chamber and mark the position of the solvent
chromatography (Whatman 3M paper, 13.5 cm x 7 cm; solvent front. Allow the chromatogram to dry. Measure the position of
system, petroleum ether:acetone (9:1, v/v); chromatography each pigment band and the solvent front and draw a full-scale
chamber, 250 ml beaker with watch glass; stapler; scissors); picture of the completed chromatogram in your notebook. Since
materials for thin-layer chromatography (silica gel plates, 10 cm each chromatogram is complete in a few minutes, it is pbssible to
x 10 cm; solvent system, petroleum ether:dioxane:2-propanol experiment with the amount of extract applied to the paper or
(70:30:10, v/v/v); chromatography chamber). plate. If good separation is not attained and pigment bands are
very dark, try a chromatogram with less extract. On the other
Experimental Procedure hand, if the bands are light and difficult to distinguish, prepare
A Extraction of the Pigments This procedure should be com- and develop a chromatogram with more extract. Each pigment is
pleted in subdued lighting! Obtain 5 tree leaves or equivalent eluted from the paper or TLC plate using acetone. With the
amount of grass or other plant tissue, tear them into small pieces paper chromatogram, use a scissors to cut each colored band
and place in a mortar or homogenizer. Measure 15 ml of acetone from the paper. Cut each paper strip into smaller pieces and
and pour into the container. Add approximately 0.5 g of pure place pieces from each band into separate, labeled test tubes
sand and grind the mixture thoroughly until the solution containing 4 ml of acetone. Cover the tubes with a cork and
allow to sit for 5-10 minutes with gentle mixing at 1-2 minute
intervals. Proceed directly to Part D. To extract the pigments
C A U T I O N : USE OF A C E T O N E from the TLC plate, use a metal spatula or razor blade to scrap
the silica gel at each colored band into labeled test tubes
Exposure to acetone by inhalation, ingestion, or skin containing 4 ml of acetone. Cover with a stopper and allow to sit
absorption is harmful. Contact with eyes will cause severe for 5-10 minutes with mixing at intervals. Centrifuge or filter
irritation. First aid: eyes - - flush with copious amounts of each solution using fluted filter paper. Proceed directly to
water for at least 15 minutes; skin - - flush with water for a Part D.
few minutes; inhalation - - remove to fresh air. Acetone is D Measurement of Visible Region Absorption Spectra Using
volatile and extremely flammable. No open flames should be glass cuvets, measure the absorption spectrum of each fraction
in the vicinity of your work. In case of fire, use a CO2 from 400 to 700 nm. A cuvet containing acetone should be used
extinguisher. Dispose of acetone extraction, and solutions in as reference. A Pasteur pipet may be used to transfer solutions
the organic waste container. to and from the cuvet but be careful not to scratch the inside of
the glass cuvet with the pipet. After all spectra have been
becomes a deep green (about 10 minutes). The plant cell walls of obtained, dispose of the acetone solutions in the waste organic
cellulose are tough, so you need to be persistent. After contz~iner in the laboratory.
extraction is complete, add 1-2 g of anhydrous sodium sulfate to
remove water from the extract. (What is the source of the Analysis of Results
water?) Rapidly filter the extract by gravity through fluted filter A Extraction of the Pigments Describe the color of the extract.
paper. Measure the volume of the filtered extract and rapidly Explain the action of acetone in the extraction process. What is
proceed to Part B. the purpose of the sand?
B Determination of Pigment Concentration In order to B Determination of Pigment Concentration Using the ab-
determine the chlorophyll and carotenoid content of your sorbance data obtained on the crude extraction mixture and the
extract, you must measure the absorbance at several wave- equations provided earlier, calculate the concentrations of
lengths, 661.6 nm, 644.8 nm and 470 nm. If you are using a chlorophyll a, chlorophyll b, and total carotenoids.
single ,beam spectrophotometer, use a cuvet containing acetone C Chromatography and Elution of Pigments Calculate the
to zero the instrument. A double beam instrument should have a mobility of each pigment relative to the solvent front (Re):
cuvet containing acetone in the reference beam and the acetone
extract solution in the sample beam. If desired, it is instructive to distance from the origin migrated by a compound
Rf =
obtain a complete spectrum of the extract in the range, distance from origin migrated by solvent
400-700 nm. This can be compared to the spectrum obtained for
each of the individual pigments in Part D. Explain the relative order of the Rf values. Can you make any
C Chromatography and Elution of Pigments The general general statements about the polarity of each pigment?
