Professional Documents
Culture Documents
Primary Syphilis in The Male Urethra: A Case Report
Primary Syphilis in The Male Urethra: A Case Report
Uploaded by
Alifia Ramadanty ArdinuriOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Primary Syphilis in The Male Urethra: A Case Report
Primary Syphilis in The Male Urethra: A Case Report
Uploaded by
Alifia Ramadanty ArdinuriCopyright:
Available Formats
Clinical Infectious Diseases
BRIEF REPORT
Primary Syphilis in the Male Urethra: Health—Seattle and King County Sexually Transmitted Disease
Clinic seeking evaluation of a 4-day history of urethral irrita-
A Case Report tion. He reported 5 sex partners (4 new) in the past 2 months,
Laura C. Chambers,1,a, Sujatha Srinivasan,2,a Sheila A. Lukehart,3,4 condomless insertive oral and anal sex at his last sexual episode,
Negusse Ocbamichael,5 Jennifer L. Morgan,5 M. Sylvan Lowens,5
David N. Fredricks,2,3 Matthew R. Golden,3,5 and Lisa E. Manhart1
and recent contact with HIV. He did not report urethral dis-
1
Department of Epidemiology, University of Washington, 2Fred Hutchinson Cancer Research
charge or dysuria, but acknowledged a 9-month history of “jock
Center, 3Department of Medicine and 4Department of Global Health, University of itch.” The clinician noted scant urethral discharge; erythema of
Washington, and 5Public Health—Seattle & King County HIV/STD Program, Washington
the meatus and distal urethra; a dry, itchy, macular rash with
We documented urethral Treponema pallidum infection in a well-demarcated borders on the inner thighs and groin crease;
man with nongonococcal urethritis and a negative syphilis and normal inguinal lymph nodes. First-void urine, 2 urethral
serology using broad-range bacterial polymerase chain reaction swab specimens, and a serum specimen were collected. On a
(PCR) and sequencing, targeted PCR, and immunofluorescence Gram-stained slide of urethral exudates, the clinician observed
microscopy. He subsequently seroconverted for syphilis. Early no Gram-negative intracellular diplococci (GNID), but >20
syphilis may present as urethritis. Urethral T. pallidum shed- polymorphonuclear leukocytes (PMNs) per high-power field
ding can occur before seroconversion. (HPF). The patient was diagnosed with NGU and tinea cruris,
Keywords. 16S rRNA gene PCR; nongonococcal urethritis; provided azithromycin (1g) for the NGU, and advised to use
primary syphilis; Treponema pallidum. an over-the-counter antifungal for the tinea cruris. Nucleic acid
amplification tests (NAATs) for urethral Chlamydia trachoma-
The incidence of syphilis has increased markedly among men tis, Neisseria gonorrhoeae, and Mycoplasma genitalium were
who have sex with men (MSM) in many urban areas of high-in- performed on the urine sample (Aptima; Hologic, Inc.; San
come nations. In 2015, the incidence of primary and secondary Diego). A rapid plasma reagin (RPR) test to screen for syphilis
syphilis among MSM in the United States was approximately was performed on the serum specimen (Macro-Vue, Becton,
309 cases per 100 000 persons [1]. In King County, Washington, Dickson, and Company, Franklin Lakes). All tests were neg-
the incidence among MSM doubled from 450 to 901 cases per ative. The remaining urine and a dry urethral swab specimen
100 000 persons between 2005 and 2015 [2]. were frozen for future testing.
With increases in syphilis cases, clinicians may see rare pres- Although the study consisted of a single visit, the patient
entations more frequently. This report describes a case of non- returned for 4 additional non-study visits due to continued
gonococcal urethritis (NGU) in which Treponema pallidum urethral symptoms (12, 15, and 25 days after the initial NGU
was the only known pathogen noted. The patient had enrolled diagnosis) and non-urethral complaints (54 days after the initial
in a cross-sectional study on the etiology of NGU that uti- NGU diagnosis). On day 12, he reported that his urethral symp-
lized broad-range 16S ribosomal RNA (rRNA) gene polymer- toms had resolved within 1 week of azithromycin therapy but
ase chain reaction (PCR) with sequencing to characterize the had returned 2 days earlier (day 10) and worsened to include
male urethral microbiota. Detection of T. pallidum by PCR and urethral irritation, urethral itch, and dysuria. His partner had
sequencing alerted us to this case. been treated as a contact to NGU (antibiotic and partner[s]
unknown), and he had resumed sporadic sexual activity on day
CLINICAL NARRATIVE 7 (condomless penile and oral exposures). The clinician noted
scant, clear urethral discharge; a very red and inflamed urethra;
A 28-year-old, non-Hispanic, White, human immunodeficiency
a red glans penis; a dry, macular groin rash; and bilaterally-en-
virus (HIV)-negative, circumcised MSM presented to the Public
larged inguinal lymph nodes. A urethral Gram stain revealed
>20 PMNs/HPF, but no GNID. The patient was diagnosed with
Received 26 July 2018; editorial decision 30 August 2018; accepted 5 September 2018 ; recurrent NGU and prescribed a 14-day course of moxifloxacin.
