Professional Documents
Culture Documents
8 Juni Folic Acid GDM 2020
Uploaded by
Ario DaniantoOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
8 Juni Folic Acid GDM 2020
Uploaded by
Ario DaniantoCopyright:
Available Formats
Journal of Diabetes Mellitus, 2021, 11, 26-39
https://www.scirp.org/journal/jdm
ISSN Online: 2160-5858
ISSN Print: 2160-5831
Gestational Diabetes Mellitus and Folic Acid
Supplementation in Pregnant Women:
A Systematic Review
Stephanie Farah1#, Obey Albaini1#, Karl Jallad2*
1
Lebanese American University Medical Center, Beirut, Lebanon
2
Obstetrics and Gynecology and Urology, Lebanese American University Medical Center, Beirut, Lebanon
How to cite this paper: Farah, S., Albaini, Abstract
O. and Jallad, K. (2021) Gestational Di-
abetes Mellitus and Folic Acid Supplemen- Background: Gestational diabetes mellitus (GDM) is a common pregnancy
tation in Pregnant Women: A Systematic disorder screened for between the 24th and 28th weeks of gestation using oral
Review. Journal of Diabetes Mellitus, 11,
glucose tolerance test. GDM has maternal and fetal health implications. Ob-
26-39.
https://doi.org/10.4236/jdm.2021.111003
jective: To assess the relation between folic acid supplementation in pregnant
women and the risk of developing GDM. Search Strategy: The search em-
Received: December 14, 2020 ployed topic-based strategies designed for each database in June 2020. Data-
Accepted: February 6, 2021
bases searched were Pubmed, Cochrane Library, Embase and Lebanese
Published: February 9, 2021
American University online database. Selection Criteria: Studies eligible
Copyright © 2021 by author(s) and were those targeting the association of GDM development and folic acid sup-
Scientific Research Publishing Inc. plementation, including pregnant women who have developed GDM and
This work is licensed under the Creative
pregnant women who were on folic acid supplementation and developed
Commons Attribution International
License (CC BY 4.0).
GDM. Both interventional and observational studies were included. Data
http://creativecommons.org/licenses/by/4.0/ Collection and Analysis: Two reviewers extracted the data independently. A
Open Access third reviewer checked the data for consistency and clarity. Data extracted in-
cluded the sample characteristics, sample size and outcomes. Cohen’s κ was
used to assess agreement between reviewers. All tools and processes were pi-
loted prior to use. Risk of bias was assessed using the Newcastle-Ottawa Scale.
Data was presented in a tabulated form. Main Results: Six studies showed a
proportional relation between folic acid intake and GDM, two reported a
protective effect, and one cohort found no association. Conclusion: The in-
consistent results made the formulation of a definitive conclusion difficult.
Hence, larger studies are needed.
Keywords
Folic Acid, Gestational Diabetes
Both are co-authors who contributed equally to the paper.
#
DOI: 10.4236/jdm.2021.111003 Feb. 9, 2021 26 Journal of Diabetes Mellitus
S. Farah et al.
1. Introduction
Gestational diabetes mellitus (GDM) is a common pregnancy disorder that is
screened for between the 24th and 28th weeks of gestation, using oral glucose to-
lerance test (OGTT) [1] (Table 1 and Table 2). It is estimated that 6% of preg-
nancies in the United States are complicated by GDM [2]. Data clearly reflect
that the rates of GDM in the US are lower in white women compared to other
races and ethnicities [3]. Unfortunately, over the past few decades, the preva-
lence of GDM worldwide has increased [4], most likely due to elevated maternal
age and body mass index (BMI) [5].
GDM increases the risk of preeclampsia, which was present in 9.8% of preg-
nant women with a fasting blood sugar (FBS) level less than 115 mg/dL, but in
18% of women with FBS above 115 mg/dL, as one study shows [6]. Similarly, in
another study, the need for cesarean delivery was just 9.5% in controls who were
not affected by GDM, but 17% in women with diet-controlled GDM, and 25% in
patients who required anti-diabetic medication [7].
GDM does not only affect the maternal health status. The fetus is at an accen-
tuated risk of neonatal hypoglycemia, hyperbilirubinemia, shoulder dystocia,
traumatic delivery and stillbirth [8]. Alarming is the increased risk of developing
maternal diabetes mellitus postpartum, with all its consequences on the overall
mortality. This risk was described to be seven-times higher the general popula-
tion in one study [9].
Table 1. Diagnostic values for gestational diabetes mellitus [1].
