Professional Documents
Culture Documents
Corrosion of Zn in Acidic Solution
Uploaded by
Leo NguyễnOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Corrosion of Zn in Acidic Solution
Uploaded by
Leo NguyễnCopyright:
Available Formats
Question 4.
a. Given pH=1
[Zn2+] = 1·10–5 M
c = –0.12 V/decade,a = 0.15 V/decade
i0H2 = 2·10–10 A cm–2, i0Zn = 1·10–8 A cm–2
The cathodic and anodic reaction:
2+¿+2 e❑ Zn¿
anode Z n ↔
+ ¿+2 e❑ H 2 ¿
cathode 2 H ↔
Tafel Equation for the anodic process:
i corr
ŋa =β a log
( ) i oZn
=Ecorr −EZ n 2+ ¿
/ Zn¿
Tafel Equation for the anodic process:
i corr
ŋc =β c log
( ) i oH
2
=E corr −E H +¿
/ H 2¿
EZ n 2 +¿
/Zn=E ¿
2+ ¿ 0 0.059
Zn /Zn + lg ¿¿ ¿
2
EH +¿
/ H2 = E +¿ 0 0.059
¿
H / H2 + lg ¿ ¿¿¿
2
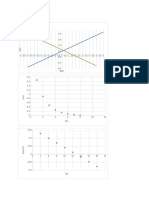
Construct the Evans diagram:
Hydrogen Reaction:
Slope is the cathodic Tafel coefficient βc =-0.12
Electrode potential from Nernst equation E H +¿
/ H2=−0.059(V )¿
Zn Dissolution:
Slope is the anodic Tafel coefficient βa=0.15
Electrode potential from Nernst equation E Z n 2 +¿
/Zn=−0.908(V )¿
EH +¿
/ H2 , ioH ¿
2
2H++2e- -> H2
Zn-> Zn2++2e-
E Zn 2+ ¿
ECorr , i corr
/ Zn,ioZn ¿
i prot
Figure 4.1 the Evans diagram for the corrosion of Zn
Based on the Evans diagram, the following values are estimated:
corrosion potential Ecorr =−0.63(V )
corrosion current density i corr =2 x 10−6 (Ac m−2)
b. Calculate the protection current density
The protection current density Iprot can be estimated by the Evans diagram to be 2x10 -3 A cm-2
c. when the pH of the residue is double, and the activity of Zn 2+ is squared
the activity of Zn2+ is squared([Zn2+] = 1·10–10 M):
EZ n 2 +¿
/Zn=E ¿
2+ ¿ 0 0.059
Zn /Zn + lg ¿¿ ¿
2
EH +¿
/ H2 = E +¿ 0 0.059
¿
H / H2 + lg ¿ ¿¿¿
2
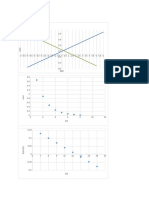
Construct the Evans diagram:
Hydrogen Reaction:
Slope is the cathodic Tafel coefficient βc =-0.12
Electrode potential from Nernst equation E H +¿
/ H2=−0.118(V )¿
Zn Dissolution:
Slope is the anodic Tafel coefficient βa=0.15
Electrode potential from Nernst equation E Z n 2 +¿
/Zn=−1.055(V )¿
EH +¿
/ H2 , ioH ¿
2
2H++2e- -> H2
Zn-> Zn2++2e-
ECorr , i corr
E Zn
2+ ¿
/ Zn,ioZn ¿
Figure 4.2 the Evans diagram for the corrosion of Zn when the pH of the residue is double,
and the activity of Zn2+ is squared
Based on figure 4.2 ,the corrosion potential(E corr )of the system∧corrosioncurrent density i corr
is estimated to be -0.64V and 5.3 x 10−6 Ac m−2 respectively. ¿
You might also like
- Tables of the Function w (z)- e-z2 ? ex2 dx: Mathematical Tables Series, Vol. 27From EverandTables of the Function w (z)- e-z2 ? ex2 dx: Mathematical Tables Series, Vol. 27No ratings yet
- Electrodes and Reduction PotentialsDocument10 pagesElectrodes and Reduction PotentialsluisNo ratings yet
- Solution Manual for an Introduction to Equilibrium ThermodynamicsFrom EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsNo ratings yet
- Electrochemistry 2Document14 pagesElectrochemistry 2Wella YektiNo ratings yet
- Electrode Potential SummaryDocument1 pageElectrode Potential SummaryNooran ShamsNo ratings yet
- Assignment Lesson 7 UNIT 02 Standard Cell PotentialDocument4 pagesAssignment Lesson 7 UNIT 02 Standard Cell PotentialCRUZ, Rochelle Joy V.No ratings yet
