Professional Documents
Culture Documents
Solution in One Page
Solution in One Page
Uploaded by
Ss0 ratings0% found this document useful (0 votes)
6 views2 pagesOriginal Title
solution-in-one-page.doc
Copyright
© © All Rights Reserved
Available Formats
DOC, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views2 pagesSolution in One Page
Solution in One Page
Uploaded by
SsCopyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
You are on page 1of 2
Solutions
Gas in solid Solution of hydrogen in palladium
Solid Solutions
Liquid in solid Amalgam of mercury with sodium
Mass %, ppm, mole fraction and molality are independent of temperature, whereas molarity
Temp. Vs Conc.
depends on temperature. This is because volume depends on temperature.
At a constant temperature, the solubility of a gas in a liquid is directly proportional to the pressure
Henry’s law. of the gas. p = KH . x
KH = Henry’s law constant ( greater the K H value means lower the solubility.)
Application of 1.To increase the solubility of CO2 in soft drinks, the bottle is sealed under high pressure.
Henry’s law. 2. To avoid bends, the tanks used by scuba divers are filled with air diluted with helium
Temp and Solubilty Solubility of gas increases with decrease of temperature. It is due to this reason that aquatic species
of gas are more comfortable in cold waters rather than in warm waters.
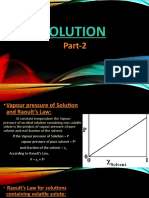
Raoult’s law for The partial vapour pressure of each component in the solution
volatile liquids is directly proportional to its mole fraction p 1 α x1 p 1 = p 10 x 1
The solutions which obey Raoult’s law over the entire range of concentration are known as ideal
Ideal Solutions solutions. ( For ideal solution ΔmixH = 0, ΔmixV = 0)
Example : Solution of n-hexane and n-heptane,
Positive deviation : A-B interactions are weaker than those between A-A or B-B,
Example - Mixtures of ethanol and acetone
Non-ideal Solutions
Negative deviations : Forces between A-A and B-B are weaker than those between A-B
Example- mixture of phenol + aniline. a mixture of chloroform +acetone
Mixtures have same composition in liquid and vapour phase and boil at a constant temp.
Azeotropes minimum boiling azeotrope(positive deviation) eg- 95% aq ethanol
maximum boiling azeotrope(negative deviation) eg- 68% aq nitric acid
Colligative Depend on the number of solute particles not upon their nature.
properties
Relative Lowering Roult’s Law: Relative lowering of vapour pressure is equal to the mole fraction of the solute
of
Vapour Pressure
Elevation of Boiling
point Kb is called Boiling Point Elevation Constant or Molal Elevation Constant (Ebullioscopic
Constant). The unit of is K kg /mol.
Kf, which depends on the nature of the solvent is
Depression of
known as Freezing Point Depression Constant or
Freezing Point
Molal Depression Constant or Cryoscopic
Constant. The unit of Kf is K kg/mol
Osmosis Solvent flows through the semi permeable membrane from pure solvent to the solution.
The extra pressure applied on the solution that just stops the flow of solvent is called osmotic
Osmotic pressure
pressure of the solution
Osmotic pressure(π)
Isotonic solutions Two solutions having same osmotic pressure
Hypertonic Higher osmotic pressure than a particular soln
Hypotonic Lower osmotic pressure than a particular soln
The direction of osmosis can be reversed if a pressure larger than the osmotic pressure is applied to
Reverse Osmosis the solution side. That is, now the pure solvent flows out of the solution
Application : Desalination of sea water
van’t Hoff factor ratio of normal molar mass to experimentally determined molar mass or as the ratio of observed
(i) colligative property to the calculated colligative property.
