Professional Documents
Culture Documents
A Single-Event Microkinetic Model For Xylene Isomerisation and Ethylbenzene Dealkylation
Uploaded by
Aor PimpornOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
A Single-Event Microkinetic Model For Xylene Isomerisation and Ethylbenzene Dealkylation
Uploaded by
Aor PimpornCopyright:
Available Formats
A single-event microkinetic model for xylene isomerisation and
ethylbenzene dealkylation
K. Toch, J. W. Thybaut, B. Vandegehuchte, G. B. Marin
Ghent University, Laboratory for Chemical Technology, Krijgslaan 281-S5, 9000 Ghent,
Belgium; tel. +32 9 264 55 63, e-mail: Joris.Thybaut@UGent.be
Xylenes are very important petrochemical products that are encountered in the
production of phtalic acid anhydride (from o-xylene) and terephtalic acid (from p-xylene) [1].
Especially the latter component is of high industrial relevance since it is an intermediate in the
production of polyethylene terephtalate (PET). Because the amounts in which the three xylene
isomers are obtained from, e.g., catalytic reforming, thermal cracking, cokes oven effluent
gases,... are not matching with the market demand, processes have been developed to convert
the less desired isomer, i.e., m-xylene, into the more commercially interesting o-xylene and,
mainly, p-xylene. Inevitably, also some ethylbenzene is contained in the xylenes mixture and,
hence, ethylbenzene dealkylation occurs during the xylene isomerisation.
As part of this work a fundamental Single-Event MicroKinetic (SEMK) model for xylene
isomerisation and ethylbenzene dealkylation has been constructed. It accounts for all
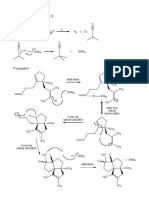
elementary steps occurring in the reaction network, see Figure 1, i.e., physisorption of the
reactants into the zeolite pores, 78 (de)-protonation, 24 1-2 methyl shift, 16 exocyclic β-
scission, and 113 transalkylation reactions at the acid sites and 18 aromatic hydrogenation on
the metal sites between 18 aromatic components, 18 cycloalkanes, 78 aromatic carbenium
ions and 1 alkane. The corresponding reaction network has been generated using an
automated reaction network generation program.
The model has been tested against an experimental data set on a Pt/H-ZSM5 zeolite. The
temperature was varied between 350 to 400°C, the total pressure ranged from 0.4 to 1.2 MPa,
and the hydrogen to hydrocarbon ratio was between 1 and 5. One activation energy per
reaction family was obtained by regression for the 1-2 methyl shift, exocyclic β-scission,
CH3 CH3
CH3 H3C
CH3
CH3 CH3
Physisorption
Physisorption Zeolite
CH3
CH3
Metal sites CH3 Acid sites
CH3 Methylshift
CH3
+
CH3 CH3 (de-)Protonation
CH3
Chemisorption (de-)Protonation CH3
(de-)Hydrogenation
+
CH3
CH3 CH3 CH3
CH3 CH3
CH3
CH3
+
CH3
Chemisorption H3C CH3 CH3
+
+
CH3
Transalkylation Dealkylation
CH3 (de-)Protonation
CH3
H3C CH3
Physisorption
CH3 Physisorption
CH3 CH3
CH3 H3C CH3 C 2H 6
Figure 1: Reaction network considered in xylene isomerisation and ethylbenzene
dealkylation
Table 1: Assumed variation in degrees of freedom and estimated activation
energies/reaction enthalpies for the considered reaction families
variation in degrees Activation
of freedom Energy/Reaction
Enthalpy (kJ mol-1)
protonation loss of all -136.30 ± 0.27
translational freedom
methyl shift no changes 114.03 ± 0.62
β-scission gain of 1 translational 173.60 ± 0.12
degree of freedom
transalkylat loss of 1 translational 133.27 ± 0.37
ion degree of freedom
hydrogenat loss of 1 translational 74.57 ± 0.45
ion degree of freedom
Figure 2: Parity diagrams for ethylbenzene conversion (left) and xylene losses
transalkylation and hydrogenation reactions, while quasi equilibrium was assumed for
(de)-protonation leading to a reaction enthalpy to be estimated. The corresponding
preexponential factors were obtained by considering the changes involved in degrees of
freedom between the reactant(s) and the species in the transition state or the reaction product,
see Table 1. The parameter values related to physisorption in the zeolite pores and chemical
adsorption on the Pt sites were taken from the literature [2-3].
