Professional Documents
Culture Documents
Competant Cells and Transformation
Uploaded by
Swetha Ramesh0 ratings0% found this document useful (0 votes)
31 views3 pagesProtocol
Original Title
Competant cells and Transformation
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentProtocol
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
31 views3 pagesCompetant Cells and Transformation
Uploaded by
Swetha RameshProtocol
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 3
Protocol 25
Preparation and Transformation of Competent
E. coli using Calcium Chloride
Tw. FOLLOWING SIMPLE AND RAPID VARIATION OF THE TECHNIQUE published by Cohen et al. (1972)
is frequently used to prepare batches of competent bacteria that yield 3 x 10° to 2 x 10” trans.
formed colonies/ug of supercoiled plasmid DNA. This efficiency of transformation is high
enough to allow all routine cloning in plasmids to be performed with ease. Competent cells made
by this procedure may be preserved at ~70°C, although there may be some deterioration in the
efficiency of transformation during prolonged storage.
MATERIALS _
Buffers and Solutions
Please see Appendix 1 for components of stock sol
Dilute stock solutions to the appropriate concentrat
CaCI,2H,0 (7 9)
‘When preparing competent calls, thaw a 10-mlaiguot ofthe stock solution and dilute ito 100 ml with
90 mi of pure HO. Sterilz the solution by filtration through a prerinsed Nalgene fer (0.45-u1m pore
sire), an then chill it 10 (FC.
or
Standard transformation buifer (TFB) (please see Protocol 23, Step 1)
For many strains of E.coli, standard TFB (Hanahan 1983) may be used instead of CaCl, with equivalent
tor better results,
MgCl,-CaCl, solution, ice cold
ions, buffers, and reagents.
Media
LB or SOB medium for initial growth of culture
SOB agar plates comaining 20 m¥i MgSO, and the appropriate antibiotic
Standard SOB contains 10 mM MgSO,
SOC medium
Approximately 1 ml of this medium i required for each transformation reaction,
Nucleic Acids and Oligonucleotides
Plasmid DNA (recombinant plasmid)
‘Construct using one of the methods described in Protocols 17 through 22 ofthis chapter
1116
Protocol 25: Preparation and Transformation of Competent E. coli using Calcium Chloride 16117
Centrifuges and Rotors
Somall GSA rotor or equivalent
Special Equipment
Polypropylene tube (50 mb, chilled in ice
Polypropylene tubes (17 x 100 mm; Falcon 2059), chilled in ice
Water bath preset to 42°C
METHOD
A IMPORTANT All steps in this procedure should be carried out aseptically.
Preparation of Cells
1. Pick a single bacterial colony (2-3 mm in diameter) from a plate that has been incubated for
16-20 hours at 37°C. Transfer the colony into 100 ml of LB broth or SOB medium ina |-liter
flask. Incubate the culture for 3 hoursat 37#C with vigorous agitation, monitoring the growth
of the culture. As a guideline, 1 ODgoy of a culture of E, coli strain DH1 contains ~10” bacte-
riafml.
For efficient transformation, it eset hat the numberof viable celle not exced 10 cell
which for most strains of Ecos equvslent to an OD, of -04, To ensure thatthe culture does
not grow t0a higher density, measre the ODja of the etre every 15-20 minutes, Plot a gph
ofthe data so thatthe time when the OD, ofthe cure approaches an be predicted with
Some accuracy Begin to harvest the culture hen the OD, teaches 0.35
Because the relationship between the OD yy and the numberof viable cells varies substantially
from strain to strain the specrophotomte must be calibrate by measuring the OD, fa grow
ing eultre ofthe particular stein of Eclat diferent times ints growth eye and determining
‘he number of ible clls at each of thesetimes by plating distin ofthe culture on LBagar pts
intheabsence of antibiotics
2, Transfer the bacterial cells to sterile, disposable, ice-cold 50-ml polypropylene tubes. Cool the
cultures to 0°C by storing the tubes on ice for 10 minutes.
3. Recover the cells by centrifugation at 2700g (4100 rpm in a Sorvall GSA rotor) for 10 min-
tutes at $C
4. Decant the medium from the cel pellets. Stand the tubes in an inverted position on a pad of
paper towels for 1 minute to allow the last traces of media to drain away.
5. Resuspend each pellet by swirling or gentle vortexing in 30 ml of ice-cold MgCl,-CaCl, sol
tion (80 mM MgCl, 20 mM CaCl).
6. Recover the cells by centrifugation at 2700g (4100 rpm in a Sorvall GSA rotor) for 10 min-
utes at 4°C,
7. Decant the medium from the cell pellets. Stand the tubes in an inverted position on a pad of
paper towels for I minute to allow the last traces of media to drain away:
8. Resuspend the pellet by swirling or gentle vortexing in 2 ml of ice-cold 0.1 M CaCl, (or TFB)
for each 50 ml of original culture.
9. At this point, either use the cells directly for transformation as described in Steps 10 through 16
below or dispense into aliquots and freeze at 70°C {please see Protocol 23, Step 12).
1118 Chapter 1: Plasmids and Their Usefulness in Molecular Cloning
Transformation
For most strains of &.colf(except for MC1061}, TFB may be used at this stage instead of
‘suivant or beter results,
the cells may be stored at 4°C in CaCl, solution for 24-48 hours (Dagert and Ehrlich 1979) The
efficiency of transformation increases four- to sixfold during the fist 12-24 hous of storage and
thereafter decreases tothe orginal level
Include all of the appropriate positive and negative controls (please see the panel on BACTERIAL
TRANSFORMATION in Protocol 23)
10.
i.
12.
13,
14.
15.
16.