Value of van’t Hoff NaCl, KCl = 2 ; BaCl2 CaCl2 = 3 ; Na3PO4 = 4 ; Al2(SO4)3 , K4[Fe(CN)6] = 5
factor(i) CH3COOH ( in benzene) = 1/2
You might also like
- Advanced Pharmaceutical analysisFrom EverandAdvanced Pharmaceutical analysisRating: 4.5 out of 5 stars4.5/5 (2)
- Creep of Outlet Pigtail Tubes of Steam Reformer and Grain Size Effect On Creep of An Incoloy 800H MaterialDocument12 pagesCreep of Outlet Pigtail Tubes of Steam Reformer and Grain Size Effect On Creep of An Incoloy 800H MaterialOwais Malik100% (1)
- Solution - in - One - Page (1) .Doc - 20240222 - 235427 - 0000Document4 pagesSolution - in - One - Page (1) .Doc - 20240222 - 235427 - 0000Naaz ChoudharyNo ratings yet
- Solution in One PageDocument2 pagesSolution in One Pageraiprisha06No ratings yet
- Learning Material (CHEMISTRY)Document141 pagesLearning Material (CHEMISTRY)aayanNo ratings yet
- Solution Revision FINAL-1Document15 pagesSolution Revision FINAL-1Madhavilatha LoganathanNo ratings yet
- Lecture - 5 - Module 1Document17 pagesLecture - 5 - Module 1Zaira Eliz GonzalesNo ratings yet
- Module 1 Lecture 5Document17 pagesModule 1 Lecture 5Amirs AmjadNo ratings yet
- 2001 SolutionDocument12 pages2001 SolutionSaghar FaridNo ratings yet
- Meeting 6-7Document30 pagesMeeting 6-7Aldo FirmansyahNo ratings yet
- Chapter-02 SolutionDocument13 pagesChapter-02 Solutionshrey4602No ratings yet
- Solutions NCERTDocument16 pagesSolutions NCERTPrecisive OneNo ratings yet
- As NotesDocument95 pagesAs NotesJude JosephNo ratings yet
- Solution of Non-ElectrolytesDocument133 pagesSolution of Non-Electrolytesneha_dand1591No ratings yet
- Colligative PropertiesDocument41 pagesColligative PropertiesJoshua SagunNo ratings yet
- Wa0245 1Document45 pagesWa0245 1lm7032478No ratings yet
- Prop of SolnDocument54 pagesProp of SolnRodayna HosniNo ratings yet
- Chemistry: SolutionDocument68 pagesChemistry: SolutionSatyajit RoutNo ratings yet
- Colligative Properties: Vapour Pressure Boiling Point Freezing Point Osmotic PressureDocument27 pagesColligative Properties: Vapour Pressure Boiling Point Freezing Point Osmotic PressureRSNo ratings yet
- 2-Excellent Chemistry Assignment SolutionsDocument5 pages2-Excellent Chemistry Assignment SolutionsSachin B SNo ratings yet
- Colligative Properties: Vapour Pressure Boiling Point Freezing Point Osmotic PressureDocument27 pagesColligative Properties: Vapour Pressure Boiling Point Freezing Point Osmotic PressureNaman GuptaNo ratings yet
- Solutions Class 12Document12 pagesSolutions Class 12Åmìßhã PŕãťãpNo ratings yet
- Colligative PropertyDocument26 pagesColligative PropertyKshama SharmaNo ratings yet
- Learning Materials of Chemistry For Board ExamDocument80 pagesLearning Materials of Chemistry For Board Examnwork0274No ratings yet
- Revision Book 1 Chemistry 2024 ExamsDocument19 pagesRevision Book 1 Chemistry 2024 ExamsKonicNo ratings yet
- Colligative Properties and Determination of Molar MassesDocument7 pagesColligative Properties and Determination of Molar MassesRoshini FelixNo ratings yet
- Minimum Learning Material CBSE ChemistryDocument50 pagesMinimum Learning Material CBSE ChemistryAntur RakhaNo ratings yet
- Key Concepts: TotalDocument18 pagesKey Concepts: TotalSachin Kumar67% (3)
- SolutionsDocument9 pagesSolutionsKota Venkata SukumarNo ratings yet
- Principal Sir (Notes Solution)Document4 pagesPrincipal Sir (Notes Solution)Shourya PastorNo ratings yet
