Professional Documents
Culture Documents
1.4 - Introduction To Spectroscopy - Chemistry LibreTexts
Uploaded by
AhmadulhaqOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
1.4 - Introduction To Spectroscopy - Chemistry LibreTexts
Uploaded by
AhmadulhaqCopyright:
Available Formats
1/25/2021 1.
4: Introduction to Spectroscopy - Chemistry LibreTexts
1.4: Introduction to Spectroscopy
Realizing that light may be considered to have both wave-like and particle-like characteristics, it is useful to consider that a
given frequency or wavelength of light is associated with a "light quanta" of energy we now call a photon. As noted in the
following equations, frequency and energy change proportionally, but wavelength has an inverse relationship to these
quantities.
c
ν = (1.4.1)
λ
and
ΔE = hν (1.4.2)
with
ν is the frequency of light
λ is the wavelength of ligt
c is the speed of light (3 × 10 m/sec )
8
ΔE is the transition energy (difference of energies between the initial and final states)
h is Planck's constant (h = 6.626069 × 10 J s )
−34
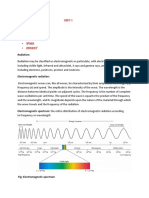
To "see" a molecule, we must use light having a wavelength smaller than the molecule itself (roughly 1 to 15 angstroms). Such
radiation is found in the X-ray region of the spectrum, and the field of X-ray crystallography yields remarkably detailed
pictures of molecular structures amenable to examination. The chief limiting factor here is the need for high quality crystals of
the compound being studied. The methods of X-ray crystallography are too complex to be described here; nevertheless, as
automatic instrumentation and data handling techniques improve, it will undoubtedly prove to be the procedure of choice for
structure determination. The spectroscopic techniques described below do not provide a three-dimensional picture of a
molecule, but instead yield information about certain characteristic features. A brief summary of this information follows:
Ultraviolet-Visible Spectroscopy: Absorption of this relatively high-energy light causes electronic excitation. The easily
accessible part of this region (wavelengths of 200 to 800 nm) shows absorption only if conjugated π electron systems are
present.
Infrared Spectroscopy: Absorption of this lower energy radiation causes vibrational and rotational excitation of groups of
atoms. within the molecule. Because of their characteristic absorptions, identification of functional groups is easily
accomplished.
Nuclear Magnetic Resonance (NMR) Spectroscopy: Absorption in the low-energy radio-frequency part of the spectrum
causes excitation of nuclear spin states. NMR spectrometers are tuned to certain nuclei (e.g. 1H, 13C, 19F & 31P). For a given
type of nucleus, high-resolution spectroscopy distinguishes and counts atoms in different locations in the molecule.
Contributors
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
https://chem.libretexts.org/Courses/University_of_Illinois_Springfield/Introduction_to_Organic_Spectroscopy/1%3A_Introduction_to_Organic_Spectros… 1/1
You might also like
- RIBA Outline Plan of Work ExplainedDocument20 pagesRIBA Outline Plan of Work ExplainedkenNo ratings yet
- Inspection and Test Plan Steel Sheet Pile DriDocument6 pagesInspection and Test Plan Steel Sheet Pile DriSofda Imela100% (1)
- Construction Materials and Testing: "WOOD"Document31 pagesConstruction Materials and Testing: "WOOD"Aira Joy AnyayahanNo ratings yet
- Organic Spectroscopy 1Document215 pagesOrganic Spectroscopy 1Rajendran PKNo ratings yet
- SpectrosDocument49 pagesSpectrosKen LamNo ratings yet
- Saturated Absorption Spectroscopy Experiment (SASDocument26 pagesSaturated Absorption Spectroscopy Experiment (SASPrakash ShanbogNo ratings yet
