Professional Documents
Culture Documents
Murphy2016 Bronquiectasias-Rx
Uploaded by
carlaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Murphy2016 Bronquiectasias-Rx
Uploaded by
carlaCopyright:
Available Formats
R e s i d e n t s ’ S e c t i o n • R ev i ew
Murphy et al.
Imaging of Cystic Fibrosis and Pediatric Bronchiectasis
Residents’ Section
Review
Downloaded from www.ajronline.org by Queen's Univ on 02/23/16 from IP address 130.15.241.167. Copyright ARRS. For personal use only; all rights reserved
Residents
Imaging of Cystic Fibrosis and
inRadiology
Pediatric Bronchiectasis
Kevin P. Murphy 1,2 Key Points
Michael M. Maher 1,2 1. CT is superior to pulmonary function tests and chest radiography for the assessment and
Owen J. O’Connor 1,2 monitoring of cystic fibrosis (CF)–related lung disease and, also, of pediatric bronchiectasis
not caused by CF (hereafter referred to as non-CF bronchiectasis).
Murphy KP, Maher MM, O’Connor OJ 2. Low-dose CT protocols that impart radiation doses similar to those used in chest radiog-
raphy are feasible for the surveillance of patients with bronchiectasis.
3. Chest radiography is still most commonly used as the first-line imaging examination of
choice for the assessment of acute complications related to bronchiectasis.
4. Pulmonary MRI, with or without the use of inhaled hyperpolarized gas, can be per-
formed to obtain functional information, and, in dedicated centers, it may yield imaging re-
sults comparable to those obtained by CT.
5. Gastrointestinal and pancreaticobiliary manifestations of CF are observed with greater
frequency in adults, because of increased life expectancy.
horacic manifestations of cystic considerably. It is estimated that individuals
T fibrosis (CF) and pediatric bron-
chiectasis not caused by CF
(hereafter known as “non-CF
born since the year 2000 will have a medi-
an life expectancy in excess of 50 years [1].
Non-CF bronchiectasis in childhood occurs
bronchiectasis”) are commonly encountered less commonly in the developed world, with a
by radiologists. In addition, abdominal mani- frequency of approximately 87 cases per mil-
festations (e.g., hepatic steatosis, cirrhosis, lion population and accounting for 9.6% of
intussusception, and meconium ileus) are new referrals to a tertiary pediatric respira-
well-recognized as being associated with CF, tory clinic in one reported series [2, 3].
and with improved survival of patients with
CF, manifestations previously thought to be Pathophysiologic Basis of
very unusual (e.g., development of carcinoma Cystic Fibrosis
or lymphoma) are being encountered with CF arises from an inherited defect in the
Keywords: bronchiectasis, cystic fibrosis,
somewhat increased frequency. Non-CF CF transmembrane conductance regulator
high-resolution CT, low-dose CT, pulmonary MRI bronchiectasis is increasingly diagnosed in (CFTR) protein, which is located on chromo-
patients at a younger age, and these individu- some 7. When defective, this protein leads to
DOI:10.2214/AJR.15.14437 als consequently undergo imaging more of- reduced transport of chloride by the trans-
ten over a longer period. We provide a brief membrane. The δ-F508 deletion is the cause
Received January 25, 2015; accepted after revision
May 20, 2015. review of the imaging approaches and find- of approximately 70% of cases of CF [1, 4,
ings for these patient cohorts. 5]. Affected patients have thickened viscous
1
Department of Radiology, Cork University Hospital, and, sometimes, inspissated secretions at the
Wilton, Cork, Ireland. Address correspondence to Disease Epidemiologic Profile mucosal surfaces, leading to lung destruc-
O. J. O’Connor (oj.oconnor@ucc.ie).
CF is the most common autosomal reces- tion, gastrointestinal malfunction, and exo-
2
Department of Radiology, University College Cork, sive disease among white individuals, with crine insufficiency. CF typically presents in
Cork, Ireland. a frequency of approximately 1 in 2400 live the neonatal period with respiratory compro-
births [1]. Respiratory disease remains the mise caused by thickened secretions or with
AJR 2016; 206:448–454
primary cause of morbidity and mortality, be- bowel obstruction secondary to meconium
0361–803X/16/2063–448 ing responsible for more than 80% of deaths. ileus. Patients with less severe manifesta-
As a result of improved therapies, the life ex- tions may present with recurrent respiratory
© American Roentgen Ray Society pectancy of patients with CF has improved infections in childhood and failure to thrive.
448 AJR:206, March 2016
Imaging of Cystic Fibrosis and Pediatric Bronchiectasis
Non-CF bronchiectasis develops through non-CF bronchiectasis, the predominant find- or tortuous vessels thought to be responsible
a cycle of events known as the Cole vicious ing is cylindric bronchiectasis [13], whereas for bleeding or an active bleeding source are
cycle [6]. It is believed that a respiratory in- cylindric bronchiectasis and cystic bronchi- used in the management of major hemopty-
jury, which most commonly is infection and ectasis are seen in association with CF [1]. In sis (expectoration of 30–300 mL of blood) or
which sometimes occurs in a susceptible CF, bilateral bronchiectasis with disease pre- massive hemoptysis (expectoration of > 300
host, leads to non-CF bronchiectasis through dominant in the upper lobe is routinely noted mL of blood) [20] (Fig. 9). Physicians debate
impaired mucociliary clearance, chronic in- (reportedly, bronchiectasis of the right lung is whether embolization is required for non-
Downloaded from www.ajronline.org by Queen's Univ on 02/23/16 from IP address 130.15.241.167. Copyright ARRS. For personal use only; all rights reserved
fection, exaggerated inflammatory response, slightly worse than that of the left lung, albeit massive hemoptysis, because most cases are
and airways destruction [6]. The principal for unknown reasons); however, other chang- self-limiting. In addition, embolization of an
causes of non-CF bronchiectasis include pre- es do not result in the observation of a simi- actively bleeding vessel or pseudoaneurysm
vious pneumonia, immunodeficiency, aspira- lar gradient [8, 14, 15]. Non-CF bronchiecta- only versus embolization of abnormal ves-
tion, and ciliary dyskinesia, with upward of sis has a variable and often localized pattern sels is also debated [20, 21].