- Mod 3.2 Colligative, Tonicity and Mod 4 Solubility (PDF - Io)Document62 pagesMod 3.2 Colligative, Tonicity and Mod 4 Solubility (PDF - Io)Charles DapitoNo ratings yet
- ColligativeDocument24 pagesColligativeHarry WinstonNo ratings yet
- Ways of Expressing Concentrations of SolutionsDocument57 pagesWays of Expressing Concentrations of SolutionsKearly Joy VictorioNo ratings yet
- X-Ray Diffraction Document (1) (4) - 1Document9 pagesX-Ray Diffraction Document (1) (4) - 1MEEZAN TVNo ratings yet
- Solutions Notes PDFDocument21 pagesSolutions Notes PDFSMELLY CATNo ratings yet
- Arindam Das Bs-15 253 B.SC Chemistry Honors Supervised By:-Dr. S MuniDocument25 pagesArindam Das Bs-15 253 B.SC Chemistry Honors Supervised By:-Dr. S MuniArindam DasNo ratings yet
- SolutionsDocument24 pagesSolutionsSRILAKSHMI K sNo ratings yet
- Chemistry PPT SolutionDocument28 pagesChemistry PPT Solutionnaukul rajNo ratings yet
- Colligative Properties: Nathaniel P. DugosDocument32 pagesColligative Properties: Nathaniel P. DugossololexzibNo ratings yet
- 02 - Colligative PropertiesDocument46 pages02 - Colligative PropertiesamirNo ratings yet
- เอกสารประกอบรายวิชา pc205 ครั้งที่ 5 solutionDocument57 pagesเอกสารประกอบรายวิชา pc205 ครั้งที่ 5 solutionVeliyana Londong AlloNo ratings yet
- Colligative PropertiesDocument28 pagesColligative PropertiesSachin S RaneNo ratings yet
- Random Notes Class 12 ChemistryDocument87 pagesRandom Notes Class 12 Chemistryankitajamatia06No ratings yet
- Solution NotesDocument17 pagesSolution NotesBabli KumariNo ratings yet
- Power Grid Campus Biharsharif Nalanda: D.A.V Public SchoolDocument28 pagesPower Grid Campus Biharsharif Nalanda: D.A.V Public SchoolAnindya BhattacharyaNo ratings yet
- 2 Solutions 97cDocument66 pages2 Solutions 97cRubiNo ratings yet
- Changes in Vapour Pressure. (Vapour Pressure Lowering) : V - P Depends Only On The SolventDocument5 pagesChanges in Vapour Pressure. (Vapour Pressure Lowering) : V - P Depends Only On The SolventMarthy DayagNo ratings yet
- Solution (Part 2)Document24 pagesSolution (Part 2)saptarshi senNo ratings yet
- Chemistry Q2Document3 pagesChemistry Q2Kristel Anne Magaru-Macauggal-LugoNo ratings yet
- Chapter10 - Properties of SolutionsDocument48 pagesChapter10 - Properties of SolutionsXiaohan TangNo ratings yet
- MolarityDocument7 pagesMolarityMacxieNo ratings yet
- Unit 2 Solutions: Points To RememberDocument15 pagesUnit 2 Solutions: Points To RememberHarpreet kaurNo ratings yet
- Properties of Matter and SolutionDocument16 pagesProperties of Matter and SolutionSurya PrakashNo ratings yet
- 12 Chemistry Solutions Notes VLDocument46 pages12 Chemistry Solutions Notes VLPiyushNo ratings yet
- Final PDF For Chemistry Class XiiDocument172 pagesFinal PDF For Chemistry Class XiiSANJAY PARMARNo ratings yet
- Solution ChemistryDocument27 pagesSolution ChemistryZarahbeth Claire G. ArcederaNo ratings yet
- Liquid SolutionDocument16 pagesLiquid SolutionRaju SinghNo ratings yet
- 12 Chemistry Keypoints Revision Questions Chapter 2Document15 pages12 Chemistry Keypoints Revision Questions Chapter 2aesthetic rushNo ratings yet
- Differential EquationsDocument2 pagesDifferential EquationsSsNo ratings yet
- Integration and Application: X X DXDocument3 pagesIntegration and Application: X X DXSsNo ratings yet
- Krishna Dhan Sharma, PGT (Physics), K.V.BagafaDocument10 pagesKrishna Dhan Sharma, PGT (Physics), K.V.BagafaSsNo ratings yet