- Temu 1Document36 pagesTemu 1Farida UtamiNo ratings yet
- 1 Basics of Atomic Structure-3-19Document17 pages1 Basics of Atomic Structure-3-19Raj KishoreNo ratings yet
- Chapter 7 Electronic Structure of AtomsDocument78 pagesChapter 7 Electronic Structure of AtomsYunNo ratings yet
- Moleculer and Atomic SpectrosDocument78 pagesMoleculer and Atomic Spectrosjomi sultonzoda0% (1)
- NONLINEAR SPECTROSCOPYDocument43 pagesNONLINEAR SPECTROSCOPYThien Phu Nguyen NguyenNo ratings yet
- Plasmonics SelectedTopicsDocument52 pagesPlasmonics SelectedTopicsDominic LeeNo ratings yet
- Wave Particle DualityDocument32 pagesWave Particle DualityDavid ThaiNo ratings yet
- PCV - Ch1Document27 pagesPCV - Ch1Yathin gowda 8971095275No ratings yet
- Light Scattering by Polymer Solutions: Light Waves - A Brief ReviewDocument23 pagesLight Scattering by Polymer Solutions: Light Waves - A Brief ReviewYashanshu GautamNo ratings yet
- Unit Spectra of Atoms: StructureDocument32 pagesUnit Spectra of Atoms: Structureতুমি রবে নীরবেNo ratings yet
- What Is It?Document6 pagesWhat Is It?PharmaEducationNo ratings yet
- Report On Raman ScatteringDocument7 pagesReport On Raman ScatteringsilentShoeNo ratings yet
- Atomic Spectroscopy Sahin EbookDocument11 pagesAtomic Spectroscopy Sahin EbookWW JeonNo ratings yet
- Rosenzweig 1998 0154Document19 pagesRosenzweig 1998 0154Particle Beam Physics LabNo ratings yet
- 3.12 The Photoelectric Effect StudentDocument4 pages3.12 The Photoelectric Effect StudentHasan AslamNo ratings yet
- Lesson 7.1 Electromagnitic RadiationDocument7 pagesLesson 7.1 Electromagnitic Radiationharpermills.17No ratings yet
- Worksheet 9 - Electromagnetic Radiation and The Bohr AtomDocument4 pagesWorksheet 9 - Electromagnetic Radiation and The Bohr Atomvisa1032No ratings yet
- Atomic StructureDocument49 pagesAtomic StructureFatimaNo ratings yet
- An Introduction to Energy Dispersive X-ray Spectroscopy (EDX or EDSDocument9 pagesAn Introduction to Energy Dispersive X-ray Spectroscopy (EDX or EDScopilliNo ratings yet
- Burgot G., Burgot J.-L. - General Analytical Chemistry - SeparationDocument60 pagesBurgot G., Burgot J.-L. - General Analytical Chemistry - SeparationAytekin GaribliNo ratings yet
- Molecular Spectroscopy: Introduction and General Principles: A. GoodmanDocument10 pagesMolecular Spectroscopy: Introduction and General Principles: A. GoodmanSam peterNo ratings yet
- On A Heuristic Point of View About The Creation and Conversion of LightDocument8 pagesOn A Heuristic Point of View About The Creation and Conversion of LightZach EspirituNo ratings yet
- Basics of Laser-Plasma Interaction: A Selection of TopicsDocument24 pagesBasics of Laser-Plasma Interaction: A Selection of TopicsNoamanNo ratings yet
- Module 3: Molecular Spectroscopy Lecture 12: Electronic SpectrosDocument9 pagesModule 3: Molecular Spectroscopy Lecture 12: Electronic SpectrosAnmol SahuNo ratings yet
- Atomic Physics NotesDocument89 pagesAtomic Physics Notesawais33306No ratings yet
- Relativistic Doppler Effect of Light and Matter Waves: Keeyung LeeDocument5 pagesRelativistic Doppler Effect of Light and Matter Waves: Keeyung LeeRuslan TrocinNo ratings yet
- UV-Visible Spectrophotometric Method and Validation of Organic CompoundsDocument4 pagesUV-Visible Spectrophotometric Method and Validation of Organic Compoundsianatul khafidlahNo ratings yet
- General Chemistry: Atoms First: Periodicity and The Electronic Structure of AtomsDocument55 pagesGeneral Chemistry: Atoms First: Periodicity and The Electronic Structure of AtomsMinh PhamNo ratings yet
- 1H NMRDocument64 pages1H NMRhussemadl41No ratings yet
- CHMBD 449 - Organic Spectral: AnalysisDocument18 pagesCHMBD 449 - Organic Spectral: AnalysisIleana ManciuleaNo ratings yet