- Redox PDFDocument41 pagesRedox PDFAYAN KUMARNo ratings yet
- Electrochemistry 12 Formula SheetDocument6 pagesElectrochemistry 12 Formula SheetFranknire IgNo ratings yet
- Chap 2-2. Mixed Potential TheoryDocument16 pagesChap 2-2. Mixed Potential Theory맛있는감자No ratings yet
- Lecture 8Document61 pagesLecture 8Ti GraNo ratings yet
- 3a-Redox ChemistryDocument40 pages3a-Redox ChemistryAbhisar UpadhyayNo ratings yet
- 3 2 Pourbaix Diagram PDFDocument31 pages3 2 Pourbaix Diagram PDFfadelomar28_gmail_coNo ratings yet
- Chapter 2Document51 pagesChapter 2Adugnaw BiksNo ratings yet
- Eh, Ph, and the chemistry of natural watersDocument17 pagesEh, Ph, and the chemistry of natural watersLeila EspinosaNo ratings yet
- Redox and pE – pH DiagramsDocument31 pagesRedox and pE – pH DiagramsAna Luisa Garnica SalgadoNo ratings yet
- Lesson 15Document109 pagesLesson 15anil ariNo ratings yet
- Corrosion ThermodynamicsDocument59 pagesCorrosion ThermodynamicstehtnicaNo ratings yet
- Physical Chemistry 2 - Kinetics of Electrochemical ProcessesDocument44 pagesPhysical Chemistry 2 - Kinetics of Electrochemical ProcessesNguyễn Thu HàNo ratings yet
- Standard Hydrogen Electrode PresentationDocument23 pagesStandard Hydrogen Electrode PresentationKishore KishoreNo ratings yet
- Chap 5. Redox TitrationDocument46 pagesChap 5. Redox TitrationKoasa NishikiNo ratings yet
- ELECTROCHEMISTRYDocument10 pagesELECTROCHEMISTRYISLAM I. Fekry100% (2)
- 1st Yr 2007 RedoxDocument66 pages1st Yr 2007 RedoxAriyanti NaissaissNo ratings yet
- Electrolysis in Aqueous SolutionDocument15 pagesElectrolysis in Aqueous SolutionEdon BediNo ratings yet
- CH 17Document42 pagesCH 17Bông Cải XanhNo ratings yet
- Lecture 5 - Redox Reactions, Latimer and Frost DiagramsDocument50 pagesLecture 5 - Redox Reactions, Latimer and Frost DiagramsDaksh GuptaNo ratings yet
- Lecture 02b Oxidation-ReductionDocument41 pagesLecture 02b Oxidation-ReductionVivi AisahNo ratings yet
- Lecture 578 Oxidation-ReductionDocument41 pagesLecture 578 Oxidation-ReductionDika Virga SaputraNo ratings yet
- Electrochemistry - 2 - 1Document6 pagesElectrochemistry - 2 - 1Mandeep PediredlaNo ratings yet
- Wa0031.Document40 pagesWa0031.SefalikaNo ratings yet
- Electrochemistry FinalDocument76 pagesElectrochemistry Finalsmudgegaming4989No ratings yet
- CH 17Document43 pagesCH 17ዝምታ ውስጤ ነውNo ratings yet
- Electrochemistry ExerciseDocument2 pagesElectrochemistry ExerciseNuraina NabihahNo ratings yet
- Redox Reactions & ElectrochemistryDocument16 pagesRedox Reactions & ElectrochemistryEzhil MukilNo ratings yet
- P08 ADocument6 pagesP08 ADana CapbunNo ratings yet
- Last Minute Revision Notes ChemistryDocument9 pagesLast Minute Revision Notes Chemistrytechwithtarun477No ratings yet
- Chapter - 12 - Electrochemistry 2Document58 pagesChapter - 12 - Electrochemistry 2Gabrielle Dio ErdiansyahNo ratings yet
- Chap 5. REDOX TITRATIONDocument62 pagesChap 5. REDOX TITRATIONT.N NgânNo ratings yet
- Electrochemistry Complete NCERTDocument20 pagesElectrochemistry Complete NCERTNitesh YadavNo ratings yet
