Professional Documents
Culture Documents
Friedel-Crafts Reactions - Alkylation and Acylation
Friedel-Crafts Reactions - Alkylation and Acylation
Uploaded by
ahumanbeinginearthOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Friedel-Crafts Reactions - Alkylation and Acylation
Friedel-Crafts Reactions - Alkylation and Acylation
Uploaded by
ahumanbeinginearthCopyright:
Available Formats
Friedel-Crafts reactions 1
FRIEDEL-CRAFTS REACTIONS - AN OVERVIEW
General
thoughts • Friedel-Crafts reactions involve electrophilic substitution of aromatic rings
• there are two types - Alkylation and Acylation
• alkylation involves the substitution of alkyl groups such as CH3, C2H5 and C3H7
• acylation involves the substitution of acyl groups such as CH3C=O
• in both cases a catalyst is needed
• this is because the attacking species isn’t a strong enough electrophile
• it hasn’t enough positive character to persuade benzene to react
• haloalkanes and acyl chlorides have polar bonds but the C isn’t positive enough
Oδ−
δ+ δ− δ+ δ−
CH3 CH2 Cl CH 3 C Cl
a haloalkane an acyl chloride
• the catalyst makes the attacking species more positive
• anhydrous aluminium chloride is the catalyst
• it works because it is a Lewis acid
• in AlCl3 the aluminium is electron deficient - it has 6 electrons in its outer shell
Knockhardy Publishing
• in both cases the reagent has a polar C-Cl bond
• the carbon atom has a δ+ charge but it isn’t enough to tempt the benzene
• the aluminium chloride increases the charge so that benzene become interested
Action
of AlCl3 • the aluminium atom is electron deficient with only 6 in its outer shell
• it acts as a Lewis acid as it can accept a lone pair to make up its octet
Cl Cl
Cl Al Cl Al Cl
Cl Cl
BEFORE AFTER
incomplete octet complete octet
trigonal planar shape tetrahedral shape
• it can do this by attracting a chlorine atom away from a C-Cl bond
• the more the Cl is attracted by the AlCl3 the more polar the C-Cl bond gets
• in the extreme case it pulls the chlorine right off leaving a C+ behind
Alkylation RCl + AlCl3 AlCl4¯ + R+
Acylation RCOCl + AlCl3 AlCl4¯ + RCO+
• the aromatic ring will now attack and electrophilic substitution takes place
© KNOCKHARDY SCIENCE 2015
2 Friedel-Crafts reactions
Alkylation substitutes an alkyl (e.g. methyl, ethyl) group
reagents a haloalkane (RX) and anhydrous aluminium chloride AlCl3
conditions room temperature; dry inert solvent (ether)
electrophile a carbocation R+ (e.g. CH3+)
equation C6H6 + C2H5Cl ——> C6H5C2H5 + HCl
mechanism
CH 2 CH 3 CH2 CH 3 CH 2 CH 3
H
+ + H
Industrial
method The industrial preparation of similar compounds is slightly different
Alkenes are used instead of haloalkanes - see other notes
Knockhardy Publishing
Acylation substitutes an acyl (e.g. ethanoyl) group
the aluminium chloride catalyst acts in the same as with alkylation
reagents an acyl chloride (RCOCl) and anhydrous AlCl3
conditions reflux 50°C; dry inert solvent (ether)
electrophile RC+= O ( e.g. CH3C+O )
product carbonyl compound (aldehyde or ketone)
equation C6H6 + CH3COCl ——> C6H5COCH3 + HCl
mechanism
O
O O
C CH3
C CH3 C CH3
H
+ + H
© KNOCKHARDY SCIENCE 2015
You might also like
- Design of Cumene Producing PlantDocument57 pagesDesign of Cumene Producing PlantAylin Uçar89% (18)