30% of cases remaining idiopathic [7]. Pa- of lung involvement, depending on its cause Increased use of CT for assessment and
tients typically are seen with recurrent low- (Fig. 3). ABPA is classically central. Bronchi- monitoring of CF, combined with an in-
er respiratory chest infections or asthmalike ectasis related to immunodeficiency is typi- creased life expectancy of patients with CF,
wheezing and dyspnea. cally more global, whereas bronchiectasis re- has resulted in increased cumulative radia-
lated to a foreign body is localized. Features tion exposures [22]. Over the past 15 years,
Imaging Strategies of mild bronchiectasis, such as the absence there has been almost a sixfold increase in
CT of bronchial tapering, a bronchoarterial ratio the use of CT for patients with CF [22]. Pa-
The use of CT for the evaluation of CF and greater than 1, or visibility of bronchi within tient age at the time of the first CT exami-
non-CF bronchiectasis has many advantages. 1 cm of the pleural surface, may be the only nation has also decreased, from a mean age
For patients with normal results of pulmo- findings [16]. of 20 years for individuals born before 1980,
nary function tests, CT frequently can depict A number of CF CT scoring systems exist, to a mean of 1.9 years for those born after
many features of CF, the severity of which but the Bhalla and Brody systems are com- 1997 [23]. Dose reduction strategies that are
can also be scored on CT. Disease severity monly used [8, 17]. Most scoring systems use not specific to the imaging of bronchiectasis,
scores determined by CT have been shown the presence, severity, and extent of bron- such as z-axis automated tube current modu-
to be more predictive of future lung disease chiectasis, bronchial wall thickening, mu- lation and iterative reconstruction techniques,
severity than have those determined by spi- cus plugging, consolidation or atelectasis, or are being used, in addition to low-dose proto-
rometry or radiography [8, 9]. This finding hyperinflation to calculate a severity score. cols that have been optimized for use in the
has important implications for patient care. The presence of abscess, air trapping (Fig. 4), follow-up of bronchiectasis. Thoracic CT is
Similarly, compared with CT, radiography pleural effusion, and bullae may also be in- particularly suited for use in dose-optimized
is less sensitive for the diagnosis of allergic cluded [18]. protocols, because of the high inherent con-
bronchopulmonary aspergillosis (ABPA), Potential complications of CF and non-CF trast between lung parenchyma and vessels
because many of the observations that can be bronchiectasis include bacterial, fungal, or or bronchi and because of the lower attenu-
made on radiography are nonspecific and are mycobacterial infection and mycetoma (Fig. ation of the CT beam, compared with CT of
commonly present in patients without this 5), ABPA, pleural effusion, pneumothorax, the abdomen. Thin-section nonhelical CT
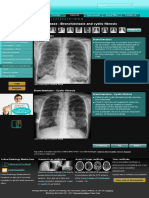
complication of CF [10] (Figs. 1A and 1B). hemoptysis, and pulmonary hypertension. consisting of six or seven images is a feasible
CT, on the other hand, is more specific for Appearances indicative of infection on CT and effective method for performing a diag-
ABPA, particularly unenhanced CT, when include air-space disease (Fig. 3), tree-in-bud nostic CT examination at an effective radia-
hyperdense material is observed in patients opacification (Fig. 6), ground-glass opacifi- tion dose of 0.14 mSv, which approximates
with dilated airways. cation (Fig. 7A), patchy nodular opacification the dose used in posteroanterior and lateral
The European Cystic Fibrosis Society rec- (Figs. 7A and 7B), and cavitation or abscess chest radiographs [24]. Newer iterative re-
ommends that patients with stable CF under- formation. constructive techniques will likely allow he-
go an annual chest radiographic examination It is worth remembering, however, that lical CT of the entire chest to be performed
[11]. The British Thoracic Society does not rec- bronchiectasis is a risk factor for atypical with radiation doses similar to those used in
ommend routine imaging surveillance for as- pulmonary infection, just as it is for the de- chest radiography.
ymptomatic patients with non-CF bronchiec- velopment of hemoptysis. Angiogenesis and
tasis, except when humoral immunodeficiency bronchial artery hypertrophy commonly Chest Radiography
is present [12]. For patients with deteriorating occur in association with CF, as a result of Radiography is inferior to CT for the as-
pulmonary function, CT is widely considered long-term inflammation. Hemoptysis gener- sessment of patients with known bronchiec-
to be the best imaging modality and is recom- ally is caused by exacerbations of infectious tasis or those with suspected disease. Nev-
mended by the aforementioned guidelines for disease caused by CF. Although bronchos- ertheless, radiography remains a useful
the assessment of bronchiectasis and the depic- copy and CT angiography are often used to modality for assessing the pulmonary com-
tion of associated complications [11, 12]. assess and localize the source of bleeding plications associated with bronchiectasis,
The most common CT finding in patients in patients without CF who have hemopty- because of its low cost, availability, low ra-
with CF is air trapping, followed by bronchi- sis, this approach is debated for patients with diation dose, and speed of acquisition [20].
ectasis, mucous plugging, consolidation, and, CF [19] (Fig. 8). Percutaneous endovascular Several radiographic scoring systems for CF
uncommonly, cysts or bullae [8] (Fig. 2). In embolization of abnormally large (≥ 3 mm) exist, with the Chrispin-Norman and Bras-
AJR:206, March 2016 449
Murphy et al.