- StickercamerajsoncacheDocument17 pagesStickercamerajsoncacheSsNo ratings yet
- 5 Unit I ElectrostaticsDocument11 pages5 Unit I ElectrostaticsSsNo ratings yet
- n1 V - n2 U n2 N 1 R: 5 Marks Questions Physics Class XiiDocument4 pagesn1 V - n2 U n2 N 1 R: 5 Marks Questions Physics Class XiiSsNo ratings yet
- ElectrochemistryDocument10 pagesElectrochemistrySsNo ratings yet
- Answers / Solutions / Key KEY For Worksheet-1 (Ray Optics)Document5 pagesAnswers / Solutions / Key KEY For Worksheet-1 (Ray Optics)SsNo ratings yet
- Formulae at A Glance: Unit-Iv-Electromagnetic Induction and A.CDocument13 pagesFormulae at A Glance: Unit-Iv-Electromagnetic Induction and A.CSsNo ratings yet
- Electrochemistry: R Cell RDocument15 pagesElectrochemistry: R Cell RSsNo ratings yet
- Unit Ix: Electronic Devices Formulae of This UnitDocument10 pagesUnit Ix: Electronic Devices Formulae of This UnitSsNo ratings yet
- PC Ray Group Value Based Questions With Answers Chemical KineticsDocument2 pagesPC Ray Group Value Based Questions With Answers Chemical KineticsSsNo ratings yet
- CBSE Board Paper Solution-2020Document51 pagesCBSE Board Paper Solution-2020SsNo ratings yet
- Renu Verma PDFDocument12 pagesRenu Verma PDFSsNo ratings yet
- SolutionDocument3 pagesSolutionSsNo ratings yet
- Sandmayers ReactionDocument6 pagesSandmayers ReactionSsNo ratings yet
- Bharti Public School: Assignment-Maths (Xii) DerivativesDocument1 pageBharti Public School: Assignment-Maths (Xii) DerivativesSsNo ratings yet
- Assignment Section-A (ONE MARK EACH)Document3 pagesAssignment Section-A (ONE MARK EACH)SsNo ratings yet
- Electricity: Presented by Kajol Kiran Srichandan Class 10 THDocument27 pagesElectricity: Presented by Kajol Kiran Srichandan Class 10 THSsNo ratings yet
- Bharti Public School Class-XII (Maths) Assignment-Inverse Trigonometric FunctionsDocument1 pageBharti Public School Class-XII (Maths) Assignment-Inverse Trigonometric FunctionsSsNo ratings yet
- E: 2 ?e - FO ( 'C4t1: +D - T-JL.-DDocument2 pagesE: 2 ?e - FO ( 'C4t1: +D - T-JL.-DSsNo ratings yet
- F + H O HF + O: # 2naoh (Cold and Dilute) + CL Nacl +naocl +H O # 6naoh (Hot and Conc.) + 3Cl 5nacl +naclo +H ODocument4 pagesF + H O HF + O: # 2naoh (Cold and Dilute) + CL Nacl +naocl +H O # 6naoh (Hot and Conc.) + 3Cl 5nacl +naclo +H OSsNo ratings yet
- Group-2 Value Based Questions Subject: Chemistry Class XIIDocument6 pagesGroup-2 Value Based Questions Subject: Chemistry Class XIISsNo ratings yet
- Kendriya Vidyalaya Nmo-3, BBSR: by A.K.PANDADocument2 pagesKendriya Vidyalaya Nmo-3, BBSR: by A.K.PANDASs100% (3)
- 1 Mark QuestionsDocument19 pages1 Mark QuestionsSsNo ratings yet
- Aldehyde Ketone and AcidDocument15 pagesAldehyde Ketone and AcidSsNo ratings yet
- Cl-Xii - Chem: Brush Up With Some Organic Concepts - (Organic - MLL) MM - 10, Time Allowed - 15 Minutes Name - Roll No - Marks ScoredDocument7 pagesCl-Xii - Chem: Brush Up With Some Organic Concepts - (Organic - MLL) MM - 10, Time Allowed - 15 Minutes Name - Roll No - Marks ScoredSsNo ratings yet
- Answers To 2.8 Exercises 2.8 Exercise 1: CL CL Cl. CLDocument3 pagesAnswers To 2.8 Exercises 2.8 Exercise 1: CL CL Cl. CLSsNo ratings yet
- Selected Questions of Chapter Aldehyde K Solved Sample Papers For Class 12 ChemistryDocument33 pagesSelected Questions of Chapter Aldehyde K Solved Sample Papers For Class 12 ChemistrySsNo ratings yet
- 2M8 U0-V-U0: Technical Data Sheet Conveyor and Process BeltsDocument2 pages2M8 U0-V-U0: Technical Data Sheet Conveyor and Process Beltsnavneet jainNo ratings yet
