67% found this document useful (3 votes)
2K views33 pagesMICROMERITICS
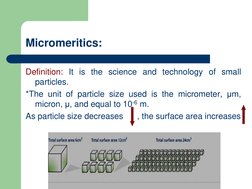
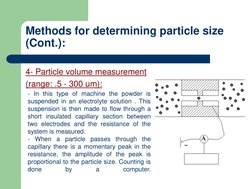
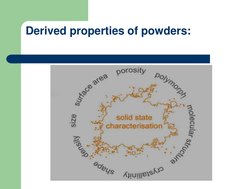
The document discusses micromeritics which is the science of small particles and how their size, shape, and surface area can impact properties like dissolution, absorption, stability, and flowability. Several methods for measuring particle size are described ranging from microscopy to sieving to sedimentation. Factors that influence powder flow properties like size, shape, surface forces, and additives are also outlined.
Uploaded by
sandymhCopyright
© Attribution Non-Commercial (BY-NC)
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
67% found this document useful (3 votes)
2K views33 pagesMICROMERITICS
The document discusses micromeritics which is the science of small particles and how their size, shape, and surface area can impact properties like dissolution, absorption, stability, and flowability. Several methods for measuring particle size are described ranging from microscopy to sieving to sedimentation. Factors that influence powder flow properties like size, shape, surface forces, and additives are also outlined.
Uploaded by
sandymhCopyright
© Attribution Non-Commercial (BY-NC)
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
- Micromeritics