field systems among the most commonly intestinal obstruction resulting from inspis- References
used [18, 25]. The parameters assessed are sated contents and occurs most commonly in 1. Dodge JA, Lewis PA, Stanton M, Wilsher J. Cystic
similar to those assessed by CT scoring sys- the terminal ileum [31]. The small bowel ap- fibrosis mortality and survival in the UK: 1947–
tems. Bronchiectasis and, in particular, cy- pears echogenic on prenatal ultrasound im- 2003. Eur Respir J 2007; 29:522–526
lindric bronchiectasis classically appear as ages, and meconium mixed with gas creates 2. Säynäjäkangas O, Keistinen T, Tuuponen T,
tram-track opacification when observed lon- a bubbly appearance on radiograph, possi- Kivelä SL. Evaluation of the incidence and age
gitudinally or as a signet ring when viewed bly in association with proximal small bow-
Downloaded from www.ajronline.org by Queen's Univ on 02/23/16 from IP address 130.15.241.167. Copyright ARRS. For personal use only; all rights reserved
distribution of bronchiectasis from the Finnish
en face on radiography (Fig. 10). el dilatation or with postperforation perito- hospital discharge register. Cent Eur J Public
neal calcification. Microcolon may be seen Health 1998; 6:235–237
MRI and Other Alternatives on images generated during a contrast-en- 3. Eastham KM, Fall AJ, Mitchell L, Spencer DA.
Although CT is considered the reference hanced enema examination. Distal intestinal The need to redefine non-cystic fibrosis bronchi-
standard for assessing bronchiectasis, pul- obstruction syndrome (previously known as ectasis in childhood. Thorax 2004; 59:324–327
monary MRI is increasingly being used [26, “meconium ileus equivalent”) occurs outside 4. Wang Y, Wrennall JA, Cai Z, Li H, Sheppard DN.
27]. MRI is less sensitive for the depiction of the neonatal period and has characteristics Understanding how cystic fibrosis mutations disrupt
small airways disease but can be used to as- similar to those of meconium ileus [32]. Pa- CFTR function: from single molecules to animal
sess functional changes, such as alterations tients with CF are also susceptible to intus- models. Int J Biochem Cell Biol 2014; 52:47–57
in pulmonary ventilation, which have led susception, appendiceal thickening, hepatic 5. Burgel P-R, Bellis G, Olesen H, et al. Fu-
many researchers to advocate the use of MRI steatosis, cirrhosis, microgallbladder, chole- ture trends in cystic fibrosis demography in 34 Eu-
for the follow-up of morphologic changes in lithiasis, biliary dilatation, fatty replacement ropean countries. Eur Respir J 2015; 46:133–141
CF and non-CF bronchiectasis. Routine T1- (steatosis) and atrophy of the pancreas (Fig. 6. Cole PJ. Inflammation: a two-edged sword—the
and T2-weighted turbo spin-echo sequences 12), pancreatitis, and nephrolithiasis [31–33]. model of bronchiectasis. Eur J Respir Dis Suppl
can be combined with inhaled agents (in par- Of interest, the frequency of acute appendi- 1986; 147:6–15
ticular, hyperpolarized helium) for imaging citis is lower in patients with CF than in the 7. Zaid AA, Elnazir B, Greally P. A decade of non-
purposes [28] (Figs. 11A and 11B). The use general population (1–2% vs 7%) [32]. With cystic fibrosis bronchiectasis 1996–2006. Ir Med J
of inhaled agents, such as hyperpolarized he- an increased life expectancy for patients with 2010; 103:77–79
lium or xenon, helps yield functional infor- CF, abdominal manifestations and compli- 8. Brody AS, Klein JS, Molina PL, Quan J, Bean JA,
mation about gas exchange. The most notable cations of CF are being seen more often in Wilmott RW. High-resolution computed tomogra-
disadvantages of MRI are the increased time adults [32, 33]. There is an increased inci- phy in young patients with cystic fibrosis: distribu-
and costs associated with image acquisition, dence of colonic, small intestinal, pancreat- tion of abnormalities and correlation with pulmo-
in addition to its limited usefulness in pa- ic, and hepatocellular cancer in patients with nary function tests. J Pediatr 2004; 145:32–38
tients receiving ventilation support. The use CF, compared with the age-matched popula- 9. Sanders DB, Li Z, Brody AS, Farrell PM. Chest
of higher-field MRI for patients with non- tion [34, 35]. computed tomography scores of severity are as-
CF bronchiectasis has been evaluated, with sociated with future lung disease progression in
MRI findings having good correlation with Conclusion children with cystic fibrosis. Am J Respir Crit
CT scores [29]. CT is the imaging method of choice for Care Med 2011; 184:816–821
FDG PET and FDG PET/CT have been the diagnosis, assessment, and surveillance 10. Cortese G, Malfitana V, Placido R, et al. Role of
used in research to quantify inflammatory of bronchiectasis and its associated compli- chest radiography in the diagnosis of allergic bron-
changes related to exacerbations of CF and cations and findings in patients with CF and chopulmonary aspergillosis in adult patients with
responses to antibiotic therapy [30]. Stan- other pediatric conditions. Modified low- cystic fibrosis. Radiol Med 2007; 112:626–636
dardized uptake values were positively cor- dose CT protocols should be considered, giv- 11. Smyth AR, Bell SC, Bojcin S, et al. European
related with CT scores for CF and were neg- en the potential for high cumulative exposure Cystic Fibrosis Society Standards of Care: Best
atively correlated with forced expiratory to radiation in this cohort. Chest radiography Practice guidelines. J Cyst Fibros 2014; 13(suppl
volume in 1 second [30]. This approach can is commonly used, particularly for monitor- 1):S23–S42
delineate areas of active infection and can ing acute complications of CF, but it provides 12. Pasteur MC, Bilton D, Hill AT. British Thoracic