Figure 2 illustrates the model’s adequacy in reproducing the main experimental
responses, i.e., the ethylbenzene conversion, mainly by dealkylation (exocyclic β-scission)
into benzene and ethane, and the xylene losses by disproportionation (transalkylation)
reactions. The model parameters are in line with literature reported values [4]: from the acid
catalyzed reactions, methyl shifts have the lowest activation energy while exocyclic β-scission
has the highest. The protonation enthalpy obtained indicates a strong chemisorption of the
aromatic reactants on the acid sites compared to that of alkenes on a reference USY zeolite.
In this work, the SEMK methodology has been applied for the first time to xylene
isomerisation and ethylbenzene dealkylation. Only minor modifications related to the new
reaction families encountered needed to be accounted for the available reaction network
generation and regression tools to come to an adequate simulation of the main and the minor
responses. It illustrates the versatility of the method in the assessment of catalytic kinetics.
References
[1] Chen, N.Y., Ind. Eng. Chem. Res., 2001. 40(20) 4157.
[2] Thybaut, J.W., M. Saeys, and G.B. Marin, Chem. Eng. J., 2002. 90(1-2) 117.
[3] Reshetnikov, S. I., S. B. Ilyin, A.A. Ivanov, and A. S. Kharitonov, React. Kinet.
Catal. Lett., 2004. 83(1) 157.
[4] Thybaut, J. W., G. B. Marin, G. V. Baron, P. A. Jacobs, and J. A. Martens, J. Catal.,
2001. 202(2) 324.
You might also like
- Neutral PH Aqueous Redox Flow Battery Materials - FF078EDocument1 pageNeutral PH Aqueous Redox Flow Battery Materials - FF078EMonika Bartocha WróblewskaNo ratings yet
- Name ReactionDocument7 pagesName ReactionSoumya KhatriNo ratings yet
- IUPAC Nomenclature 1Document2 pagesIUPAC Nomenclature 1samarthNo ratings yet
- Kuliah-1 NMRDocument26 pagesKuliah-1 NMRTitah Aldila BudiastantiNo ratings yet
- FitoquimiaDocument2 pagesFitoquimialuisgerardo000000No ratings yet
- Trabajo de QuimicaDocument2 pagesTrabajo de QuimicaNicolas MartinezNo ratings yet
- TPDocument39 pagesTPARAMAYO JuanNo ratings yet
- Estructura de Compuestos Biologicos-Parte 2: F.-EsteroidesDocument7 pagesEstructura de Compuestos Biologicos-Parte 2: F.-EsteroidesffhkeNo ratings yet
- Practice Problems On Alkane Nomenclature: CH CHDocument2 pagesPractice Problems On Alkane Nomenclature: CH CHRishav Sasmal100% (1)
- S20 Ac Quim 10 Viellard EstebanDocument1 pageS20 Ac Quim 10 Viellard Estebanesteban viellardNo ratings yet
- Parte 4Document1 pageParte 4Esteban Mauricio Viellard CorralesNo ratings yet
- JoDocument1 pageJoJoana Marie BeltranNo ratings yet
- DM pp61-80Document20 pagesDM pp61-80MLUNGISI MkhwanaziNo ratings yet
- Problem Set 3, Question 2 InitiationDocument1 pageProblem Set 3, Question 2 InitiationRudra Shankha NandyNo ratings yet
- Trabajo Quimica Superior NAVIDADDocument13 pagesTrabajo Quimica Superior NAVIDADSebastian GuerraNo ratings yet