procedures for paper and thin-layer chromatography are similar. D Measurement of Visible Absorption Spectrum Prepare a
Obtain a piece of chromatography paper (13.5 x 7 cm) or a thin- table of spectral data (wavelengths of major peaks and absorb-
layer plate (10 x 10 cm). With a pencil, draw a very light line ante) for each pigment. Using these data and the standard
1.5 cm from and parallel to the long edge of the paper or TLC spectra in Figs 3 and 4, try to assign the identity of each pigment
plate. Using a Pasteur pipet or tapered capillary, evenly apply isolated. Now that each compound is identified, is there a
the acetone extract along the line. Begin on the pencilled line correlation between the experimental Rf value and polarity as
approximately 1 cm from the edge and stop approximately 1 cm determined from the structure? Which pigments are more polar,
from the opposite edge. The narrower the line, the better your those near the top or those near the bottom of the chromato-
separation will be. Allow the first coating of extract to dry and gram?
repeat above procedure with another application of extract.
Repeat a third time if the line is not dark green. Allow the paper Study Questions
to dry completely, roll it into a circle with the pencil line and (1) What other solvents in addition to acetone could be used for
extract outside and on the circular edge. Place the paper (extract extracting plant photosynthetic pigments?
line down) into a chamber containing no more than 1 cm of (2) Predict the relative Rf values for thin-layer chromatography
BIOCHEMICAL EDUCATION 18(4) 1990
206
of a mixture of chlorophylls a and b, f3-carotene, lutein, In this paper we describe the simulation of radioactive tracer
neoxanthin, and violaxanthin. Assume that the solvent is the incorporation and distribution. The simulation can be used in
same as used in this experiment. isolation, and makes a useful introduction to the topic of
(3) Repeat Question 2 (above) except substitute paper chroma- metabolic analysis by using labelled precursors. However, most
tography with petroleum ether:acetone (9:1) as developing benefit will be derived if an actual experiment is carried out in
solvent. conjunction with the simulation. We therefore present a brief
(4) How does acetone disrupt the plant pigments from their description of a suitable experimental system which we have
natural state as pigment-protein complexes in the chloroplasts? found reliable.
(5) What extraction solvent system would you use if you wanted
to isolate the intact protein-pigment complexes? Describe Experimental System
experimental techniques which could be used to determine the The experiment involves photosynthetic incorporation of radio-
molecular weight of each protein-pigment complex. active CO2 by algal cells. Strains of Chlorella or Chlamydomonas
are probably most useful, although any photosynthetically
References competent alga could be used. Details of suitable strains, media
tGoodwin, T W (1983) 'Introduction to Plant Biochemistry', Second and growth conditions can be found in references 3-5.
Edition, Pergamon Press, Oxford, pp 96-103 The experiment requires access to a scintillation counter and
2Lichtenthaler, H K (1987) in 'Methods in Enzymology', Academic facilities to work with 14COa. The labelling apparatus is simple to
Press, New York, vol 148, pp 350-382 construct, and modifications may be incorporated to suit
3Mathews, C and van Holde, K (1990) 'Biochemistry', Benjamin- materials available. A key safety point is that release of 14CO2 to
Cummings, Redwood City, CA, pp 643-669 the atmosphere should be prevented. A suitable apparatus is
4Stryer, L (1988) 'Biochemisty', Third Edition, W H Freeman, New shown in Fig 1.