also help differentiate CF from fibrosis. Lim- less information than does CT. The use of Society guideline for non-CF bronchiectasis. Tho-
ited access, high cost, and radiation dose are MRI with inhaled hyperpolarized gas yields rax 2010; 65(suppl 1):i1–i58
clear constraints that prohibit widespread use additional functional information, compared 13. Habesoglu MA, Ugurlu AO, Eyuboglu FO. Clini-
of FDG PET and FDG PET/CT. with CT; however, this type of imaging ex- cal, radiologic, and functional evaluation of 304
amination is not widely available. Abdomi- patients with bronchiectasis. Ann Thorac Med
Abdominal Imaging in Patients With nal manifestations of CF are increasingly be- 2011; 6:131–136
Cystic Fibrosis ing encountered as a result of increased life 14. Simanovsky N, Cohen-Cymberknoh M, Shoseyov
Abdominal complications related to gas- expectancy for patients with CF. D, et al. Differences in the pattern of structural
trointestinal or pancreaticobiliary disease re- abnormalities on CT scan in patients with cystic
main common in patients with CF [31]. Neo- Acknowledgment fibrosis and pancreatic sufficiency or insufficien-
natal meconium ileus occurs in 10–15% of We thank Casey Sams (Division of Pediat- cy. Chest 2013; 144:208–214
patients with CF and can be diagnosed on the ric Imaging, Department of Radiology, Uni- 15. Mott LS, Park J, Gangell CL, et al. Distri-
basis of clinical, radiologic, or sonographic versity of North Carolina) for providing MR bution of early structural lung changes due to cys-
findings. Meconium ileus is characterized by images. tic fibrosis detected with chest computed tomog-
450 AJR:206, March 2016
Imaging of Cystic Fibrosis and Pediatric Bronchiectasis
raphy. J Pediatr 2013; 163:243–248.e1–3 cades. Chest 2012; 141:1575–1583 ment of chest high-field magnetic resonance imaging
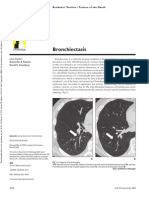
16. Javidan-Nejad C, Bhalla S. Bronchiectasis. R adiol 23. Donadieu J, Roudier C, Saguintaah M, Maccia C, in children and young adults with noncystic fibrosis
Clin North Am 2009; 47:289–306 Chiron R. Estimation of the radiation dose from chronic lung disease: comparison to high-resolution
17. Bhalla M, Turcios N, Aponte V, et al. Cystic fibro- thoracic CT scans in a cystic fibrosis population. computed tomography and correlation with pulmo-
sis: scoring system with thin-section CT. Chest 2007; 132:1233–1238 nary function. Invest Radiol 2009; 44:532–538
Radiology 1991; 179:783–788 24. O’Connor OJ, Vandeleur M, McGarrigle AM, et 30. Amin R, Charron M, Grinblat L, et al. Cystic fibro-
18. Vult von Steyern K, Björkman-Burtscher IM, Gei- al. Development of low-dose protocols for thin- sis: detecting changes in airway inflammation with
Downloaded from www.ajronline.org by Queen's Univ on 02/23/16 from IP address 130.15.241.167. Copyright ARRS. For personal use only; all rights reserved
jer M. Radiography, tomosynthesis, CT and MRI section CT assessment of cystic fibrosis in pediat- FDG PET/CT. Radiology 2012; 264:868–875
in the evaluation of pulmonary cystic fibrosis: an ric patients. Radiology 2010; 257:820–829 31. Chaudry G, Navarro OM, Levine DS, Oudjhane
untangling review of the multitude of scoring sys- 25. Terheggen-Lagro S, Truijens N, van Poppel N, K. Abdominal manifestations of cystic fibrosis in
tems. Insights Imaging 2013; 4:787–798 Gulmans V, van der Laag J, van der Ent C. Corre- children. Pediatr Radiol 2006; 36:233–240
19. Flume PA, Mogayzel PJ Jr, Robinson KA, lation of six different cystic fibrosis chest radio- 32. Liong SY, Awad D, Jones AM, Sukumar SA. The
Rosenblatt RL, Quittell L, Marshall BC. Cystic graph scoring systems with clinical parameters. adult cystic fibrosis patient with abdominal pain:
fibrosis pulmonary guidelines: pulmonary com- Pediatr Pulmonol 2003; 35:441–445 what the radiologist needs to know. Clin Radiol
plications: hemoptysis and pneumothorax. Am J 26. Puderbach M, Eichinger M. The role of advanced im- 2011; 66:132–139
Respir Crit Care Med 2010; 182:298–306 aging techniques in cystic fibrosis follow-up: is there a 33. Robertson MB, Choe KA, Joseph PM. Review of the
20. Ketai LH, Mohammed TL, Kirsch J, et al. ACR place for MRI? Pediatr Radiol 2010; 40:844–849 abdominal manifestations of cystic fibrosis in the
appropriateness criteria hemoptysis. J Thorac 27. Montella S, Maglione M, Bruzzese D, et al. Mag- adult patient. RadioGraphics 2006; 26:679–690
Imaging 2014; 29:W19–W22 netic resonance imaging is an accurate and reli- 34. Johannesson M, Askling J, Montgomery SM, Ek-
21. Chun JY, Belli AM. Immediate and long-term able method to evaluate non-cystic fibrosis paedi- bom A, Bahmanyar S. Cancer risk among patients
outcomes of bronchial and non-bronchial system- atric lung disease. Respirology 2012; 17:87–91 with cystic fibrosis and their first-degree relatives.
ic artery embolisation for the management of hae- 28. Sun Y, O’Sullivan BP, Roche JP, et al. Using hy- Int J Cancer 2009; 125:2953–2956
moptysis. Eur Radiol 2010; 20:558–565 perpolarized 3He MRI to evaluate treatment effi- 35. Maisonneuve P, Marshall BC, Knapp EA, Lowen-
22. O’Connell OJ, McWilliams S, McGarrigle A, et cacy in cystic fibrosis patients. J Magn Reson Im- fels AB. Cancer risk in cystic fibrosis: a 20-year
al. Radiologic imaging in cystic fibrosis: cumula- aging 2011; 34:1206–1211 nationwide study from the United States. J Natl
tive effective dose and changing trends over 2 de- 29. Montella S, Santamaria F, Salvatore M, et al. Assess- Cancer Inst 2013; 105:122–129
Fig. 1—53-year-old woman with asymmetric
bronchiectasis secondary to cystic fibrosis (CF) who
underwent contrast-enhanced CT for monitoring
of chronic progressive deterioration of pulmonary
function.