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- Organic Chemistry Multiple Choice QuestionsDocument4 pagesOrganic Chemistry Multiple Choice QuestionsRonald Angelo LopezNo ratings yet
- Lectures Ch4 Ns PDFDocument85 pagesLectures Ch4 Ns PDFPatricio VillarrealNo ratings yet
- Friedel & Crafts: CHEM2401, CHEM2911, CHEM2915Document23 pagesFriedel & Crafts: CHEM2401, CHEM2911, CHEM2915Kage InuNo ratings yet
- 4alkyl Halides and AlcoholsDocument85 pages4alkyl Halides and AlcoholssharmimiameerasanadyNo ratings yet
- Notes Alkanes +alkenesDocument44 pagesNotes Alkanes +alkenesLucas “Khumalo” KaunduNo ratings yet
- Organic Chemistry - Alkanes: Hydrocarbons (Compounds Containing Only C and H)Document6 pagesOrganic Chemistry - Alkanes: Hydrocarbons (Compounds Containing Only C and H)Jojo LeongNo ratings yet
- Asil Halida (Halida Asam)Document22 pagesAsil Halida (Halida Asam)9062Hasnan KurniawanNo ratings yet
- 6 8 Acyl Chlorides and Acid AnhydridesDocument5 pages6 8 Acyl Chlorides and Acid AnhydridesPedro Moreno de SouzaNo ratings yet
- CHAPTER 6 Alkyl Halides and Aryl HalidesDocument150 pagesCHAPTER 6 Alkyl Halides and Aryl HalidesexpertwritersNo ratings yet
- Hydrocarbon NotesDocument187 pagesHydrocarbon Notessamay gujratiNo ratings yet
- 5 Alkyl HalideDocument53 pages5 Alkyl Haliderusnah chungNo ratings yet
- Lecture CycloalkanesDocument21 pagesLecture CycloalkanesDain GutierrezNo ratings yet
- Alkyl HalidesDocument15 pagesAlkyl HalidesDjdj DjdjNo ratings yet
- Alkyl Halides, Alocholes, Phenols, Ethers, AminesDocument11 pagesAlkyl Halides, Alocholes, Phenols, Ethers, AminesMohammadHussainKhanNo ratings yet
- Haloalkanes & HaloarenesDocument8 pagesHaloalkanes & Haloarenesrajtarabap55No ratings yet
- Alkyle Halides Full ChapterDocument13 pagesAlkyle Halides Full Chapterwajid123No ratings yet
- Acyl Chloride PresentationDocument20 pagesAcyl Chloride PresentationGanga Jones Manodon DucyogenNo ratings yet
- Structure of Aldehydes and KetonesDocument42 pagesStructure of Aldehydes and KetonesPaul Jhon EugenioNo ratings yet
- Alkyl HalideDocument54 pagesAlkyl HalideNelvianaNo ratings yet
- Halogenoalkanes, Nucleophilic Substitution, Elimination Reactions, Uses and CFC Problems PDFDocument7 pagesHalogenoalkanes, Nucleophilic Substitution, Elimination Reactions, Uses and CFC Problems PDFGrace KamauNo ratings yet
- AlkanesDocument28 pagesAlkanesLeticia0% (1)
- The Chemistry of HaloalkanesDocument42 pagesThe Chemistry of HaloalkanesMervinboNo ratings yet
- Carbonyl Compounds Aldehydes and Ketones2Document6 pagesCarbonyl Compounds Aldehydes and Ketones2Sachitra WijethungaNo ratings yet
- Chapter 6 Nucleophilic Substitution On Saturated CarbonsDocument46 pagesChapter 6 Nucleophilic Substitution On Saturated CarbonsNUR HAZWANI BINTI MOHAMAD SANI / UPMNo ratings yet
- Haloalkanes Haloarnes NotesDocument44 pagesHaloalkanes Haloarnes Noteshareharanbt22No ratings yet
- Lecture-1 Alkyl Halide DinushaDocument44 pagesLecture-1 Alkyl Halide DinushaNadunKodikaraNo ratings yet
- Alkynes: Organic ChemistryDocument45 pagesAlkynes: Organic ChemistrySaima NajmNo ratings yet
- Formula Sheet Hydrocarbons 1Document6 pagesFormula Sheet Hydrocarbons 1unknownqueen714No ratings yet
- Ch8 Elimination ReactionDocument25 pagesCh8 Elimination Reactionsitiaisyah harizamrryNo ratings yet
- Carboxylic Acid and Their DerivatesDocument10 pagesCarboxylic Acid and Their Derivatesvita iftitahiyahNo ratings yet