- (EN) Tribol 1100Document2 pages(EN) Tribol 1100gerardoctavaraNo ratings yet
- Analysis of Composite Box GirdersDocument169 pagesAnalysis of Composite Box Girderspmc_lbm4440No ratings yet
- Basic Chemistry ReviewDocument5 pagesBasic Chemistry ReviewArman Neil J. BudogNo ratings yet
- Intro-EMH 211 - Thermodynamics - 2019 PDFDocument20 pagesIntro-EMH 211 - Thermodynamics - 2019 PDFMuhammad Adib HaikalNo ratings yet
- Tut 04Document2 pagesTut 04Ebert AroneNo ratings yet
- Concrete Technology Unit 3Document33 pagesConcrete Technology Unit 3sainathNo ratings yet
- Materials System SpecificationDocument6 pagesMaterials System SpecificationAwais CheemaNo ratings yet
- Filter Cloth CatalogueDocument8 pagesFilter Cloth CatalogueChandra Sekar RNo ratings yet
- Rheological Modelling of Complex Fluids. I. The Concept of Effective Volume Fraction RevisitedDocument9 pagesRheological Modelling of Complex Fluids. I. The Concept of Effective Volume Fraction RevisitedodexNo ratings yet
- Portal Frame Knee Connection Report SampleDocument5 pagesPortal Frame Knee Connection Report SampleozbuildNo ratings yet
- Caryophyllene: Woody, Spicy, Earthy, DryDocument1 pageCaryophyllene: Woody, Spicy, Earthy, DryFauzan FebriyantoNo ratings yet
- ASTM B 75 2011 - STD Spec For Seamless Copper TubeDocument8 pagesASTM B 75 2011 - STD Spec For Seamless Copper TubenVent QualityNo ratings yet
- EN - PSX 60 Part A.SDS - SGS PDFDocument16 pagesEN - PSX 60 Part A.SDS - SGS PDFnaspauzanNo ratings yet
- TED (21) - 5121 Revision 2021 Model Question Paper Adhesive TechnologyDocument3 pagesTED (21) - 5121 Revision 2021 Model Question Paper Adhesive TechnologySam JhonsonNo ratings yet
- Electrical ConductivityDocument19 pagesElectrical ConductivityHARISH KNo ratings yet
- Mts Extensometer CatalogDocument44 pagesMts Extensometer CatalogSWAPNIL PATILNo ratings yet
- Performance Evaluation of An Oil Fired Boiler A Case Study in Dairy Industry.Document8 pagesPerformance Evaluation of An Oil Fired Boiler A Case Study in Dairy Industry.atul100% (8)
- SalinityDocument8 pagesSalinityKarl GustavNo ratings yet
- Inadvertent Mixing of Incompatible Chemicals IncidentDocument10 pagesInadvertent Mixing of Incompatible Chemicals IncidentAkmal AriffinNo ratings yet
- C708-08 Standard Specification For Nuclear-Grade Beryllium Oxide PowderDocument4 pagesC708-08 Standard Specification For Nuclear-Grade Beryllium Oxide PowderAlabbas FadhelNo ratings yet
- Metamorphism and MetastomatismDocument10 pagesMetamorphism and Metastomatismbilal ahmadNo ratings yet
- Irc 112Document24 pagesIrc 112Bharath Reddy ChinthiReddy100% (1)
- What Is The Best Book On Semiconductor Devices - QuoraDocument4 pagesWhat Is The Best Book On Semiconductor Devices - QuoraTapasRoutNo ratings yet
- Back To Basics: An Introduction To Metal Recycling: Aluminum CansDocument3 pagesBack To Basics: An Introduction To Metal Recycling: Aluminum CansRakesh Ranjan MishraNo ratings yet
- Solutions 06 - Practice Sheet With SolutionDocument3 pagesSolutions 06 - Practice Sheet With SolutionDhruv TonyNo ratings yet
- Design Symbols - Spark PlugsDocument2 pagesDesign Symbols - Spark Plugsmechkarov1100% (1)
- Powder Metallurgy PracticalDocument15 pagesPowder Metallurgy PracticalAzhar Ali100% (1)
- Plaquette Maguin GB Nveau Logo-2Document11 pagesPlaquette Maguin GB Nveau Logo-2Yohannes GebreNo ratings yet