A, Posterior thoracic coronal CT image displayed on
lung windows shows advanced CF bronchiectasis
with volume loss in left lower lobe. There is less
severe cylindric bronchiectasis (arrows) in right
lower lobe.
B, Chest radiograph shows asymmetric cystic and
cylindric bronchiectasis (arrowheads), left lung
volume loss, and small effusion (straight arrow).
Chest port (curved arrow) is also shown.
A B
AJR:206, March 2016 451
Murphy et al.
Downloaded from www.ajronline.org by Queen's Univ on 02/23/16 from IP address 130.15.241.167. Copyright ARRS. For personal use only; all rights reserved
Fig. 2—24-year-old man with cystic fibrosis who was undergoing annual Fig. 3—2-year-old boy with bronchiectasis secondary to
surveillance contrast-enhanced CT. Midthoracic axial CT image on lung window hypogammaglobulinemia-related immunodeficiency who presented with
setting shows typical findings of advanced bronchiectasis: advanced cylindric complicated respiratory infection. Lower thoracic axial contrast-enhanced CT
bronchiectasis (arrows), which is most marked in right upper lobe, with associated image displayed on lung windows shows varicoid bronchiectasis (curved arrow)
volume loss and bronchial wall thickening (arrowheads). Chest port is shown in involving anterior segment of right upper lobe with associated air-space disease
right anterior chest wall. (straight arrows).
Fig. 4—22-year-old man with cystic fibrosis complicated by acute deterioration
in symptom severity. Axial unenhanced CT image acquired at level of upper lobes
and displayed on lung windows shows mosaic attenuation (arrows) resulting from
small airways disease with central cylindric bronchiectasis (arrowheads) and
adjacent patchy air-space disease. Left-sided lung parenchymal volume loss is
also shown.
Fig. 5—27-year-old woman with advanced bronchiectasis secondary to Fig. 6—16-year-old boy with cystic fibrosis with clinical symptoms of lower
cystic fibrosis. Contrast-enhanced CT was performed to investigate subacute respiratory chest infection. Lung basal axial unenhanced CT image displayed on
exacerbation of respiratory symptoms. Axial CT image displayed on lung window lung windows shows tree-in-bud opacities (straight arrows) and ground-glass
shows mycetoma (arrows) just below carinal level in superior segment of right opacification (curved arrows) in peripheral right lower lobe. Mild lower lobe
lower lobe. bronchiectasis (arrowheads) is also evident.
452 AJR:206, March 2016
Imaging of Cystic Fibrosis and Pediatric Bronchiectasis
Downloaded from www.ajronline.org by Queen's Univ on 02/23/16 from IP address 130.15.241.167. Copyright ARRS. For personal use only; all rights reserved
A B
Fig. 7—26-year-old woman with cystic fibrosis who underwent imaging because of clinical deterioration suggestive of infection.
A, Axial contrast-enhanced CT image of midthorax displayed on lung windows shows multiple peripheral peribronchovascular nodular
opacities (arrows) in addition to mosaic attenuation (arrowheads). These opacities were confirmed to be secondary to atypical mycobacterial
infection after bronchoscopy.
B, Chest radiograph shows same nodular opacities (arrows) seen on CT, although opacities are less clear on radiographic image. Chest port
(arrowhead) is also shown.
Fig. 8—42-year-old man with bronchiectasis caused by cystic fibrosis who was
admitted to hospital with significant hemoptysis. Axial contrast-enhanced CT
image of upper lobes displayed on soft-tissue windows shows markedly enlarged
bronchial arteries (arrowheads) in addition to air-space disease and ground-
glass opacification (arrows) from hemorrhage in right upper lobe. Successful
embolization of branches of right bronchial artery was performed.
Fig. 9—18-year-old woman with bronchiectasis not caused by cystic fibrosis who was admitted to hospital
with moderate volume hemoptysis. Digital subtraction angiogram of thoracic aorta and branches shows mildly
enlarged intercostobronchial artery (arrow) in right upper lobe and abnormal blush caused by extravasation of
contrast medium (arrowheads). This artery was selectively embolized with resultant symptom cessation.
AJR:206, March 2016 453
Murphy et al.
Fig. 10—27-year-old woman with cystic fibrosis who underwent chest radiography for assessment of subacute
deterioration of pulmonary status. Radiographic image shows tram-track opacities (arrowheads) due to
cylindric bronchiectasis predominantly in upper lobe. Chest port (arrow) is also shown.
Downloaded from www.ajronline.org by Queen's Univ on 02/23/16 from IP address 130.15.241.167. Copyright ARRS. For personal use only; all rights reserved
Fig. 11—20-year-old man with cystic fibrosis
with subacute deterioration of pulmonary status.
(Courtesy of Sams C, University of North Carolina,
Chapel Hill, NC)
A, Chest radiograph shows lung hyperexpansion
and mild cylindric bronchiectasis (arrows) but no
evidence of pneumonia.
B, Contemporaneous coronal spin-echo MR
image confirms left midzone scarring and
atelectasis (curved arrow), bronchial wall
thickening (arrowheads), and upper zone cylindric
bronchiectasis with some plugging (straight arrows).
A B
Fig. 12—21-year-old woman with cystic fibrosis who underwent contrast-
enhanced CT of thorax for evaluation of pulmonary disease. Lowermost axial
upper abdominal CT image displayed on soft-tissue windows shows steatosis
of pancreas (straight arrows) and liver nodularity secondary to cirrhosis (curved
arrows), in addition to splenomegaly and venous collaterals (arrowheads) resulting
from portal hypertension.