- Mixed Opioid TRIsDocument3 pagesMixed Opioid TRIsdissentionNo ratings yet
- Yellow-Green All Green Plants Except Bacteria Blue-Green All Higher Plants and Green Algae Green Diatoms Green Red Algae Pale Blue BacteriaDocument19 pagesYellow-Green All Green Plants Except Bacteria Blue-Green All Higher Plants and Green Algae Green Diatoms Green Red Algae Pale Blue BacteriaAnonymous jdC36sKP57No ratings yet
- How To Find No of Structural Isomers by S.K.sinha See Chemistry Animations at HTTP://WWW - Openchemistry.inDocument2 pagesHow To Find No of Structural Isomers by S.K.sinha See Chemistry Animations at HTTP://WWW - Openchemistry.inmyiitchemistry81% (16)
- IUPAC Name 18th Sep 15Document8 pagesIUPAC Name 18th Sep 15samarthNo ratings yet
- Basics of Photochemistry and Norrish Type I Reaction: Presented By: Dr. Nidhi VashisthaDocument12 pagesBasics of Photochemistry and Norrish Type I Reaction: Presented By: Dr. Nidhi Vashisthanidhi vashisthaNo ratings yet
- Taller Quimica 6Document1 pageTaller Quimica 6Paula Andrea Saenz HernandezNo ratings yet
- Electromatic Aromatic SubstitutionDocument5 pagesElectromatic Aromatic SubstitutionSagar paulNo ratings yet
- 10 Organic NomenclatureDocument45 pages10 Organic NomenclatureAnubhab100% (2)
- General Organic Chemistry: Solution To Subjective ProblemsDocument6 pagesGeneral Organic Chemistry: Solution To Subjective ProblemsMD IMRANNo ratings yet
- DR Rafiq Zakaria Campus, Maulana Azad College, DR Ahmad Zaheer Lecture SeriesDocument97 pagesDR Rafiq Zakaria Campus, Maulana Azad College, DR Ahmad Zaheer Lecture Seriesmohsin abdul azizNo ratings yet
- Practical Ken e SansDocument6 pagesPractical Ken e SansJoe JulianNo ratings yet
- 32 Methods For Ring ContractionDocument11 pages32 Methods For Ring ContractionNisargaNo ratings yet
- HPLCDocument20 pagesHPLCNugroho HartonoNo ratings yet
- Quaternary Ammonium Salts PDFDocument11 pagesQuaternary Ammonium Salts PDFBridgett Lanette RobinsonNo ratings yet
- Ketone Retrosynthesis SECTION 1Document1 pageKetone Retrosynthesis SECTION 1Ana-Marija BartolincicNo ratings yet
- s19 Ac Quim 10 Viellard Esteban 2Document1 pages19 Ac Quim 10 Viellard Esteban 2Esteban Mauricio Viellard CorralesNo ratings yet
- HC y AlcoholesDocument2 pagesHC y AlcoholesGonzalo HernandezNo ratings yet
- Ejercicios de Nomenclatura de Alcoholes.Document3 pagesEjercicios de Nomenclatura de Alcoholes.Diego Fernando Ardila ArizaNo ratings yet
- Estructuras Quimicas OrganicasDocument2 pagesEstructuras Quimicas OrganicasCamil SolerNo ratings yet
- Triple ReuptakeDocument1 pageTriple ReuptakedissentionNo ratings yet
- Hydrocarbons Pyqs SolnsDocument12 pagesHydrocarbons Pyqs Solnssaadvik1121No ratings yet
- 51LC S13 Elimination Background PDFDocument4 pages51LC S13 Elimination Background PDFButterlesstoastNo ratings yet