- There Are Three Convenient Ways To Graphically Summarize An Element's Redox PropertiesDocument22 pagesThere Are Three Convenient Ways To Graphically Summarize An Element's Redox PropertiesAdnan BukhariNo ratings yet
- Electrochemistry 12Document19 pagesElectrochemistry 12Manas ChhabraNo ratings yet
- Electrochemical Cells and Corrosion ReviewDocument31 pagesElectrochemical Cells and Corrosion ReviewpkNo ratings yet
- Electrochemistry: e So Conventional Current K PotentiometerDocument9 pagesElectrochemistry: e So Conventional Current K PotentiometerRica Janelle Rioflorido MarticioNo ratings yet
- Oxidation Number: Na, Be, K, PB, H, O, PDocument40 pagesOxidation Number: Na, Be, K, PB, H, O, Pjoe 45No ratings yet
- Last Minute Revision Notes Chemistry PDFDocument8 pagesLast Minute Revision Notes Chemistry PDFBibash Shrestha67% (3)
- 分析電化學講義1Document33 pages分析電化學講義1ylliwqNo ratings yet
- 5 Electrochemistry PDFDocument21 pages5 Electrochemistry PDFP. E. I. AcademicsNo ratings yet
- 1501 Electrode Potential: The Spontaneity of Electron Transfer Relationship Between E, GandkDocument21 pages1501 Electrode Potential: The Spontaneity of Electron Transfer Relationship Between E, GandkJuan Martínez0% (1)
- Lec BalancingredoxmrxnDocument2 pagesLec BalancingredoxmrxnMs. BNo ratings yet
- Question 801367Document4 pagesQuestion 801367niveditasingh2472No ratings yet
- Solved Problems in Quantum MechanicsDocument3 pagesSolved Problems in Quantum MechanicsMalvado Aun Mas MalvadoNo ratings yet
- Questionnaire Lab Session 4 RedoxDocument4 pagesQuestionnaire Lab Session 4 RedoxNameanxa AngelsNo ratings yet
- Quick Revision Notes ChemistryDocument9 pagesQuick Revision Notes Chemistrybobby wNo ratings yet
- 2021 Plasma Ju CDocument80 pages2021 Plasma Ju CFikret SaricNo ratings yet
- Chapter 19 ElectrochemistryDocument33 pagesChapter 19 ElectrochemistryStar LightNo ratings yet
- 8 ElectrochemistryDocument58 pages8 ElectrochemistryLutfiah HaninNo ratings yet
- Solution of Tutorial 2 - Uncontrolled Rectifier CircuitsDocument15 pagesSolution of Tutorial 2 - Uncontrolled Rectifier CircuitsBalestier HillNo ratings yet
- Electrochemistry - DPP 04Document2 pagesElectrochemistry - DPP 04MehulNo ratings yet
- Chapter 19 ElectrochemistryDocument33 pagesChapter 19 ElectrochemistryIntan NuraeniNo ratings yet
- CHEMICAL ENGINEERING COVER SHEETDocument14 pagesCHEMICAL ENGINEERING COVER SHEETLeo NguyễnNo ratings yet
- Assignment 8Document10 pagesAssignment 8Leo NguyễnNo ratings yet
- Question 3Document2 pagesQuestion 3Leo NguyễnNo ratings yet
- UN Sustainable Development Goals & Essential PBL DesignDocument1 pageUN Sustainable Development Goals & Essential PBL DesignLeo NguyễnNo ratings yet
- Ssessment Over HeetDocument10 pagesSsessment Over HeetLeo NguyễnNo ratings yet
- CHEMICAL ENGINEERING COVER SHEETDocument14 pagesCHEMICAL ENGINEERING COVER SHEETLeo NguyễnNo ratings yet
- Question 3Document2 pagesQuestion 3Leo NguyễnNo ratings yet
- Assignment 8Document10 pagesAssignment 8Leo NguyễnNo ratings yet
- Decadence of Victorian Masculinity, or Dandyism in Oscar Wilde's Lady Windermere's FanDocument16 pagesDecadence of Victorian Masculinity, or Dandyism in Oscar Wilde's Lady Windermere's FanLeo NguyễnNo ratings yet