454 AJR:206, March 2016
You might also like
- Euphoria 2021 F K Anyone Whos Not A Sea Blob Part 2 Jules Script Teleplay Written by Sam Levinson and Hunter SchaferDocument40 pagesEuphoria 2021 F K Anyone Whos Not A Sea Blob Part 2 Jules Script Teleplay Written by Sam Levinson and Hunter SchaferMadalena Duarte7No ratings yet
- American History 2017-10 PDFDocument76 pagesAmerican History 2017-10 PDFCristian OvanisofNo ratings yet
- Contract of LeaseDocument4 pagesContract of LeaseIelBarnachea100% (5)
- A Short Guide For Feature Engineering and Feature SelectionDocument32 pagesA Short Guide For Feature Engineering and Feature SelectionjainnitkNo ratings yet
- Embedded System in Automobile VehiclesDocument17 pagesEmbedded System in Automobile Vehiclessam clastineNo ratings yet
- 920 FullDocument17 pages920 FullHeru SigitNo ratings yet
- Advances in Interventional Diagnostic Bronchoscopy For Peripheral Pulmonary LesionsDocument15 pagesAdvances in Interventional Diagnostic Bronchoscopy For Peripheral Pulmonary LesionsAlaaNo ratings yet
- Chest X-Ray - Pulmonary Disease - Bronchiectasis and Cystic FibrosisDocument1 pageChest X-Ray - Pulmonary Disease - Bronchiectasis and Cystic FibrosisNang KhamNo ratings yet
- Mondoni Et AlDocument11 pagesMondoni Et AlpandylouisputraNo ratings yet
- Review: The Changing Face of Pneumonia Maids PatientsDocument9 pagesReview: The Changing Face of Pneumonia Maids Patientsmail junkNo ratings yet
- Rad RecogDocument6 pagesRad RecogsiscaNo ratings yet
- AsdfDocument2 pagesAsdfMagma SanggiriNo ratings yet
- Ajr 175 6 1751533Document4 pagesAjr 175 6 1751533nursofiatrierlianaNo ratings yet
- Endobronchial Ultrasound For The Diagnosis and Staging of Lung Cancer (Methods)Document7 pagesEndobronchial Ultrasound For The Diagnosis and Staging of Lung Cancer (Methods)Gio Mari MarcialNo ratings yet
- Postoperative Pulmonary Complication As An.21Document6 pagesPostoperative Pulmonary Complication As An.21DrRitoban Saha BhowmickNo ratings yet
- Bronkiektasis PDFDocument14 pagesBronkiektasis PDFHalida Batik AlunaNo ratings yet
- Boonsarngsuk - Factors Affecting The Diagnostic Yield of Flexible Bronchoscopy Without Guidance in Pulmonary Nodules or MassesDocument6 pagesBoonsarngsuk - Factors Affecting The Diagnostic Yield of Flexible Bronchoscopy Without Guidance in Pulmonary Nodules or MassesXaralyn XaviereNo ratings yet
- Thoracic Radiology: # (149) # (149) # (149) Nestor L. Muller, MD, PHDDocument6 pagesThoracic Radiology: # (149) # (149) # (149) Nestor L. Muller, MD, PHDPutri Amelia RizqiNo ratings yet
- Bronchial and Arterial Sleeve Resection For Centrally-Located Lung CancersDocument10 pagesBronchial and Arterial Sleeve Resection For Centrally-Located Lung CancersJovelyn SagangNo ratings yet
- Ofstead ArticleDocument10 pagesOfstead ArticleAndrei ModreanuNo ratings yet
- Case VBCTDocument7 pagesCase VBCTPari Pengda BaliNo ratings yet
- Lood Eosinophil Percentage As A Predictor of Response Toinhaled Corticosteroid in BronchiectasisDocument8 pagesLood Eosinophil Percentage As A Predictor of Response Toinhaled Corticosteroid in BronchiectasiskarlosNo ratings yet
- Bronkoskopi PediatrikDocument4 pagesBronkoskopi PediatrikAnonymous lSWQIQNo ratings yet
- Imaging of Pulmonary Viral Pneumonia: Online CMEDocument22 pagesImaging of Pulmonary Viral Pneumonia: Online CMEsofiaNo ratings yet
- Combining Deep Learning and Coherent Anti-Stokes Raman Scattering Imaging For Automated Differential Diagnosis of Lung CancerDocument11 pagesCombining Deep Learning and Coherent Anti-Stokes Raman Scattering Imaging For Automated Differential Diagnosis of Lung Cancersyirah97No ratings yet
- Pulmonary PerspectiveDocument8 pagesPulmonary PerspectiveTony AndersonNo ratings yet
- 2001 Broncoscopia - British - Thoracic - Society PDFDocument21 pages2001 Broncoscopia - British - Thoracic - Society PDFMartín FleiNo ratings yet
- Aspecto Linfonodal Fujiwara 2010Document7 pagesAspecto Linfonodal Fujiwara 2010VickNo ratings yet
- Hepatic Fibrosis Evaluation With SemiquantitativeDocument9 pagesHepatic Fibrosis Evaluation With Semiquantitativeehsanulazim50buetNo ratings yet
- Imaging in Non-Cystic Fibrosis Bronchiectasis and Current LimitationsDocument10 pagesImaging in Non-Cystic Fibrosis Bronchiectasis and Current LimitationsRivaldy BobihuNo ratings yet
- Lung Adenocarcinoma With Solitary MetastDocument88 pagesLung Adenocarcinoma With Solitary MetastMikmik bay BayNo ratings yet
- Linical Eat Res Pri Ar L NG Cancer Presenting As P L Nar Cnsliatin Iicingpne NiaDocument5 pagesLinical Eat Res Pri Ar L NG Cancer Presenting As P L Nar Cnsliatin Iicingpne NiaYusi RizkyNo ratings yet
- BS-Net: Learning COVID-19 Pneumonia Severity On A Large Chest X-Ray DatasetDocument28 pagesBS-Net: Learning COVID-19 Pneumonia Severity On A Large Chest X-Ray Datasetjeremy joshuaNo ratings yet