- Anic Chemistry Alkyl HalidesDocument15 pagesAnic Chemistry Alkyl Halideseamcetmaterials100% (6)
- Friedel-Crafts Alkylation: (X CL, BR, I)Document2 pagesFriedel-Crafts Alkylation: (X CL, BR, I)kalloliNo ratings yet
- Name Eactions FinalDocument33 pagesName Eactions FinalAli Akand AsifNo ratings yet
- Chapter 11 Alkynes: Lecture Notes Chem 51B S. KingDocument12 pagesChapter 11 Alkynes: Lecture Notes Chem 51B S. KingHuấnĐìnhNguyễnNo ratings yet
- Halo Alkanes & HaloarenesDocument30 pagesHalo Alkanes & HaloarenesEarNo ratings yet
- Alkyl Halides & Aryl Halides-01 - TheoryDocument32 pagesAlkyl Halides & Aryl Halides-01 - TheoryRaju SinghNo ratings yet
- Overview of The Reactions of Carbonyl Compounds: - Topical Outline of CoverageDocument54 pagesOverview of The Reactions of Carbonyl Compounds: - Topical Outline of CoverageveronashaqNo ratings yet
- Chapter 2-2Document29 pagesChapter 2-2AdellNo ratings yet
- Ketones and Aldehydes: Organic ChemistryDocument56 pagesKetones and Aldehydes: Organic ChemistryHezron BumbunganNo ratings yet
- Formation of C-C Bonds by Acid-Catalyzed CondensationsDocument21 pagesFormation of C-C Bonds by Acid-Catalyzed CondensationsThabiso GwijiNo ratings yet
- Chapter 2 Alkanes - 2Document49 pagesChapter 2 Alkanes - 2farah amaniNo ratings yet
- chm207 chp5Document92 pageschm207 chp5Muhd Mirza HizamiNo ratings yet
- 10장Document37 pages10장sungyeon heoNo ratings yet
- Chapter 8: Chemistry of Alkynes (C H) Bonding & HybridizationDocument11 pagesChapter 8: Chemistry of Alkynes (C H) Bonding & HybridizationimPERFECTme09No ratings yet
- Hydro CarbonsDocument133 pagesHydro Carbonsanikesh JainNo ratings yet
- Halogen Compounds NarayanaDocument107 pagesHalogen Compounds NarayanaVindya Vahini Namala100% (1)
- Acyl ChloridesDocument20 pagesAcyl ChloridesGiannaNo ratings yet
- Carbonyl Compounds1Document25 pagesCarbonyl Compounds1Lyana TaylorNo ratings yet
- ORsMs L04 - Fall 2022Document14 pagesORsMs L04 - Fall 2022Yousef EssamNo ratings yet
- Chemistry 202: Reactions of Alkenes, Part 1Document28 pagesChemistry 202: Reactions of Alkenes, Part 1CindyNo ratings yet
- P1 AlkenesDocument74 pagesP1 AlkenesShirl Angelee OcampoNo ratings yet
- Thing To Remember - Haloalkane - StudentsDocument10 pagesThing To Remember - Haloalkane - StudentspoornaNo ratings yet
- Carbonyl ReactionDocument34 pagesCarbonyl Reactionmichot feleguNo ratings yet
- Alkyl HalideDocument30 pagesAlkyl HalideMalaika KhalidNo ratings yet
- Functional GR Analysis - Alkanes and AlkenesDocument9 pagesFunctional GR Analysis - Alkanes and AlkenesMakeedaNo ratings yet
- Reactions of AlcoholsDocument23 pagesReactions of Alcoholsinushanth inuNo ratings yet
- Case Based Question Haloalkanes HaloarenesDocument3 pagesCase Based Question Haloalkanes HaloarenesFake GamerNo ratings yet
- Aldehydes and Ketones - Properties, Reactions, Identification and 2,4-DNP PDFDocument9 pagesAldehydes and Ketones - Properties, Reactions, Identification and 2,4-DNP PDFStephenNo ratings yet
- Chapter 6: Organohalogens: Alkyl Halide Vinyl Halide Aryl HalideDocument13 pagesChapter 6: Organohalogens: Alkyl Halide Vinyl Halide Aryl HalidecikguhafidzuddinNo ratings yet
- Alkanes 2Document14 pagesAlkanes 2Hasen umerNo ratings yet
- Unit 6 - Review Worksheet AnswersDocument9 pagesUnit 6 - Review Worksheet AnswersDương Thị Ngọc HiềnNo ratings yet