- APhO 2018Document88 pagesAPhO 2018Aditya MondalNo ratings yet
- Теоретический тур PDFDocument18 pagesТеоретический тур PDFVokerleeNo ratings yet
- Optical Transition Radiation: 'Work Supported Physics DepartmentDocument35 pagesOptical Transition Radiation: 'Work Supported Physics DepartmentParticle Beam Physics LabNo ratings yet
- Capitolo 1 - IntroduzioneDocument7 pagesCapitolo 1 - IntroduzioneAntonino GiuffridaNo ratings yet
- X RaysDocument11 pagesX RaysCSF1No ratings yet
- Electromagnetic in bifrefringeant systemDocument2 pagesElectromagnetic in bifrefringeant systemjedusableNo ratings yet
- HBTDocument52 pagesHBTFabiana MonteiroNo ratings yet
- Paper - EntropiaDocument25 pagesPaper - EntropiaAlejandra CarlosamaNo ratings yet
- 2019-Key Physics Equations and Experiments Explained and Derived by Energy Wave EquationsDocument29 pages2019-Key Physics Equations and Experiments Explained and Derived by Energy Wave Equationsjoao pedro GomesNo ratings yet
- PhysRevLett 71 1994Document4 pagesPhysRevLett 71 1994PedroNo ratings yet
- SpectrosDocument14 pagesSpectrosmaxwell amponsahNo ratings yet
- Unit 2+12 TARGET SHEET HL Atomic StructureDocument2 pagesUnit 2+12 TARGET SHEET HL Atomic Structurediya joshiNo ratings yet
- Worksheet 10 PDFDocument4 pagesWorksheet 10 PDFJosh FlorentinoNo ratings yet
- Atoms and NucleiDocument12 pagesAtoms and NucleiBablu ChaudharyNo ratings yet
- Spectroscopy & Characterization of Polymers: Chain MicrostructureDocument10 pagesSpectroscopy & Characterization of Polymers: Chain MicrostructureMustansar CheemaNo ratings yet
- Absorption of γ-Rays: Determining Half-Value ThicknessDocument19 pagesAbsorption of γ-Rays: Determining Half-Value ThicknessAbhrajit MahapatraNo ratings yet
- Fundamental and Harmonic Microbunching Measurements in A High-Gain, Self-Amplified, Spontaneous Emission Free-Electron LaserDocument14 pagesFundamental and Harmonic Microbunching Measurements in A High-Gain, Self-Amplified, Spontaneous Emission Free-Electron LaserParticle Beam Physics LabNo ratings yet
- Unit 4 Lasers - Properties and Applications of Coherent Light SourcesDocument21 pagesUnit 4 Lasers - Properties and Applications of Coherent Light SourcesNele 40No ratings yet
- The Spectrum of A Single Photoionized CloudDocument17 pagesThe Spectrum of A Single Photoionized Cloudchewbacca1409No ratings yet
- Unit 4 Lasers: Properties of A Laser Beam 1. CoherenceDocument17 pagesUnit 4 Lasers: Properties of A Laser Beam 1. CoherenceMeghana Chowdary ArumilliNo ratings yet
- Unit-1 Learning Objective Radiation Units Work EnergyDocument31 pagesUnit-1 Learning Objective Radiation Units Work EnergyManisha khanNo ratings yet
- 1 Introduction To Quantum Mechanics Unit IDocument12 pages1 Introduction To Quantum Mechanics Unit IyomamaNo ratings yet
- The Electron Density and Chemical Bonding in Organic Compounds by X-Ray DiffractionDocument58 pagesThe Electron Density and Chemical Bonding in Organic Compounds by X-Ray DiffractionJesus Isaac Castillo SanchezNo ratings yet
- Physics NotesDocument14 pagesPhysics NotesDeniz TopNo ratings yet
- Spectroscopy of Organic CompoundsDocument36 pagesSpectroscopy of Organic Compoundsnandhini_lgc0% (1)
- Negative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 2: Gravitational and Inertial Control, #2From EverandNegative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 2: Gravitational and Inertial Control, #2No ratings yet
- Negative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 3: Gravitational and Inertial Control, #3From EverandNegative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 3: Gravitational and Inertial Control, #3No ratings yet
- Infrared Spectroscopy ExplainedDocument5 pagesInfrared Spectroscopy ExplainedAhmadulhaqNo ratings yet