- Neonatal Lung Disorders: Pattern Recognition Approach To DiagnosisDocument12 pagesNeonatal Lung Disorders: Pattern Recognition Approach To DiagnosisMeiriyani LembangNo ratings yet
- The Role of Imaging in The Diagnosis of Bronchiectasis: The Key Is in The DistributionDocument3 pagesThe Role of Imaging in The Diagnosis of Bronchiectasis: The Key Is in The DistributionrosmeniNo ratings yet
- Characteristics Feature of Chronic Obstructive Pulmonary Disease Correlation With Clinical FindingDocument6 pagesCharacteristics Feature of Chronic Obstructive Pulmonary Disease Correlation With Clinical FindingCentral Asian StudiesNo ratings yet
- CT Features of Community-Acquired Pneumonia at The Emergency DepartmentDocument8 pagesCT Features of Community-Acquired Pneumonia at The Emergency DepartmentpachomdNo ratings yet
- PIIS0012369222040818Document12 pagesPIIS0012369222040818JUAN NARVAEZ RUELANo ratings yet
- The Radiological Diagnosis of Bronchiectasis: What 'S in A Name?Document9 pagesThe Radiological Diagnosis of Bronchiectasis: What 'S in A Name?Nabil Sangga BuanaNo ratings yet
- Arya - Usefulness and Safety of Transbronchial Biopsy With Large Forceps During Flexible BronchosDocument5 pagesArya - Usefulness and Safety of Transbronchial Biopsy With Large Forceps During Flexible BronchosXaralyn XaviereNo ratings yet
- Jurnal 1Document6 pagesJurnal 1Ahmad Fari Arief LopaNo ratings yet
- Clinical Review: Radiological Review of PneumothoraxDocument5 pagesClinical Review: Radiological Review of PneumothoraxChe yallyNo ratings yet
- 248 FullDocument2 pages248 Fullabutalebheba95No ratings yet
- Uro 2Document10 pagesUro 2danielNo ratings yet
- Imaging in Chronic Obstructive Pulmonary DiseaseDocument10 pagesImaging in Chronic Obstructive Pulmonary DiseaseAsniar RNo ratings yet
- Lung Intervention by Using CT Fluoroscopy With Repository FrameworkDocument5 pagesLung Intervention by Using CT Fluoroscopy With Repository FrameworkerpublicationNo ratings yet
- Histopathologic Analysis of Ct-Guided Core Needle Biopsy in Radiologically Detected Suspicious Mediastinal and Lung Mass: Two Years' Study in Tertiary HospitalDocument4 pagesHistopathologic Analysis of Ct-Guided Core Needle Biopsy in Radiologically Detected Suspicious Mediastinal and Lung Mass: Two Years' Study in Tertiary HospitalDesiree MejicaNo ratings yet
- Effectiveness of Chest Physiotherapy in The Management of BronchiectasisDocument15 pagesEffectiveness of Chest Physiotherapy in The Management of Bronchiectasiskim suhoNo ratings yet
- ACCP Lung Cancer 2013Document25 pagesACCP Lung Cancer 2013Hendarsyah SuryadinataNo ratings yet
- Retropharyngeal AbscessDocument1 pageRetropharyngeal AbscessDominique Ishmaielle Sumillano HibionadaNo ratings yet
- Key Points: Diagnostic Imaging in Adult Non-Cystic Fibrosis BronchiectasisDocument8 pagesKey Points: Diagnostic Imaging in Adult Non-Cystic Fibrosis BronchiectasisAmirah SilinoNo ratings yet
- Radiology of Chest Wall MassesDocument11 pagesRadiology of Chest Wall MassesDevina BumiNo ratings yet
- Pediatric Point-of-Care Lung UltrasonographyDocument8 pagesPediatric Point-of-Care Lung UltrasonographycristianNo ratings yet
- The Positive Result of Cytology Brushing at Flexible Fiberoptic Bronchoscopy Compared With Transthoracic Needle Aspiration in Central Lung TumorDocument10 pagesThe Positive Result of Cytology Brushing at Flexible Fiberoptic Bronchoscopy Compared With Transthoracic Needle Aspiration in Central Lung TumorBayuptrNo ratings yet
- Peng Et Al-2020-Intensive Care Medicine PDFDocument2 pagesPeng Et Al-2020-Intensive Care Medicine PDFTeodor ŞişianuNo ratings yet
- Bronchoscopic Drainage of A Malignant Lung Abscess: ASE EportDocument4 pagesBronchoscopic Drainage of A Malignant Lung Abscess: ASE EportAlfani FajarNo ratings yet
- Should All Initial Episodes of Hemoptysis Be Evaluated by Bronchoscopy? YesDocument4 pagesShould All Initial Episodes of Hemoptysis Be Evaluated by Bronchoscopy? YesAnisa Hanifatin RahayuNo ratings yet
- Endobronchial Ultrasound: What Is It and When Should It Be Used?Document6 pagesEndobronchial Ultrasound: What Is It and When Should It Be Used?Legenda AkNo ratings yet
- Imaging of Pancreaticobiliary MaljunctionDocument15 pagesImaging of Pancreaticobiliary MaljunctionMaria Ximena SilvaNo ratings yet
- Nomogram Model To Predict Pneumothorax After CompuDocument7 pagesNomogram Model To Predict Pneumothorax After CompueugeniaNo ratings yet
- 23rd Respimirror Bronchiectasis PDFDocument16 pages23rd Respimirror Bronchiectasis PDFmeddyaaNo ratings yet
- 23rd Respimirror BronchiectasisDocument16 pages23rd Respimirror BronchiectasismeddyaaNo ratings yet
- Hydropneumothorax SupineDocument4 pagesHydropneumothorax SupineVioline MartaliaNo ratings yet
- Case Emfisema ParuDocument3 pagesCase Emfisema ParuMuhammad SyukurNo ratings yet