- Errors and Uncertainties 2017-PDocument50 pagesErrors and Uncertainties 2017-PLeo NguyễnNo ratings yet
- Subject: Chemistry Class: XI Chapter: Redox Reactions Top ConceptsDocument7 pagesSubject: Chemistry Class: XI Chapter: Redox Reactions Top Conceptsaustinfru7No ratings yet
- Kamiastricity - The Effeciency of KamiasDocument15 pagesKamiastricity - The Effeciency of KamiasRobby Lastimosa100% (1)
- Corrosion Testing of Aerosol ProductsDocument4 pagesCorrosion Testing of Aerosol ProductsEdgardo Ed RamirezNo ratings yet
- ASTM Code For Corrosion TestDocument4 pagesASTM Code For Corrosion TestPRERNA SINGH100% (1)
- Lecture8-Environmental Degradation and Material SelectionDocument29 pagesLecture8-Environmental Degradation and Material SelectionRUGERO KeslyneNo ratings yet
- Introduction To Structural Integrity PrintableDocument68 pagesIntroduction To Structural Integrity Printablegerard correaNo ratings yet
- WWW - Thermadyne ArcairDocument1 pageWWW - Thermadyne ArcairJohn Alexander Bonilla AngelNo ratings yet
- Chemical Engineering Journal: Yingshi Zhu, Fengxia Deng, Shan Qiu, Fang Ma, Yanshi Zheng, Lei GaoDocument11 pagesChemical Engineering Journal: Yingshi Zhu, Fengxia Deng, Shan Qiu, Fang Ma, Yanshi Zheng, Lei GaoFlori DediuNo ratings yet
- Thermal Analysis Techniques and Solid State ReactionsDocument14 pagesThermal Analysis Techniques and Solid State Reactionsد.حاتممرقهNo ratings yet
- Basics & Timing-PmDocument120 pagesBasics & Timing-PmWiwik Puji Lestari100% (1)
- ElectrogravimetryDocument10 pagesElectrogravimetrypatriciaNo ratings yet
- Lab 21Document2 pagesLab 21Nor Ashikin IsmailNo ratings yet
- 7build Your Own Mud BatteryDocument3 pages7build Your Own Mud BatterySKNo ratings yet
- Mete Alp Yıldırım EXP 10 ReportDocument7 pagesMete Alp Yıldırım EXP 10 ReportAlp YıldırımNo ratings yet
- Vimal - CV - 14062022Document2 pagesVimal - CV - 14062022Sajid UddinNo ratings yet
- Unit 1 Ionic Equilibrium and ElectrochemistryDocument74 pagesUnit 1 Ionic Equilibrium and ElectrochemistryShubham SharmaNo ratings yet
- Chemistry ProjectDocument10 pagesChemistry ProjectAwais AnwarNo ratings yet
- Phy Projects 3Document12 pagesPhy Projects 3ArjunanRajakumarRNo ratings yet
- CORROSION CONTROL METHODSDocument37 pagesCORROSION CONTROL METHODSRohan PolNo ratings yet
- Lecture 03 Electrochemical Kinetics ZCDocument20 pagesLecture 03 Electrochemical Kinetics ZCNguyễn Duy LongNo ratings yet
- واجب شامل للمقررDocument30 pagesواجب شامل للمقررOsama AlkinaneNo ratings yet
- Lecture 3 Kinetics PDFDocument69 pagesLecture 3 Kinetics PDFmanishtubNo ratings yet
- Energy and AIDocument16 pagesEnergy and AIjabir sulaimanNo ratings yet
- Battery Energy - 2023 - Choi - Highly Textured and Crystalline Materials For Rechargeable Li Ion BatteriesDocument26 pagesBattery Energy - 2023 - Choi - Highly Textured and Crystalline Materials For Rechargeable Li Ion BatteriesChinnasamy M100% (1)
- Device Corrosion: Enumerate The Electrical Contact Degradation and The Types of Mechanism Involved in ItDocument10 pagesDevice Corrosion: Enumerate The Electrical Contact Degradation and The Types of Mechanism Involved in ItSreekanth KrishnamurthyNo ratings yet