‘To transform the CaCl,-treated cells directly, transfer 200 ul of each suspension of competent
cells to a sterile, chilled 17 x 100-mm polypropylene tube using a chilled micropipette tip.
Add DNA (no more than 50 ng in a volume of 10 tl or less) to each tube. Mix the contents
of the tubes by swirling gently. Store the tubes on ice for 30 minutes.
‘Transfer the tubes to a rack placed in a preheated 42°C circulating water bath, Store the tubes
in the rack for exactly 90 seconds, Do not shake the tubes.
Heat shock isa crucial step. Ii very important thatthe cells be raised to exactly the right tem>
perature atthe correct rate
Rapidly transfer the tubes to an ice bath, Allow the cells to chill for 1-2 minutes.
Add 800 ul of SOC medium to each tube. Incubate the cultures for 45 minutes in a water bath
set at 37°C to allow the bacteria to recover and to express the antibiotic resistance marker
encoded by the plasmid
The cells may be gent
recovery period,
y agitated (50 cycle'minute or less ina rotary shaker) at 376C: during the
lf screening by a-complementation, proceed to Protocol 27 for plating.
Transfer the appropriate volume (up to 200 ul per 90-mm plate) of transformed competent
cells onto agar SOB medium containing 20 mM MgSO, and the appropriate antibiotic:
‘When selecting for resistance to tetracycline, the entire transformation mixture maybe speed on
a single plate or plate in tp agar. this case, collect he bacteria by centrifuging fo 20 seconds
2 room temperature in a miseofe, and then gently resuspend the el pellet in 10 pl of SOC
‘medium by tapping the sides ofthe tbe.
{A IMPORTANT Seize a ben ls rod by coping it ino ethanol and then in he fame of «Bunsen
tue. When the od fas cook o room temperature, spread the transformed cells gery ove the
surface ofthe agar plate.
When selecting for resistance to ampicillin, transformed cel shouldbe plated at ove density 10"
colonies per 90-mm plate),and the pats should not be incubated for more than 20 hours at 37%:
“hc eneyme f-lactamas is sereed ino the medium from ampiiln-resistan transformants and
You might also like
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Advanced Hindi GrammarDocument156 pagesAdvanced Hindi GrammarSwetha RameshNo ratings yet
- Enzyme Notes - SathyanarayanaDocument32 pagesEnzyme Notes - SathyanarayanaSwetha RameshNo ratings yet
- The Cartoon Guide To Statistics-3Document8 pagesThe Cartoon Guide To Statistics-3Swetha Ramesh100% (1)
- Cells of Immune SystemDocument14 pagesCells of Immune SystemSwetha RameshNo ratings yet
- His Tag Purification PDFDocument5 pagesHis Tag Purification PDFSwetha RameshNo ratings yet
- Varalakshmi Vratham Pooja Ebook in Tamil PDFDocument25 pagesVaralakshmi Vratham Pooja Ebook in Tamil PDFSwetha RameshNo ratings yet
- B198T45ApplicationForm PDFDocument1 pageB198T45ApplicationForm PDFSwetha RameshNo ratings yet
- Development of Neem Based Bioplastic For Food Packaging ApplicationDocument8 pagesDevelopment of Neem Based Bioplastic For Food Packaging ApplicationSwetha RameshNo ratings yet
- Processing and Storage Effects On Orange Juice Aroma: A ReviewDocument13 pagesProcessing and Storage Effects On Orange Juice Aroma: A ReviewSwetha RameshNo ratings yet
- Lymphoid OrganDocument23 pagesLymphoid OrganSwetha RameshNo ratings yet
- International WebinarDocument1 pageInternational WebinarSwetha RameshNo ratings yet
- Bioscouring in Textile IndustryDocument2 pagesBioscouring in Textile IndustrySwetha RameshNo ratings yet
- P-3116 - Immunocytochemistry Lecture Notes PDFDocument26 pagesP-3116 - Immunocytochemistry Lecture Notes PDFSwetha RameshNo ratings yet
- Notes of Lesson - Microbiology Code: BT1204 Unit - I: Introduction History of MicrobiologyDocument18 pagesNotes of Lesson - Microbiology Code: BT1204 Unit - I: Introduction History of MicrobiologySwetha RameshNo ratings yet
- Restriction Mapping - KeyDocument6 pagesRestriction Mapping - KeySwetha Ramesh100% (1)
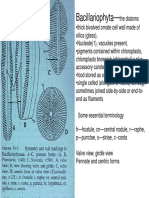
- Diatom Book-1 PDFDocument23 pagesDiatom Book-1 PDFSwetha RameshNo ratings yet
- Genome Sequencing: BY:-Anitha.Y 14KUST4002Document20 pagesGenome Sequencing: BY:-Anitha.Y 14KUST4002Swetha RameshNo ratings yet
- Notes of Lesson - Microbiology Code: BT1204 Unit - I: Introduction History of MicrobiologyDocument18 pagesNotes of Lesson - Microbiology Code: BT1204 Unit - I: Introduction History of MicrobiologySwetha RameshNo ratings yet
- Organic Farming:: Organic Inputs and Techniques: S. No. Groups ExamplesDocument23 pagesOrganic Farming:: Organic Inputs and Techniques: S. No. Groups ExamplesSwetha RameshNo ratings yet
- Unit II Microbes Structure and MultiplicationDocument22 pagesUnit II Microbes Structure and MultiplicationSwetha RameshNo ratings yet
- GE6251-Basic Civil and Mechanical Engineering-1 - 258 PDFDocument21 pagesGE6251-Basic Civil and Mechanical Engineering-1 - 258 PDFSwetha RameshNo ratings yet
- Staining NotesDocument5 pagesStaining NotesSwetha RameshNo ratings yet