- Ment 2023Document3 pagesMent 2023Rayhan ShaikhNo ratings yet
- Draw The Following Alkanes.: Hydrocarbon Nomenclature WorksheetDocument3 pagesDraw The Following Alkanes.: Hydrocarbon Nomenclature WorksheetCaseelyn Joy NantizaNo ratings yet
- Hidrocarburos MIX-01Document1 pageHidrocarburos MIX-01Abraham CastañedaNo ratings yet
- CO2 - 1S2 - 2011 - LSLL - CopieDocument5 pagesCO2 - 1S2 - 2011 - LSLL - CopiePFENo ratings yet
- 3 Naming Alkenes WsDocument2 pages3 Naming Alkenes WsJaya Chitra Degala Ramalu100% (1)
- The Fundamentals of Advanced Materials: A Novel Triazole Resin For Low Temperature-CureDocument59 pagesThe Fundamentals of Advanced Materials: A Novel Triazole Resin For Low Temperature-Curegormanirene84No ratings yet
- E2 MechanismDocument2 pagesE2 MechanismPasipanodya MuzendaNo ratings yet
- 0910 4 AbsDocument9 pages0910 4 AbsEngr Muhammad AqibNo ratings yet
- 0910 4 Abs PDFDocument9 pages0910 4 Abs PDFLAURA LUC�A ATENCIA CASTILLONo ratings yet
- 12 U Orgo - 1 - Hydrocarbon Nomenclature WorksheetDocument4 pages12 U Orgo - 1 - Hydrocarbon Nomenclature WorksheetZia Rathore100% (1)
- OrganicChemistryChapter7 PDFDocument30 pagesOrganicChemistryChapter7 PDFSeanne CruzNo ratings yet
- Samhitha Bandi UIN 132000378: Exam 2 - Extra Credit AssignmentDocument2 pagesSamhitha Bandi UIN 132000378: Exam 2 - Extra Credit AssignmentSamhitha BandiNo ratings yet
- Estructuras OrganicasDocument1 pageEstructuras OrganicasJose CNo ratings yet
- Ejercitario Notacion y NomenclaturaDocument2 pagesEjercitario Notacion y Nomenclaturaelianhearmoa23No ratings yet
- đọc tênDocument7 pagesđọc tênthái đức thắngNo ratings yet
- Drug Abuse Ag8114en MKDocument56 pagesDrug Abuse Ag8114en MKA VegaNo ratings yet
- Chemistry-JEE Adv Previous Year Paper P1 (Code-9) 2017 EzyexamsolutionDocument15 pagesChemistry-JEE Adv Previous Year Paper P1 (Code-9) 2017 EzyexamsolutionSagar MalhotraNo ratings yet
- CPP - HydrocarbonDocument13 pagesCPP - HydrocarbondivyanshjoshidpsjkpNo ratings yet
- 3 Naming Alkynes Ws KeyDocument2 pages3 Naming Alkynes Ws KeyJaya Chitra Degala RamaluNo ratings yet
- EstructurasDocument6 pagesEstructurasMORENO MANZANO ISRAELNo ratings yet
- Naming Hydrocarbons Worksheet and Key: Write The Name of Each of The Hydrocarbon Molecules Shown Below: 1) 8)Document2 pagesNaming Hydrocarbons Worksheet and Key: Write The Name of Each of The Hydrocarbon Molecules Shown Below: 1) 8)Jafrinta Irma Ruta AstariNo ratings yet
- Naming Hydrocarbons Worksheet and Key: Write The Name of Each of The Hydrocarbon Molecules Shown Below: 1) 8)Document2 pagesNaming Hydrocarbons Worksheet and Key: Write The Name of Each of The Hydrocarbon Molecules Shown Below: 1) 8)Aaron TehNo ratings yet
- Licensed Toxic Waste Collectors in SingaporeDocument27 pagesLicensed Toxic Waste Collectors in Singaporecolin_m_chanNo ratings yet
- 1201 GlyptalDocument11 pages1201 GlyptalRonald CatacoraNo ratings yet