York, pp 517-533
5Voet, D and Voet, J (1990) 'Biochemistry', John Wiley and Sons, New
York, pp 586-594
[
6Goodwin, T W (1958) Biochem J 68, 503-511
7Moore, T C (1981) 'Research Experiences in Plant Physiology - - A
U c
Laboratory Manual', Second Edition, Springer-Verlag, New York, pp
35-45 E
STswett, M (1906) Ber Dtsch Bot Ges 24, 384
B . !G
A Simulation of Radioactive Tracer Flow
/i I\
DESMOND S T NICHOLL l and PETER C L JOHN 2
1Biology Department
Paisley College o f Technology Figure I Labelling apparatus. Algal suspension (A) is placed in a
cell culture flask (B) and a magnetic flea added. Cells are
Paisley P A l 2BE, Scotland illuminated from above with fluorescent strip lights (C) and
and agitated on a magnetic stirrer (D). The neck of the flask has a
2plant Cell Biology Group rubber seal which is pierced with three syringe needles, one of
Research School o f Biological Sciences which is attached to a length of small-bore silicon tubing. A filter
The Australian National University (E) is made by plugging a syringe barrel with cotton wool. Solid
KOH pellets may be incorporated to trap any radioactive C02
P O Box 475 released. The label is introduced by syringe (F). Samples (1 ml)
Canberra A C T 2601, Australia are withdrawn at G
Introduction The isotope used is sodium [14C] bicarbonate, which can be
The use of radiolabelled compounds in the study of metabolic used at a variety of dilutions. We have found that a final
pathways has been of crucial importance in biochemistry. radioactive concentration of around 37kBq m1-1 (1 i~Ci m1-1) is
Perhaps the best known example of the use of this technique is adequate. The label is added to the cells and the experiment
the elucidation of the path of carbon during photosynthetic timed from this point. Samples are removed at intervals and
fixation of CO2, which provided information about the pathway processed using apparatus similar to that shown in Fig 2. If
which we now know as the Calvin cycle. Although this type of possible this should be carried out in a fume-hood designated for
work is time-consuming and complicated, there have been radioactive materials, although the system is designed to prevent
reports of experiments suitable for class practicals in biochem- release of labelled CO2.
istry. These include investigation of the Calvin cycle I and the When all samples have been processed, 1 ml of each is
incorporation of radiolabelled inorganic and organic precursors centrifuged in a microfuge to separate the soluble and insoluble
by E coli. 2 fractions. The remainder is retained for measuring total incor-
In undergraduate classes we have used a procedure similar to poration. After centrifugation the supernatant is removed to a
that of Hawcroft 1 to illustrate the incorporation of 14CO2 by clean tube and the pellet resuspended in 1 ml acetic methanol.
Chlorella, and to monitor the distribution of label between This gives three samples for counting to determine total
soluble and insoluble pools in the cell. We also provide a simple incorporation, counts in soluble and counts in insoluble frac-
simulation to illustrate and complement the experimental pro- tions. A typical result is shown in Fig 3. This gives a good
cess. This has proved valuable, particularly in the case of weaker indication of the incorporation of label and its distribution, but
students who have difficulty grasping the concepts involved. does not identify the products of the labelling experiment.
B I O C H E M I C A L E D U C A T I O N 18(4) 1990