- 2.3 - How The Mass Spectrometer Works - Chemistry LibreTextsDocument3 pages2.3 - How The Mass Spectrometer Works - Chemistry LibreTextsAhmadulhaqNo ratings yet
- Cis-Trans and E-Z Isomerism - Pick Your Side - StereochemistryDocument8 pagesCis-Trans and E-Z Isomerism - Pick Your Side - StereochemistryAhmadulhaqNo ratings yet
- 2 - Mass Spectrometry - Chemistry LibreTextsDocument2 pages2 - Mass Spectrometry - Chemistry LibreTextsAhmadulhaqNo ratings yet
- Chemistry - Are Allenes Optically Active - QuoraDocument3 pagesChemistry - Are Allenes Optically Active - QuoraAhmadulhaqNo ratings yet
- Cis-Trans and E-Z Isomerism - Pick Your Side - StereochemistryDocument8 pagesCis-Trans and E-Z Isomerism - Pick Your Side - StereochemistryAhmadulhaqNo ratings yet
- Understanding Time-Resolved vs Frequency-Resolved SpectroscopyDocument5 pagesUnderstanding Time-Resolved vs Frequency-Resolved SpectroscopyAhmadulhaqNo ratings yet
- Password ManagementDocument7 pagesPassword ManagementNeerav KrishnaNo ratings yet
- Nettoplcsim S7online Documentation en v0.9.1Document5 pagesNettoplcsim S7online Documentation en v0.9.1SyariefNo ratings yet
- The Manning EquationDocument10 pagesThe Manning EquationFederico LeonNo ratings yet
- Lesson 2Document10 pagesLesson 2angeliquefaithemnaceNo ratings yet
- Sand Compaction MethodDocument124 pagesSand Compaction Methodisaych33ze100% (1)
- Factory Test Report For OPzS 800 EED-20041724 2VDocument3 pagesFactory Test Report For OPzS 800 EED-20041724 2VmaherNo ratings yet
- Ginglen 2022 - Necrotizing Enterocolitis - StatPearlsDocument8 pagesGinglen 2022 - Necrotizing Enterocolitis - StatPearlsBee GuyNo ratings yet
- Plant Disease Detection Using Deep LearningDocument5 pagesPlant Disease Detection Using Deep LearningIJRASETPublicationsNo ratings yet
- ASIA IVALUE Business ProfileDocument9 pagesASIA IVALUE Business ProfileDidiek PriambudiNo ratings yet
- Rose Jean AlvarezDocument15 pagesRose Jean AlvarezMika Ela Pantaleon Doria100% (1)
- S 1804 2019 (E) - 0Document9 pagesS 1804 2019 (E) - 0Juan Agustin CuadraNo ratings yet
- SocorexDocument6 pagesSocorexTedosNo ratings yet
- 2020.07.31 Marchese Declaration With ExhibitsDocument103 pages2020.07.31 Marchese Declaration With Exhibitsheather valenzuelaNo ratings yet
- CellphoneBill PDFDocument6 pagesCellphoneBill PDFRaza KhanNo ratings yet
- Neutron SourcesDocument64 pagesNeutron SourcesJenodi100% (1)
- 7 - NIBL - G.R. No. L-15380 Wan V Kim - DigestDocument1 page7 - NIBL - G.R. No. L-15380 Wan V Kim - DigestOjie SantillanNo ratings yet
- HNM Announcement Flyer enDocument2 pagesHNM Announcement Flyer enJali SahiminNo ratings yet
- Syllabus PTSV3Document21 pagesSyllabus PTSV3Pablito Quispe RuizNo ratings yet
- Concise Operating Instructions: Frequency Converter For HOISTING - TRAVEL (Siemens)Document9 pagesConcise Operating Instructions: Frequency Converter For HOISTING - TRAVEL (Siemens)Pablo Hidalgo ValenzuelaNo ratings yet
- Basketball 2011: Johnson CountyDocument25 pagesBasketball 2011: Johnson CountyctrnewsNo ratings yet
- Physical Science 1Document25 pagesPhysical Science 1EJ RamosNo ratings yet
- Nitobond SBR (Latex)Document4 pagesNitobond SBR (Latex)Samarakoon Banda100% (1)
- 4) April 2023 Current AffairsDocument24 pages4) April 2023 Current AffairsPicturesque vibrant shadesNo ratings yet
- Lecture Euler EquationDocument33 pagesLecture Euler EquationYash RajNo ratings yet
- 2.2valves, Alarm - Ul Product IqDocument1 page2.2valves, Alarm - Ul Product Iqbhima irabattiNo ratings yet
- Science MELCsDocument42 pagesScience MELCsRanjell Allain TorresNo ratings yet
- Nigeria Emergency Plan NemanigeriaDocument47 pagesNigeria Emergency Plan NemanigeriaJasmine Daisy100% (1)