Plasma or serum glucose level; carpenter PLASMA level; national diabetes
System
and coustan conversion (mg/dl) data group conversion (mg/dl)
Unit mg/dL mmol/L mg/dL mmol/L
Fasting 95 5.3 105 5.8
1 Hour 180 10.0 190 10.6
2 Hours 155 8.6 165 9.2
3 Hours 120 7.8 145 8.0
Table 2. International Association of Diabetes and Pregnancy Study Groups Recom-
mendation the Diagnosis and Classification of Hyperglycemia in Pregnancy: one value
equal or above to any of these values from the 75-g OGTT is enough for the diagnosis of
GDM [26].
Glucose concentration threshold
Glucose measure
Mmol/L Mg/dL
Fasting 5.1 92
1 Hour 10.0 180
2 Hours 8.5 153
DOI: 10.4236/jdm.2021.111003 27 Journal of Diabetes Mellitus
S. Farah et al.
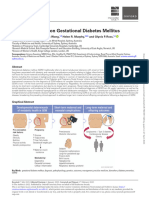
Folic acid or vitamin B9 is used as a supplement during the prenatal care,
starting prior to conception, and to be continued throughout the first trimester,
given that it decreases the risk of neural tube defects [10]. As recommendations
clearly state the need for use of folic acid by pregnant women, the doses of this
vitamin can reach 400 to 600 mcg per day [11].
Folic acid interferes with metabolism, as some studies found that alterations
in the ratio between folate and vitamin B12 can lead to fetal insulin resistance and
increased risk of obesity [12]. Interestingly, several studies suggested that in-
creased supplementation of folic acid imposed a higher risk of developing GDM
[13]-[20]. However, no systematic review was ever published regarding folate
use during pregnancy. Hence, the aim of this article is to assess the relation be-
tween the use of folic acid in pregnant women and the risk of developing GDM.
2. Methods
Design and methods used for this systematic review complied with the Preferred
Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guide-
lines. No review protocol was published. Eligibility criteria were based on the
SPIDER guidelines. It is important to note that this is a systematic review that
imposes no extra risk on patients as it just collected what was already published
in the literature without further patient recruitment.
Studies eligible for this review were those targeting the association of GDM
development and folic acid supplementation, including pregnant women who
developed GDM and pregnant women who were on folic acid supplementation
and developed GDM. Both interventional (randomized controlled trials) and
observational studies (cohort, cross sectional, case control) were included. Qua-
litative, quantitative and mixed-methods research were searched for. The search
employed topic-based strategies designed for each database in June 2020. There
was no language or geographical restrictions.
Databases searched were Pubmed, the Cochrane Library, Medline and the
Lebanese American University (LAU) online database. The search strategy
included the study population and the phenomenon of interest using terms
and keywords derived from scoping search and expertise in the subject field:
pregnant, women, pregnancy, association, folic acid, folate, tetrahydrofolate,
methenyltetrahydrofolate, folinic acid, folacine, pteroyglutamic acid, vitamin B9,
complications, gestational diabetes mellitus.
Records were managed through a specific software while two reviewers (OA
and SF) searched information sources independently and assessed identified
studies for inclusion by grading each eligibility criterion as eligible, not eligible
or might be eligible. The full text of a study was reviewed and the study consi-
dered potentially relevant, following discussion between the two reviewers, when
it could not be clearly excluded on the basis of its title or abstract. Full text was
obtained for abstracts with insufficient information or in a situation of disa-
greement. A study was included when both reviewers assessed it as satisfying the
DOI: 10.4236/jdm.2021.111003 28 Journal of Diabetes Mellitus
S. Farah et al.
inclusion criteria. A third reviewer (KJ) mediated in the event of disagreement
following discussion.
Using a standardized form, two reviewers (SF and OA) extracted the data in-
dependently. A third reviewer (KJ) checked the data for consistency and clarity.
Data extracted included the following: sample characteristics, sample size and
outcomes. Risk of bias for each included trial was independently assessed by the
same initial reviewers. The third reviewer mediated in situations of disagree-
ment. Cohen’s κ was used to assess agreement between reviewers. All tools and
processes were piloted prior to use. Risk of bias was assessed using the Newcas-
tle-Ottawa Scale (NOS).
Data was presented in a tabulated form to allow for semi qualitative compari-
son of study samples, associations and outcomes. All results were reported in the
context of overall study quality.
It is unlikely that a meta-analysis could have been possible based on the find-
ings that most of the retrieved studies are observational. The strength of the
overall body of evidence was assessed using Grading of Recommendations, As-
sessment, Development and Evaluation (GRADE) (Appendix S1).