- COPEN-9 Full Paper Upload 77Document6 pagesCOPEN-9 Full Paper Upload 77aghosh704100% (1)
- Electrochemistry PresentationDocument36 pagesElectrochemistry PresentationMuhammad HaziqNo ratings yet
- MUCLecture 2022 42033403Document9 pagesMUCLecture 2022 42033403ReedhiNo ratings yet
- User S Manual: Four & Six One and Two Litre Tank Galvanic PlantsDocument24 pagesUser S Manual: Four & Six One and Two Litre Tank Galvanic PlantsTacha Von LammNo ratings yet
- Question Bank On Energy Storage SystemDocument12 pagesQuestion Bank On Energy Storage Systemjoshinihar19100% (1)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- Piping and Pipeline Calculations Manual: Construction, Design Fabrication and ExaminationFrom EverandPiping and Pipeline Calculations Manual: Construction, Design Fabrication and ExaminationRating: 4 out of 5 stars4/5 (18)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (3)
- Meltdown: Nuclear disaster and the human cost of going criticalFrom EverandMeltdown: Nuclear disaster and the human cost of going criticalRating: 5 out of 5 stars5/5 (5)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- Nuclear Energy in the 21st Century: World Nuclear University PressFrom EverandNuclear Energy in the 21st Century: World Nuclear University PressRating: 4.5 out of 5 stars4.5/5 (3)
- Guidelines for Asset Integrity ManagementFrom EverandGuidelines for Asset Integrity ManagementRating: 5 out of 5 stars5/5 (1)
- Functional Safety from Scratch: A Practical Guide to Process Industry ApplicationsFrom EverandFunctional Safety from Scratch: A Practical Guide to Process Industry ApplicationsNo ratings yet
- Coating and Drying Defects: Troubleshooting Operating ProblemsFrom EverandCoating and Drying Defects: Troubleshooting Operating ProblemsRating: 5 out of 5 stars5/5 (1)
- The Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookNo ratings yet
- Gas-Liquid And Liquid-Liquid SeparatorsFrom EverandGas-Liquid And Liquid-Liquid SeparatorsRating: 3.5 out of 5 stars3.5/5 (3)
- Chemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastFrom EverandChemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastNo ratings yet
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- An Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksFrom EverandAn Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksRating: 5 out of 5 stars5/5 (1)
- The Periodic Table: A Very Short IntroductionFrom EverandThe Periodic Table: A Very Short IntroductionRating: 4.5 out of 5 stars4.5/5 (3)
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeFrom EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeRating: 5 out of 5 stars5/5 (1)
- Trevor Kletz Compendium: His Process Safety Wisdom Updated for a New GenerationFrom EverandTrevor Kletz Compendium: His Process Safety Wisdom Updated for a New GenerationNo ratings yet
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (14)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction in the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction in the Science of Everyday LifeRating: 4 out of 5 stars4/5 (9)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)