- Snijders 2007Document6 pagesSnijders 2007carlaNo ratings yet
- Zhang 2018Document6 pagesZhang 2018carlaNo ratings yet
- 05.03.19. (B) Efficacy and Safety of The Combination Fluticasone Propionate Plus Salmeterol in Asthmatic Preschoolers (Observational Study) - JA 2018Document9 pages05.03.19. (B) Efficacy and Safety of The Combination Fluticasone Propionate Plus Salmeterol in Asthmatic Preschoolers (Observational Study) - JA 2018carlaNo ratings yet
- Stridor in Children: Case ReportDocument7 pagesStridor in Children: Case ReportcarlaNo ratings yet
- Post-Infectious Bronchiolitis Obliterans in Children: A Review of 42 CasesDocument6 pagesPost-Infectious Bronchiolitis Obliterans in Children: A Review of 42 CasescarlaNo ratings yet
- Postinfectious Bronchiolitis Obliterans in Children.2020Document16 pagesPostinfectious Bronchiolitis Obliterans in Children.2020carlaNo ratings yet
- 04.03.19. (A) Management of Recurrent Preschool, Doctor-Diagnosed Wheeze (Review) - IJP 2018Document9 pages04.03.19. (A) Management of Recurrent Preschool, Doctor-Diagnosed Wheeze (Review) - IJP 2018carlaNo ratings yet
- Acute Disseminated Encephalomyelitis in Children: ObjectiveDocument9 pagesAcute Disseminated Encephalomyelitis in Children: ObjectivecarlaNo ratings yet
- Thoracoscopic Decortication Vs Tube Thoracostomy With Fibrinolysis For Empyema in Children: A Prospective, Randomized TrialDocument6 pagesThoracoscopic Decortication Vs Tube Thoracostomy With Fibrinolysis For Empyema in Children: A Prospective, Randomized TrialcarlaNo ratings yet
- Therapeutic Approach To The Management of Pediatric Demyelinating Disease: Multiple Sclerosis and Acute Disseminated EncephalomyelitisDocument12 pagesTherapeutic Approach To The Management of Pediatric Demyelinating Disease: Multiple Sclerosis and Acute Disseminated EncephalomyelitiscarlaNo ratings yet
- Arch Dis Child 2003 Stonehouse 122 4Document4 pagesArch Dis Child 2003 Stonehouse 122 4carlaNo ratings yet
- 10 1016@j Atherosclerosissup 2015 02 037Document6 pages10 1016@j Atherosclerosissup 2015 02 037carlaNo ratings yet
- Shock Septico en Pediatria II Enfoque Actual en ElDocument16 pagesShock Septico en Pediatria II Enfoque Actual en ElcarlaNo ratings yet
- Writing Task 2 - Discussion - Opinion EssayDocument7 pagesWriting Task 2 - Discussion - Opinion EssayTonNo ratings yet
- Tourism in India: Service Sector: Case StudyDocument16 pagesTourism in India: Service Sector: Case StudyManish Hemant DatarNo ratings yet
- Genmath Q2 Week4Document36 pagesGenmath Q2 Week4ShennieNo ratings yet
- APU CSLLT - 6 - Introduction To Assembly LanguageDocument23 pagesAPU CSLLT - 6 - Introduction To Assembly LanguageAli AtifNo ratings yet
- 1 Programming Model (Algorithms 1.1)Document10 pages1 Programming Model (Algorithms 1.1)HarshaSharmaNo ratings yet
- The Contemporary World Lesson 2Document32 pagesThe Contemporary World Lesson 2WENDELL VERGARANo ratings yet
- List of Affilited CollegesDocument28 pagesList of Affilited Collegesuzma nisarNo ratings yet
- Bank of South Sudan Act, 2011 - Bank of South SudanDocument59 pagesBank of South Sudan Act, 2011 - Bank of South SudanAnnadevaraNageswararaoNo ratings yet
- The Rook Volume XXIIIDocument40 pagesThe Rook Volume XXIIIThe RookNo ratings yet
- Internet Addiction Test (IAT)Document4 pagesInternet Addiction Test (IAT)نور اكمال عابدNo ratings yet
- Professional Resume - Sunil BhardwajDocument2 pagesProfessional Resume - Sunil BhardwajSunil KumarNo ratings yet
- Permutations & Combinations DDPDocument20 pagesPermutations & Combinations DDPVibhu100% (1)
- SaviorKitty - (Seven Deadly Sins Series 4) PrideDocument48 pagesSaviorKitty - (Seven Deadly Sins Series 4) PrideMarife LuzonNo ratings yet
- Regulations ON Academic Matters: Tezpur UniversityDocument21 pagesRegulations ON Academic Matters: Tezpur UniversitybishalNo ratings yet
- Long Quiz 1 Eim Tools, MaterialsDocument1 pageLong Quiz 1 Eim Tools, MaterialsLea Ann PalaciosNo ratings yet
- Nec Exhibition BrochureDocument4 pagesNec Exhibition BrochurejppullepuNo ratings yet
- LY Adverbs Combined ListDocument1 pageLY Adverbs Combined ListcarmencrisanNo ratings yet
- Kelompok 1 Recount TextDocument11 pagesKelompok 1 Recount TextElvina RahmayaniNo ratings yet
- Candi MerakDocument2 pagesCandi MerakEdi YantoNo ratings yet
- 5990200Document551 pages5990200Basit Ahmad bhat0% (1)
- Ece 250 Project PortfolioDocument7 pagesEce 250 Project Portfolioapi-511924847No ratings yet
- Vestel Alva B Na-127vb3 Na-147vb3 SMDocument40 pagesVestel Alva B Na-127vb3 Na-147vb3 SMjoseNo ratings yet
- Baughman Don Marianne 1977 NigeriaDocument11 pagesBaughman Don Marianne 1977 Nigeriathe missions networkNo ratings yet
- Grade 11 Reco ScriptDocument2 pagesGrade 11 Reco ScriptCecill Nicanor LabininayNo ratings yet
- Culture and CraftDocument27 pagesCulture and CraftFreya UmelNo ratings yet