You might also like
- Progress in Phytochemistry: Volume 6From EverandProgress in Phytochemistry: Volume 6L. ReinholdNo ratings yet
- Lecture 13 PPIDocument15 pagesLecture 13 PPIabeehazakeeshNo ratings yet
- Chlorophylls and Carotenoids Pigments of Photosynthetic Biomembranes PDFDocument33 pagesChlorophylls and Carotenoids Pigments of Photosynthetic Biomembranes PDFAhmad Hassan ChNo ratings yet
- 436154638-The-Study-of-Chlorophyll-Content-in-Various-Plants 4.docx - 20231113 - 184531 - 0000Document11 pages436154638-The-Study-of-Chlorophyll-Content-in-Various-Plants 4.docx - 20231113 - 184531 - 0000Saran.kNo ratings yet
- COMPARATIVE CHLOROPHYLL STUDYDocument16 pagesCOMPARATIVE CHLOROPHYLL STUDYsasidharanNo ratings yet
- PhotosynthesisDocument21 pagesPhotosynthesisprabhat ranjan mishraNo ratings yet
- FY B.Sc. Photosynthetic PigmentsDocument3 pagesFY B.Sc. Photosynthetic PigmentsVijendraNo ratings yet
- 3 5 0 Plastids (3 4)Document33 pages3 5 0 Plastids (3 4)Fitri SukmawatiNo ratings yet
- Chlorophyll - WikipediaDocument53 pagesChlorophyll - WikipediaBashiir NuurNo ratings yet
- Amount of Chlorophyll in Five LeafDocument24 pagesAmount of Chlorophyll in Five LeafRakshitha MgowdaNo ratings yet
- BiologyDocument21 pagesBiologyHrituraj banikNo ratings yet
- Algal Pigments PDFDocument5 pagesAlgal Pigments PDFmanoj_rkl_07No ratings yet
- The Study of Chlorophyll Content in Various PlantsDocument24 pagesThe Study of Chlorophyll Content in Various PlantsShubham Raj72% (54)
- CRP 101 Lecture No. 10Document18 pagesCRP 101 Lecture No. 10AnandKuttiyanNo ratings yet
- Study of Chlorophyll Content in Various PlantsDocument15 pagesStudy of Chlorophyll Content in Various PlantsKunguma Vignesh57% (7)
- Cholorophyll Content in Five Different Species of PlantDocument14 pagesCholorophyll Content in Five Different Species of Plantlatest tamil moviesNo ratings yet
- Cbse BioDocument20 pagesCbse BioHrituraj banikNo ratings yet
- Photosynthesis in Higher PlantsDocument22 pagesPhotosynthesis in Higher PlantsSourav WiseNo ratings yet
- CPH-101-Fundamentals of Crop PhysiologyDocument105 pagesCPH-101-Fundamentals of Crop PhysiologyShivansh GurjarNo ratings yet
- Photosynthesis Explained - How Plants Use Light EnergyDocument21 pagesPhotosynthesis Explained - How Plants Use Light EnergyPritamNo ratings yet
- Photosynthesis CO AssimilationDocument30 pagesPhotosynthesis CO AssimilationUttam GurungNo ratings yet
- BE210S97W7R01Document33 pagesBE210S97W7R01Ahmad HoteitNo ratings yet
- Yilmaz 2016Document5 pagesYilmaz 2016Eduardo TarangoNo ratings yet
- Ii-Photosynthesis 5Document10 pagesIi-Photosynthesis 5bkarun216No ratings yet
- PhotosynthesisDocument37 pagesPhotosynthesisKambaska BeheraNo ratings yet
- Biology Project 1Document23 pagesBiology Project 1Aashray KothaNo ratings yet
- Lecture 14 PPIDocument17 pagesLecture 14 PPIabeehazakeeshNo ratings yet
- Nishant Mishra: Dav Public School Kalinga NagarDocument24 pagesNishant Mishra: Dav Public School Kalinga NagarNishant MishraNo ratings yet
- Ivestigatory Project On Biology 12Document24 pagesIvestigatory Project On Biology 12Nishant MishraNo ratings yet
- Lichtenthal Er 1987Document33 pagesLichtenthal Er 1987Gabriel EspinozaNo ratings yet
- Importance of Chlorophyll and Other PigmentsDocument7 pagesImportance of Chlorophyll and Other PigmentsIan Gabriel MayoNo ratings yet
- Phycobiliprotein Glazer1994 PDFDocument8 pagesPhycobiliprotein Glazer1994 PDFpradeep H NNo ratings yet
- File CompleteDocument22 pagesFile CompleteRaoNo ratings yet