published online September 10, 2018.
a
L. C. C. and S. S. contributed equally to this manuscript.
NAATs of urethral specimens for the herpes simplex virus 1 and
Presented in part: The 2018 Annual Meeting of the Sexually Transmitted Infections 2 (Solana HSV 1 + 2/VZV, Quidel, San Diego) and an adeno-
Cooperative Research Centers (STI-CRC), Baltimore, Maryland, 23–24 May 2018. virus culture were negative.
Correspondence: L. C. Chambers, Department of Epidemiology, University of Washington,
325 Ninth Avenue, HMC #359931, Seattle, WA 98104 (lauracc@uw.edu). On day 15, the patient returned with gastrointestinal dis-
Clinical Infectious Diseases® 2019;68(7):1231–4 comfort and persistent urethral symptoms, including urethral
© The Author(s) 2018. Published by Oxford University Press for the Infectious Diseases Society
of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
discharge. He had discontinued moxifloxacin after 2 days due
DOI: 10.1093/cid/ciy771 to gastrointestinal symptoms and requested azithromycin. The
BRIEF REPORT • CID 2019:68 (1 April) • 1231
clinician noted scant, clear urethral discharge; a persistent rash and an enzyme immunoassay (CAPTIA Syphilis IgG, Bio-Rad
consistent with tinea cruris; and bilaterally-enlarged inguinal Laboratories, Inc., Hercules) was non-reactive. On day 54, the
lymph nodes. A urethral Gram stain had 11 PMNs/HPF and patient returned with non-urethral complaints. He did not
no GNID. The patient was diagnosed with persistent NGU and report urethral symptoms and did not have visible urethral dis-
provided azithromycin (1 g). charge. A repeat RPR titer was 1:8; a T. pallidum particle agglu-
On day 25, the patient returned with worsening urethral tination assay was reactive; and an enzyme immunoassay was
symptoms and a 3-day history of painful penile swelling. The equivocal.
clinician noted a moderate amount of clear urethral discharge,
a swollen distal penis shaft, dorsal vein and urethral indura-
RETROSPECTIVE LABORATORY WORK
tion, and a firm left inguinal lymph node. No genital or oral
lesions were noted. An on-site RPR test was reactive for syphi- As part of the NGU study, we applied broad-range 16S rRNA
lis. Motile spirochetes were observed in a drop of urethral dis- gene PCR targeting the V3-V4 region, with deep sequencing [3],
charge by darkfield microscopy in the clinic. The patient was to the urine specimen from the initial NGU visit. We detected
diagnosed with primary syphilis in the urethra and treated with 3989 T. pallidum sequence reads at 6% relative abundance
intramuscular benzathine penicillin G (single dose; 2.4 million (Figure 1). No other known pathogens were noted. The major-
units). The RPR titer was 1:16; a T. pallidum particle aggluti- ity of sequences represent skin commensal bacteria, including
nation assay (Serodia, Fujirebio, Inc., Tokyo) was reactive; Corynebacterium and Dermabacter species. Amplification of
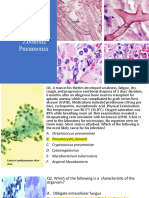
Figure 1. Evidence of urethral Treponema pallidum infection in specimens collected at the initial nongonococcal urethritis visit. A, Composition of the urethral microbiota
(relative abundances) in a first-void urine specimen. B, Treponemes visualized with fluorescence microscopy of a smear prepared from a urethral swab specimen.
1232 • CID 2019:68 (1 April) • BRIEF REPORT
2 T. pallidum gene targets, tp0548 and the 47-kDa lipoprotein using PCR during primary syphilis, in some cases prior to sero-
(tp0574) [4], confirmed the presence of the T. pallidum subspe- conversion [12]. PCR testing is typically conducted on swabs
cies pallidum. A restriction digestion analysis targeting both obtained from primary chancres or moist secondary lesions.