3. Results
3.1. Search Results
The search yielded 101 record hits. After removing duplicates, we ended up with
88 studies. We excluded 73 records during screening, with a remainder of 15 ar-
ticles. After full article review, a total of 6 studies were excluded for various rea-
sons (Table S2).
Finally, 9 articles were found to be relevant and met the inclusion criteria
(Table S1). Most of them were observational. 7 were cohort [13] [14] [17] [19]
[21] [22] [23], one had a case-control design [24] and only one was an interven-
tional randomized controlled trial (RCT) [23]. 8 studies were conducted in Chi-
na [13] [17] [19]-[24], and only one in USA [25]. All studies were published and
written in English.
The eligible studies included a minimum of 326 pregnant women [14] and a
maximum of 20,199 [25] with a mean of 5626 participants. Participants were di-
agnosed to have GDM based on different validated criteria: National diabetes
data group and 3rd International workshop conference [25], Carpenter Coustan
[19] [24], International association of diabetes and pregnancy study groups [13]
[14] [17] [21] [23].
3.2. Individual Study Outcomes
This review included one main primary outcome, which is the effect of folic acid
(FA) supplementation on GDM risk.
3.2.1. Studies Showing an Increased Risk of GDM with FA
Supplementation
The majority of the included studies showed a proportional relation between
DOI: 10.4236/jdm.2021.111003 29 Journal of Diabetes Mellitus
S. Farah et al.
folic acid intake and GDM. Zhu et al. reported a proportional correlation be-
tween the risk of GDM and daily folic acid (FA) consumption during the first
trimester (adjusted odds ratio (aOR) 2.25 [95% CI 1.35 - 3.76]); in addition,
pre-pregnancy body mass index (BMI) with folic acid play a role in increasing
GDM risk (a pre-pregnancy BMI > 25 kg/m2 with daily FA supplementation in
the first trimester had a much higher risk of GDM (odds ratio OR = 5.63 [95%
CI 2.77 - 11.46]) compared to a pre-pregnancy BMI < 25 kg/m2 and no FA sup-
plements [19]. One study found that larger folate and potassium levels in the vi-
tamin supplements used by pregnant ladies increased the risk of GDM [24]. It
explained this finding by the hypothesis that higher FA level worsened the ef-
fects of vitamin B12 deficiency as well as insulin resistance. Innovatively, Zhang
et al. demonstrated a synergistic effect of folic acid and pollution-induced GDM
during the first trimester [22].
3.2.2. Studies Targeting the Dosages and the Duration of
Supplementation
Some studies targeted the duration and the doses of FA supplementation. Cheng
et al. proved that pre-pregnancy FA supplementation for a duration of 3 months
or longer was associated with an increased risk of GDM (adjusted relative risk
(aRR): 1.72; 95% CI: 1.17 - 2.53) [13]. Another study showed an increase risk of
GDM with both higher doses and longer duration. Short duration was defined as
taking FA in the pre pregnancy period for less than 4 weeks and/or less than 16
weeks during pregnancy but before OGTT, and long duration defined as starting
supplements for at least 4 weeks and continued for at least 16 weeks during
pregnancy before OGTT [17]. The following 4 groups were studied: FA400-S
(400 - 800 mcg/day short duration), FA400-L (400 - 800 mcg/day long duration),
FA800-S (>800 mcg/day short duration), and FA800-L (>800 mcg/day for long
duration). Results showed an odds ratio OR with 95% CIs of GDM of 1.23 (0.84
- 1.80), 1.23 (0.78 - 1.94), 1.37 (0.93 - 2.01), and 2.36 (1.51 - 3.69) for FA400-S,
FA400-L, FA800-S and FA800-L, respectively, compared with non-users. After
adjusting for confounders, those with FA800-L still had significant higher risk of
GDM (adjusted OR [95% CI] 2.09 [1.30 - 3.36]). Similarly, Huang et al. showed
that a longer duration of FA (>90 days) increases GDM risk (adjusted OR 3.45
(95% CI: 1.01, 11.8) compared to a shorter duration [14].
3.2.3. Studies Showing a Decreased Risk of GDM with FA
Supplementation
On the other hand, 2 studies reported a protective effect of folic acid on GDM
risk. One showed that women with adequate total folate intake (>400 mg/day)
had a RR of GDM of 0.83 (95% CI 0.72, 0.95, P = 0.007) compared with women
with a lesser intake (<400 mg/day). The RRs of GDM for 1 - 399, 400 - 599,
and >600 mg/day of supplemental folate intake were 0.83, 0.77, and 0.70, re-
spectively, compared with no supplemental folate intake (P = 0.002). The asso-
ciation between supplemental folate intake and GDM risk largely persisted af-
ter additional adjustment for intake of multivitamins and other micronu-
DOI: 10.4236/jdm.2021.111003 30 Journal of Diabetes Mellitus
S. Farah et al.
trients, as well as among women who likely planned for the pregnancy [17].