- History: Chlorophyll (Also Chlorophyl) Is Any of Several Related GreenDocument5 pagesHistory: Chlorophyll (Also Chlorophyl) Is Any of Several Related GreenRamesh AdhikariNo ratings yet
- Spectral Properties of Chalcones II: Begüm Evranos AKSÖZ, Rahmiye ERTANDocument12 pagesSpectral Properties of Chalcones II: Begüm Evranos AKSÖZ, Rahmiye ERTANGanesamoorthy ThirunarayananNo ratings yet
- 11 Biology Notes Ch13 Photosymthesis in Higher PlantsDocument6 pages11 Biology Notes Ch13 Photosymthesis in Higher PlantsAAYUSH KUMARNo ratings yet
- Photosynthesis in Higher PlantsDocument25 pagesPhotosynthesis in Higher PlantsRaichal P BijuNo ratings yet
- Module 3 Sec 4Document18 pagesModule 3 Sec 4James MagnoNo ratings yet
- Optimization of Thin Layer Chromatography for Flavonoids and Phenolic AcidsDocument6 pagesOptimization of Thin Layer Chromatography for Flavonoids and Phenolic AcidsAndra Ch123No ratings yet
- Photosynthesis in Higher PlantsDocument5 pagesPhotosynthesis in Higher PlantsGaurav Arun RanaNo ratings yet
- Phytosynthesis: Pharmaceutical Botany With TaxonomyDocument2 pagesPhytosynthesis: Pharmaceutical Botany With TaxonomyVanessa RamosNo ratings yet
- C3 vs C4 Photosynthesis: Key DifferencesDocument5 pagesC3 vs C4 Photosynthesis: Key DifferencesParulNo ratings yet
- Chlorophylls and Other PigmentsDocument6 pagesChlorophylls and Other PigmentsHope Ladyline EspanolaNo ratings yet
- Porphyrin Basics: The Colorful Organic PigmentsDocument3 pagesPorphyrin Basics: The Colorful Organic PigmentsSumedha ThakurNo ratings yet
- 5 FotosintesiDocument29 pages5 FotosintesiSerena DamianNo ratings yet
- WEEK 2 Color ReflectionsDocument31 pagesWEEK 2 Color Reflectionsjhon achilles dugoNo ratings yet
- PHOTOBIOLOGYDocument49 pagesPHOTOBIOLOGYserimawarNo ratings yet
- Recommended Textbooks for Heteroaromatic ChemistryDocument25 pagesRecommended Textbooks for Heteroaromatic ChemistrySundas FatimaNo ratings yet
- Chloroplast Review: Milka Rahman Senior Biology Teacher MastermindDocument47 pagesChloroplast Review: Milka Rahman Senior Biology Teacher MastermindMilka RahmanNo ratings yet
- 22 Photosynthesis 1Document44 pages22 Photosynthesis 1黄心怡No ratings yet
- Botany Sci Rep PeconDocument7 pagesBotany Sci Rep PeconMargel PeconNo ratings yet
- BioenergeticsDocument36 pagesBioenergeticsmuzammalNo ratings yet
- Unit 5. Revision Notes in Accordance With Syllabus SpecificationsDocument16 pagesUnit 5. Revision Notes in Accordance With Syllabus SpecificationsShoaib Ahmed SamNo ratings yet
- Kikuchi 1999Document12 pagesKikuchi 1999jounfrank19No ratings yet
- Bio ProjectDocument13 pagesBio ProjectAdarsh Kumar Mohapatra0% (1)
- Structure and Reactions of ChlorophyllDocument8 pagesStructure and Reactions of Chlorophyllسید طاہر عباسNo ratings yet
- Energy & Equilibrium - Photosynthesis: 1. Overview of TopicDocument22 pagesEnergy & Equilibrium - Photosynthesis: 1. Overview of TopicNiharika GuptaNo ratings yet
- General Biology Quarter 2 - Week 7 Name: Rona Mae N. Betita Grade and Section: STEM-12 Vygotsky Intext Questions 11.1Document4 pagesGeneral Biology Quarter 2 - Week 7 Name: Rona Mae N. Betita Grade and Section: STEM-12 Vygotsky Intext Questions 11.1Rona Mae BetitaNo ratings yet
- Chlorophyll and Carotenoid Determination 2010Document2 pagesChlorophyll and Carotenoid Determination 2010Shahrukh Ghulam NabiNo ratings yet
- Principle of Paper ChromatographyDocument12 pagesPrinciple of Paper ChromatographyVijeta Jain100% (1)
- Data Structures and Algorithms For Approximate String Matching'Document40 pagesData Structures and Algorithms For Approximate String Matching'Lucy ZuluNo ratings yet