23S ribosomal DNA alleles [5] revealed an A2058G mutation, However, limited evidence of mucosal T. pallidum shedding in
which confers azithromycin resistance. Finally, indirect immu- the absence of positive serological tests suggests that there may
nofluorescence microscopy, using pooled T. pallidum–infected be a role for targeted NAAT of mucosal swab specimens from
rabbit sera [6] on a smear prepared from the urethral swab from persons at very high risk of syphilis. This requires further study.
the initial NGU visit, revealed numerous treponemes (Figure 1). Our report has limitations. The patient had only 1 research
visit. Specimens from subsequent visits were not available to
DISCUSSION determine the ongoing presence of T. pallidum nucleic acid in
This patient, diagnosed with NGU, had a negative syphi- his urethra. Moreover, the patient saw a different clinician at
lis serology but evidence of urethral T. pallidum infection each visit, with potential variations in the descriptions of clin-
by broad-range 16S rRNA gene PCR and sequencing, T. pal- ical findings. Finally, the patient reported condomless expo-
lidum–specific PCR, and immunofluorescence microscopy, sures during this time period. The possibility of re-infection
highlighting the urethra as the site of an active, clinically-sig- cannot be ruled out.
nificant early syphilis infection and a potential source of In conclusion, primary syphilis presenting as NGU may be
transmission, even in a seronegative man. Persistent, worsen- seen more often as the incidence of syphilis increases. Our data
ing urethritis-like symptoms in this patient despite receiving suggest that syphilis may be an occasional cause of urethritis.
azithromycin therapy are likely explained by the presence of Future research on the frequency of mucosal T. pallidum shed-
a macrolide-resistant mutation in his T. pallidum strain. This ding during early syphilis would inform our understanding of
patient’s NGU resolved after the receipt of intramuscular ben- the natural history of syphilis and the potential utility of pairing
zathine penicillin G, further supporting T. pallidum as the serological screening with PCR testing to optimize diagnoses
cause of his urethral symptoms. and reduce transmission.
The presence of T. pallidum in the patient’s urethral exudates
could have resulted from a urethral chancre that was not visible Notes
Author contributions. The manuscript concept was developed by L. C.
on clinical examination or could have been a consequence of
C., S. S., S. A. L., D. N. F., M. R. G., and L. E. M. Clinical data were compiled
mucosal shedding of the infection. An estimated 1% of primary for the manuscript by L. C. C., J. L. M., and M. S. L. Oversight of the clin-
syphilis chancres develop at the urethral meatus [7], though, ical care described in the manuscript was provided by N. O. Laboratory
to our knowledge, primary syphilis presenting as NGU in the work for the manuscript was conducted by S. S., D. N. F., and S. A. L. The
manuscript was drafted by L. C. C. and S. S. The manuscript was critically
absence of a visible chancre has not been reported. In a report revised by all authors.
from 1891, a few cases of intra-urethral primary chancre of the Acknowledgments. The authors thank the study participant; Sean Proll
fossa navicularis (dilated portion of the urethra within the glans for assistance with the figures; and the Public Health—Seattle and King
County Sexually Transmitted Disease Clinic staff. They thank Sylvia Berry,
penis) were described [8], but we did not identify any subse- Eduardo Munoz, and Dwyn Dithmer-Schreck for their contributions to the
quent reports of this clinical presentation. clinical care of the patient and Matthew Munch, Dan Domogala, Charmie
A recent report suggested that mucosal surfaces may shed Godornes, and Barbara Molini for laboratory contributions. Finally, they
thank Hologic, Inc. for donation of test kits and reagents.
T. pallidum DNA in the absence of a visible primary chancre or
Financial support. This work was supported by the National Institutes
secondary lesions [9]. However, detection of T. pallidum DNA of Health National Institute of Allergy and Infectious Diseases (funder ID
at these sites by PCR alone is not equivalent to demonstrating 100000060, grant number U19 AI113173) and the National Center for
the presence of viable, infectious organisms. Nevertheless, our Advancing Translational Sciences (funder ID 100006108, grant number
TL1 TR002318 trainee support to L. C. C.).
patient had clearly-identifiable, motile T. pallidum organisms in Potential conflicts of interest. M. R. G. received grants from Hologic, Inc.