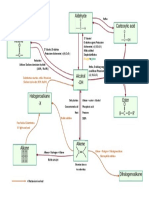
- As Chemistry Organic MindmapDocument1 pageAs Chemistry Organic MindmapDương Thị Ngọc HiềnNo ratings yet
- Carboxylic Acids and DerivativesDocument9 pagesCarboxylic Acids and DerivativesDương Thị Ngọc HiềnNo ratings yet
- 0654 (Chemistry) ChecklistDocument6 pages0654 (Chemistry) ChecklistDương Thị Ngọc Hiền0% (1)
- Chapter 16 Lecture NotesDocument15 pagesChapter 16 Lecture NotesJSGINo ratings yet
- Atomic Structure Electron Configuration QsDocument30 pagesAtomic Structure Electron Configuration QsJesulayomi BolajiNo ratings yet
- Design ReportDocument193 pagesDesign ReportNhut Nguyen100% (1)
- MCQ Chemistry PDFDocument19 pagesMCQ Chemistry PDFV Srinivasa RaoNo ratings yet
- Aluminium: Aluminium (Aluminum in American andDocument29 pagesAluminium: Aluminium (Aluminum in American andVysakh VasudevanNo ratings yet
- 2 - Group 13Document11 pages2 - Group 13Mahin ChandwaniNo ratings yet
- Inorganic Chemistry (Savemyexams)Document44 pagesInorganic Chemistry (Savemyexams)Farhan SadiqueNo ratings yet
- Preparation of Complex Salts and Double Salt A. BackgroundDocument7 pagesPreparation of Complex Salts and Double Salt A. BackgroundMaipha DeapatiNo ratings yet
- Poultry and Hatchery Farming (4116)Document9 pagesPoultry and Hatchery Farming (4116)eiribooksNo ratings yet
- EAS NotesDocument39 pagesEAS NotesSudhanshu HedaNo ratings yet
- Organic Chemistry Named Reaction InDetail by MeritnationDocument3 pagesOrganic Chemistry Named Reaction InDetail by Meritnationraohimesh94No ratings yet
- Polymer ChemistryDocument47 pagesPolymer ChemistryBapu ThoratNo ratings yet
- 525 1 Eot 3 2022Document17 pages525 1 Eot 3 2022OTTO OLIMANo ratings yet
- Group 13 AluminiumDocument48 pagesGroup 13 AluminiumLooi Chui Yean100% (1)
- Organic Chemistry II Problem Set Reaction of Substituted BenzeneDocument4 pagesOrganic Chemistry II Problem Set Reaction of Substituted BenzenesaddamixoNo ratings yet
- Specalist Process Report For Purification of ChorosilanesDocument8 pagesSpecalist Process Report For Purification of Chorosilanesdileepkumarsaidu1985No ratings yet
- Aromatic CompoundsDocument32 pagesAromatic CompoundsAmanMittal25100% (2)
- C8 - Chemical AnalysisDocument47 pagesC8 - Chemical AnalysisMr Twum. Yep that’s meNo ratings yet
- 13 THDocument10 pages13 THAman9692No ratings yet
- Organic Reaction: Addition Substitution Elimination RearrangementDocument39 pagesOrganic Reaction: Addition Substitution Elimination RearrangementJulJayaNo ratings yet
- Resonance DPPDocument6 pagesResonance DPPshambhavi26100% (2)
- Aldehyde KetonesDocument64 pagesAldehyde KetonesDebayanbasu.juNo ratings yet
- ArenesDocument4 pagesArenesNkemzi Elias NzetengenleNo ratings yet
- Organic Chemistry Final NotesDocument30 pagesOrganic Chemistry Final Notespawan khandweNo ratings yet
- Chemical Resistance (LLDPE) PDFDocument24 pagesChemical Resistance (LLDPE) PDFrubyshreeNo ratings yet
- 2023 H2 Chemical Equilibria Tutorial (QP)Document15 pages2023 H2 Chemical Equilibria Tutorial (QP)nivind88No ratings yet
- AluminioDocument14 pagesAluminiobrauliocoroNo ratings yet
- Multiple Choice Questions: Question Bank Class: Xii, Chemistry Unit 4: Haloalknaes & HaloarenesDocument27 pagesMultiple Choice Questions: Question Bank Class: Xii, Chemistry Unit 4: Haloalknaes & HaloarenesAkshita BoroNo ratings yet