- Lecture:6 Food Microbiology Microbial Spoilage of Cereals and Bakery FoodsDocument4 pagesLecture:6 Food Microbiology Microbial Spoilage of Cereals and Bakery FoodsLucy ZuluNo ratings yet
- 1.0 Carbonyl Compounds - II - Synthesis of Aldehydes and Ketones - 2020 PDFDocument15 pages1.0 Carbonyl Compounds - II - Synthesis of Aldehydes and Ketones - 2020 PDFLucy ZuluNo ratings yet
- 10 Bread Spoilage and Staling: Irene M.C. PaterasDocument2 pages10 Bread Spoilage and Staling: Irene M.C. PaterasLucy ZuluNo ratings yet
- Che2522 Test 2Document3 pagesChe2522 Test 2Lucy ZuluNo ratings yet
- Cheese Production BIO 4352: DR Sydney MalamaDocument3 pagesCheese Production BIO 4352: DR Sydney MalamaLucy ZuluNo ratings yet
- Carbohydrate MetabolismDocument52 pagesCarbohydrate MetabolismLucy ZuluNo ratings yet
- Comparison of Parametric ValuesDocument5 pagesComparison of Parametric ValuesLucy ZuluNo ratings yet
- Identification of Microorganisms Responsible For S PDFDocument3 pagesIdentification of Microorganisms Responsible For S PDFLucy ZuluNo ratings yet
- BIO 4352 - Ass - 1 - 2020 PDFDocument1 pageBIO 4352 - Ass - 1 - 2020 PDFLucy ZuluNo ratings yet
- Revised Sessional Dates 2019 - 2020 Academic Year PDFDocument3 pagesRevised Sessional Dates 2019 - 2020 Academic Year PDFLucy ZuluNo ratings yet
- BIO 4352 - Ass - 1 - 2020 PDFDocument1 pageBIO 4352 - Ass - 1 - 2020 PDFLucy ZuluNo ratings yet
- Water Treatment NotesDocument3 pagesWater Treatment NotesLucy ZuluNo ratings yet
- Fermentation - 2019 - BIO 4352Document5 pagesFermentation - 2019 - BIO 4352Lucy ZuluNo ratings yet
- Water Treatment NotesDocument3 pagesWater Treatment NotesLucy ZuluNo ratings yet
- Unix Commands Cheat Sheet PDFDocument1 pageUnix Commands Cheat Sheet PDFRosemond FabienNo ratings yet
- Lect 1 Introducing Bio InformaticsDocument97 pagesLect 1 Introducing Bio InformaticsLucy ZuluNo ratings yet
- PDF PDFDocument31 pagesPDF PDFLucy ZuluNo ratings yet
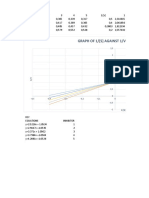
- Graph of 1/ (S) Against 1/V Competive InhibitionDocument2 pagesGraph of 1/ (S) Against 1/V Competive InhibitionLucy ZuluNo ratings yet
- The University of Zambia School of Natural Sciences Department of Biological Sciences BIO-4321Document4 pagesThe University of Zambia School of Natural Sciences Department of Biological Sciences BIO-4321Lucy ZuluNo ratings yet
- WHO-EU Comparative Drinking Water StandardsDocument4 pagesWHO-EU Comparative Drinking Water StandardsLucy ZuluNo ratings yet
- CHE2522 Course Outline - 2019-2020 Academic Year-Final 24.07.2020Document5 pagesCHE2522 Course Outline - 2019-2020 Academic Year-Final 24.07.2020Lucy ZuluNo ratings yet
- Lipid Metabolism and DigestionDocument17 pagesLipid Metabolism and DigestionLucy ZuluNo ratings yet
- Che 3111 Lecture Notes, Nitrogen Metabolism 2019 July 2020 PDFDocument34 pagesChe 3111 Lecture Notes, Nitrogen Metabolism 2019 July 2020 PDFLucy ZuluNo ratings yet
- Arabinose + Tryptophan OperonDocument58 pagesArabinose + Tryptophan OperonLucy ZuluNo ratings yet
- CHE 3122 Lecture Notes Energetics of Photosynthesis: 2018 Academic Year October 2019Document9 pagesCHE 3122 Lecture Notes Energetics of Photosynthesis: 2018 Academic Year October 2019Lucy ZuluNo ratings yet
- Translation Initiation: April 2017 BIO 3421Document109 pagesTranslation Initiation: April 2017 BIO 3421Lucy ZuluNo ratings yet
- Enzymes Used rDNA TechDocument51 pagesEnzymes Used rDNA TechLucy ZuluNo ratings yet