his urethral discharge, indicating viable bacteria were present. and GlaxoSmithKline outside of the submitted work. L. E. M. has received
Direct mucosal shedding of T. pallidum may be possible in some donations of test kits and reagents from Hologic, Inc. All other authors report
no potential conflicts. All authors have submitted the ICMJE Form for
cases, and this would have implications for our understanding Disclosure of Potential Conflicts of Interest. Conflicts that the editors con-
of the natural history and transmission of syphilis. Sexual trans- sider relevant to the content of the manuscript have been disclosed.
mission is generally thought to require direct contact between
a primary chancre or secondary lesion and a partner’s mucosal References
surface or skin site with microabrasions. 1. de Voux A, Kidd S, Grey JA, et al. State-specific rates of primary and secondary
Serological testing remains the most common method of syphilis among men who have sex with men—United States, 2015. MMWR Morb
Mortal Wkly Rep 2017; 66:349–54.
syphilis diagnosis. However, RPR is only 70–80% sensitive in 2. Public Health—Seattle and King County. 2015 King County sexually transmitted
primary syphilis [10, 11], T. pallidum–specific serological tests diseases epidemiology report. Seattle, Washington: Public Health—Seattle and
King County, 2015. Available at: https://www.kingcounty.gov/depts/health/com-
are variably sensitive in early infection [11], and increasing evi- municable-diseases/hiv-std/patients/epidemiology/~/media/depts/health/com-
dence suggests that T. pallidum nucleic acids can be detected municable-diseases/documents/hivstd/2015-STD-Epidemiology-Report.ashx
BRIEF REPORT • CID 2019:68 (1 April) • 1233
3. Golob JL, Pergam SA, Srinivasan S, et al. Stool microbiota at neutrophil recovery 8. White JM. Syphilis. In: Sajous CE, ed. Annual of the universal medical sciences
is predictive for severe acute graft vs host disease after hematopoietic cell trans- and analytical index: a yearly report of the progress of the general sanitary sciences
plantation. Clin Infect Dis 2017; 65:1984–91. throughout the world. Vol. 3. Philadelphia, Pennsylvania: F.A. Davis, 1891:F1–44.
4. Godornes C, Giacani L, Barry AE, Mitja O, Lukehart SA. Development of a mul- 9. Yang CJ, Chang SY, Wu BR, et al. Unexpectedly high prevalence of Treponema
tilocus sequence typing (MLST) scheme for Treponema pallidum subsp. pertenue: pallidum infection in the oral cavity of human immunodeficiency virus-infected
application to yaws in Lihir Island, Papua New Guinea. PLoS Negl Trop Dis 2017; patients with early syphilis who had engaged in unprotected sex practices. Clin
11:e0006113. Microbiol Infect 2015; 21:787.e1–7.
5. Lukehart SA, Godornes C, Molini BJ, et al. Macrolide resistance in Treponema 10. Dang Q, Feng J, Lu X, et al. Evaluation of specific antibodies for early diagnosis
pallidum in the United States and Ireland. N Engl J Med 2004; 351:154–8. and management of syphilis. Int J Dermatol 2006; 45:1169–71.
6. Handsfield HH, Lukehart SA, Sell S, Norris SJ, Holmes KK. Demonstration of 11. Larsen SA, Steiner BM, Rudolph AH. Laboratory diagnosis and interpretation of
Treponema pallidum in a cutaneous gumma by indirect immunofluorescence. tests for syphilis. Clin Microbiol Rev 1995; 8:1–21.
Arch Dermatol 1983; 119:677–80. 12. Read PJ, Guy R, Jeoffreys N, Baker D, Shields M, Donovan B. Treatment and out-
7. Mindel A, Tovey SJ, Timmins DJ, Williams P. Primary and secondary syphilis, comes of polymerase chain reaction-confirmed early syphilis. Sex Health 2015;
20 years’ experience. 2. Clinical features. Genitourin Med 1989; 65:1–3. 12:506–11.