- Sro 565-2006Document44 pagesSro 565-2006Abdullah Jathol100% (1)
- Hydrocarbon Processing Petrochemical Processes 2001Document144 pagesHydrocarbon Processing Petrochemical Processes 2001Alejandra Arias100% (1)
- Astm - D3230.39961-2019Document7 pagesAstm - D3230.39961-2019CarlosJayaNo ratings yet
- Kingspan IRW Maintenance Manual NADocument9 pagesKingspan IRW Maintenance Manual NAEddie MunsonNo ratings yet
- Catalytic Reforming - 2Document30 pagesCatalytic Reforming - 2Alekhya Bandaru0% (1)
- ExxonMobil EMTAM ProcessDocument2 pagesExxonMobil EMTAM ProcessAMANo ratings yet
- Understanding Solvents Part IDocument21 pagesUnderstanding Solvents Part IKakakiNo ratings yet
- 1310369102.14 Msds TdsDocument5 pages1310369102.14 Msds Tdsheru santosoNo ratings yet
- Setalux 1152 SS 60 - Emea - enDocument1 pageSetalux 1152 SS 60 - Emea - enMOHAMEDNo ratings yet
- TDS 7212 XB 60Document3 pagesTDS 7212 XB 60Shafiq LatifNo ratings yet
- ET574PTA-7.5BG7 and 2 (English)Document10 pagesET574PTA-7.5BG7 and 2 (English)우승환Woo seung hwanNo ratings yet
- Petrochemical IndustryDocument12 pagesPetrochemical IndustryamirlngNo ratings yet
- BTXDocument18 pagesBTXnabilahNo ratings yet
- On The Solvent Stress-Cracking of PolycarbonateDocument10 pagesOn The Solvent Stress-Cracking of PolycarbonateAnonymous 3aS2d8VcwZNo ratings yet
- Clearing EmbeddingDocument16 pagesClearing EmbeddingAnna Charisa HernandezNo ratings yet
- Table of Chemicals Vs Gloves (Furfural)Document8 pagesTable of Chemicals Vs Gloves (Furfural)Fouad MilanoNo ratings yet
- Metal Supported Zeolite For Heavy Aromatics Transalkylation ProcessDocument10 pagesMetal Supported Zeolite For Heavy Aromatics Transalkylation ProcessArash AbbasiNo ratings yet
- Toluene Disproportionation Reaction CatalystDocument14 pagesToluene Disproportionation Reaction CatalystVăn Đại - BKHNNo ratings yet
- FUELS IdentificationDocument34 pagesFUELS IdentificationHomero PilataNo ratings yet
- Domacryl 507 50 X/Bac: Hydroxy Acrylic ResinDocument1 pageDomacryl 507 50 X/Bac: Hydroxy Acrylic ResinmonrmNo ratings yet
- Natural Gas To BTXDocument505 pagesNatural Gas To BTXFrank Pocomucha GallardoNo ratings yet
- MX SorbexDocument2 pagesMX SorbexgshdavidNo ratings yet
- New Haven Air Toxics InventoryDocument108 pagesNew Haven Air Toxics InventoryNHUDLNo ratings yet
- Catalysts Licensing One Pager EnpdfDocument2 pagesCatalysts Licensing One Pager Enpdfprasad336No ratings yet
- HAZID TemplateDocument38 pagesHAZID TemplateMd AfzanNo ratings yet
- PolyGloss Sublimation Coating For All Hard Substrates (Glossy Finish) - DyePress Graphic SupplyDocument10 pagesPolyGloss Sublimation Coating For All Hard Substrates (Glossy Finish) - DyePress Graphic SupplyAkifa NoorNo ratings yet
- Uny MarineDocument10 pagesUny Marinemata90001No ratings yet
- Xylenes PubDocument27 pagesXylenes PubGurunath EpiliNo ratings yet