- Lipid Metabolism and DigestionDocument17 pagesLipid Metabolism and DigestionLucy ZuluNo ratings yet
- CopyofCopyofMaldeepSingh Jawanda ResumeDocument2 pagesCopyofCopyofMaldeepSingh Jawanda Resumebob nioNo ratings yet
- Annual Reading Plan - Designed by Pavan BhattadDocument12 pagesAnnual Reading Plan - Designed by Pavan BhattadFarhan PatelNo ratings yet
- MPU 2232 Chapter 5-Marketing PlanDocument27 pagesMPU 2232 Chapter 5-Marketing Plandina azmanNo ratings yet
- Risus License Information PDFDocument1 pageRisus License Information PDFSam CorbenNo ratings yet
- Yatendra Kumar Sharma ResumeDocument3 pagesYatendra Kumar Sharma ResumeDheeraj SharmaNo ratings yet
- Chapter 2 Research and DesignDocument24 pagesChapter 2 Research and Designalvin salesNo ratings yet
- FINANCIAL REPORTSDocument34 pagesFINANCIAL REPORTSToni111123No ratings yet
- Arnica The Miracle Remedy - Case RecordsDocument4 pagesArnica The Miracle Remedy - Case Recordskaravi schiniasNo ratings yet
- Infrastructure Finance Project Design and Appraisal: Professor Robert B.H. Hauswald Kogod School of Business, AUDocument2 pagesInfrastructure Finance Project Design and Appraisal: Professor Robert B.H. Hauswald Kogod School of Business, AUAida0% (1)
- Drainage Manual: State of Florida Department of TransportationDocument78 pagesDrainage Manual: State of Florida Department of TransportationghoyarbideNo ratings yet
- A Review of High School Economics Textbooks: February 2003Document27 pagesA Review of High School Economics Textbooks: February 2003Adam NowickiNo ratings yet
- Wind Hydro 2Document6 pagesWind Hydro 2Vani TiwariNo ratings yet
- Detailed Pacing Guide HMH Science Dimensions Grades K-5Document70 pagesDetailed Pacing Guide HMH Science Dimensions Grades K-5Milica PejcicNo ratings yet
- Super BufferDocument41 pagesSuper Bufferurallalone100% (1)
- Seminar On: Hadoop TechnologyDocument13 pagesSeminar On: Hadoop TechnologySAV SportsNo ratings yet
- Chapter 08-Borrowing Costs-Tutorial AnswersDocument4 pagesChapter 08-Borrowing Costs-Tutorial AnswersMayomi JayasooriyaNo ratings yet
- RCD-GillesaniaDocument468 pagesRCD-GillesaniaJomarie Alcano100% (2)
- Operational Readiness Guide - 2017Document36 pagesOperational Readiness Guide - 2017albertocm18100% (2)
- Floor CraneDocument6 pagesFloor CranejillianixNo ratings yet
- MetalCut User ManualDocument71 pagesMetalCut User Manualnammaris0% (1)
- 1967 2013 PDFDocument70 pages1967 2013 PDFAlberto Dorado Martín100% (1)
- Samples For Loanshyd-1Document3 pagesSamples For Loanshyd-1dpkraja100% (1)
- Cultural DiffusionDocument2 pagesCultural DiffusionNicole Aguarin SwinNo ratings yet
- Aptamers in HIV Research Diagnosis and TherapyDocument11 pagesAptamers in HIV Research Diagnosis and TherapymikiNo ratings yet
- Latest Information Technology Trends 2023Document5 pagesLatest Information Technology Trends 2023Salveigh C. TacleonNo ratings yet
- Airbag Inflation: The Airbag and Inflation System Stored in The Steering Wheel. See MoreDocument5 pagesAirbag Inflation: The Airbag and Inflation System Stored in The Steering Wheel. See MoreShivankur HingeNo ratings yet
- UK Journal Compares Clove & Rosemary Oil Antibacterial ActivityDocument5 pagesUK Journal Compares Clove & Rosemary Oil Antibacterial ActivityNurul Izzah Wahidul AzamNo ratings yet
- Engine Rear Oil Seal PDFDocument3 pagesEngine Rear Oil Seal PDFDIEGONo ratings yet
- Fundamentals of Accountancy Business Management 2: Learning PacketDocument33 pagesFundamentals of Accountancy Business Management 2: Learning PacketArjae Dantes50% (2)