1234 • CID 2019:68 (1 April) • BRIEF REPORT
You might also like
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5813)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (844)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (348)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1092)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Mayo Clinic CardiologyDocument1,606 pagesMayo Clinic CardiologyDunareanu Ana-alexandra89% (18)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Ovarian CystDocument26 pagesOvarian CystAlmyr RimandoNo ratings yet
- Repertory of Bowel Nosodes PDF FreeDocument8 pagesRepertory of Bowel Nosodes PDF FreeFuente DelavidaNo ratings yet
- Human Reproductive System 5esDocument10 pagesHuman Reproductive System 5esJoseph Joel AlmagroNo ratings yet
- Vandhyatva: (According To Ayurveda)Document13 pagesVandhyatva: (According To Ayurveda)INSTA SHORTSNo ratings yet
- ExosomesDocument18 pagesExosomesMahmood-S ChoudheryNo ratings yet
- Herpes Simplex VirusDocument39 pagesHerpes Simplex VirusWenalyn Grace Abella LlavanNo ratings yet
- Visit Report of DHQDocument2 pagesVisit Report of DHQUsman DastgirNo ratings yet
- Thymol Inhibits LPS-Stimulated In flammatory Response via Down-Regulation of NF-κB and MAPK Signaling Pathways in Mouse Mammary Epithelial CellsDocument9 pagesThymol Inhibits LPS-Stimulated In flammatory Response via Down-Regulation of NF-κB and MAPK Signaling Pathways in Mouse Mammary Epithelial CellsGianluca FrancoNo ratings yet
- EnuresisDocument4 pagesEnuresisDalene KirstenNo ratings yet
- Novel Tretinoin 0.05% Lotion For The Once-Daily Treatment of Moderate-To-Severe Acne Vulgaris: Assessment of Safety and Tolerability in SubgroupsDocument9 pagesNovel Tretinoin 0.05% Lotion For The Once-Daily Treatment of Moderate-To-Severe Acne Vulgaris: Assessment of Safety and Tolerability in SubgroupsFika Riskiah IskandarNo ratings yet
- Vystavka 201809Document35 pagesVystavka 201809prabhat kumarNo ratings yet
- Mental Retardation or Intellectual DisabilityDocument15 pagesMental Retardation or Intellectual DisabilityMelody ZambranoNo ratings yet
- Endodontic File Separation WordDocument5 pagesEndodontic File Separation WordGeorgiana ValeanuNo ratings yet
- Consent For Diagnostic And/or Therapeutic ParacentesisDocument2 pagesConsent For Diagnostic And/or Therapeutic ParacentesisnaveenNo ratings yet
- Interview With DR Kenneth P Stoller Hyperbaric Oxy PDFDocument11 pagesInterview With DR Kenneth P Stoller Hyperbaric Oxy PDFJoyce MulengaNo ratings yet
- Analisis ClinicosDocument6 pagesAnalisis Clinicosdr_joe23No ratings yet
- Specimen Collection Manual For Laboratory Serivces LRHDocument100 pagesSpecimen Collection Manual For Laboratory Serivces LRHPasan MaheshaNo ratings yet
- Benign Disease of The UterusDocument27 pagesBenign Disease of The UterusnyangaraNo ratings yet
- Bacterial Modification and Cancer TherapyDocument7 pagesBacterial Modification and Cancer TherapyAndrés Felipe Galindo MantillaNo ratings yet
- Modified Micro Marsupialization in Pediatric Patients: A Minimally Invasive TechniqueDocument4 pagesModified Micro Marsupialization in Pediatric Patients: A Minimally Invasive TechniquerinahpsNo ratings yet
- CV NyeriDocument1 pageCV NyeriMuhammad YunusNo ratings yet
- Comparative Study of Nifedipine and Isoxpurine As Tocolytics For Preterm LaborDocument4 pagesComparative Study of Nifedipine and Isoxpurine As Tocolytics For Preterm LaborMeitika Wahyu Wedha WatiNo ratings yet
- C Arms Cios Alpha Connect Select Fusion Mobile C Arm Machine Cios Family Flyer 02524075Document8 pagesC Arms Cios Alpha Connect Select Fusion Mobile C Arm Machine Cios Family Flyer 02524075Imam HidayatNo ratings yet
- I Need To Know About Fresh Frozen Plasma: BloodDocument1 pageI Need To Know About Fresh Frozen Plasma: BloodHumberto OmaresNo ratings yet
- Gop-020a Global Infectious Disease Outbreak Preparedness PDFDocument10 pagesGop-020a Global Infectious Disease Outbreak Preparedness PDFLakshmi BalaNo ratings yet
- Esmee Sabina 19-5372 PDFDocument3 pagesEsmee Sabina 19-5372 PDFEsme SabinaNo ratings yet
- GRAT - Fungal, HAI - Zoonotic InfectionsDocument13 pagesGRAT - Fungal, HAI - Zoonotic InfectionsDaniel Del RiscoNo ratings yet
- Manejo de La Migraña Crónica. Review 2022Document19 pagesManejo de La Migraña Crónica. Review 2022Edwin Fabian Paz UrbanoNo ratings yet
- 1 Revised Food Borne Illness Lecture 1Document5 pages1 Revised Food Borne Illness Lecture 1Tarequl Islam NishadNo ratings yet