Interestingly, another study demonstrated that an individualized supplementa-
tion of folic acid according to the polymorphisms of methylene terahydrofolate
reductase (MTHFR) and methionine synthase reductase (MTTR) can reduce the
GDM risk [23].
3.2.4. Studies Showing no Relation between the Risk of GDM and FA
Supplementation
Finally, one cohort study found no statistical significance between GDM and
folic acid supplementation after adjustment for rs1801133 polymorphisms [21].
Higher RBC folate, partly caused by MTHFR 677C T, may be associated with
increased GDM risk, even in early pregnancy; participants with the TT genotype
had the highest RBC folate concentrations. Those with heterozygous or homo-
zygous variants did not have a significantly higher risk of GDM than did women
with C alleles.
4. Discussion
Main Findings
Out of the 9 studies included in this review, 6 supported the association between
increased folic acid intake and the development of GDM during pregnancy, 2
showed an inverse relation between folate supplementation and GDM risk, while
just one found no association at all when several factors were adjusted for, in-
cluding genetics [21]. However, in the last study, the sample size was small
compared to others (n = 336), and it focused on one ethnicity in China. Hence,
further studies on different ethnicities and higher sample sizes are needed to
prove the lack of relation between the risk of GDM during pregnancy and FA
supplementation when C677T polymorphism in the MTHFR gene is considered.
This requires wide genetic studies to link the different alleles of the MTHFR
gene with the risk of developing GDM and use of FA supplements, as another
study that investigated the A1298C allele of MTHFR gene found an inverse rela-
tion between FA supplementation and GDM risk [23]. However, applying ge-
netic studies necessitates the assessment for cost-effectiveness and the identifica-
tion of the high-risk population which could benefit from genetic detection of
the different MTHFR alleles.
One very large study with 20,199 pregnancies supported the inverse correla-
tion between FA supplementation and GDM development [25]. It included the
highest number of participants (35.5% of patients in this review). The enormous
sample size gives more credibility to the results, given that this study accounted
for the different confounding factors (physical activity and different dietary life-
styles). Nonetheless, the fact that some participants were self-reporting their FA
intake definitely increased the risk of bias. In addition, the group we would be
interested in was small, as 4.1% of the patients developed GDM. Hence, we need
a similar huge study but with a case-control design where more GDM cases can
be recruited.
DOI: 10.4236/jdm.2021.111003 31 Journal of Diabetes Mellitus
S. Farah et al.
Chen Q. et al. focused on multivitamin supplementation [25], yet there is a
need to identify the overall intake of FA including diet. Zhang et al. studied air
pollution and GDM [22]. Their results raised concerns for pregnant women liv-
ing in big cities, and thus, further studies are warranted to link the level of con-
tamination in the air to the accepted doses of FA during pregnancy.
The novel cohort study suggesting that a daily FA dose of 800 mg increased
the risk of GDM can push towards a revision of the recommendations of daily
doses higher than 400 mg [17]. Two studies discussing the duration of use of
FA [13] [14] also proved the need for folic acid supplementation as it not only
can decrease the risk of neural tube defects, but also can decrease the risk of
GDM compared to no use; however, this vitamin should not be taken for more
than 3 months prior to conception [13] and for a duration less than 90 days
post-conception [14]. It is important to note that diet alone was not sufficient in
providing the needed levels of micronutrients for pregnant women, including
folate which was scarcely taken from food, as shown by Kozlowska A. [26] and
Looman M. [27].
Zhu et al. suggested that increased FA intake among pregnant ladies can lead
to GDM directly by explaining that more folate can augment the effects of vita-
min B12 deficiency, most likely focusing on insulin resistance, or indirectly via
the harmful effects of non-metabolized folate [19]. Understanding the mechan-
ism behind this process can be reached through other studies targeting the me-
tabolism of folate.
5. Interpretation
The analysis of the data in these studies combined cannot reach a conclusion on
whether or not FA supplementation pre-pregnancy and during gestation in-
crease the risk of developing GDM, as the results are inconsistent.
Aside from the articles discussed in this review, one study showed that babies
born to women with GDM had higher cord plasma concentration of folic acid,
which it attributed to an altered feto-placental metabolism of the vitamin or to a
change in the transport mechanism [28]. Another study found that pregnant
women with GDM had higher folate levels in the plasma [29].
In one article, it was shown that higher red blood cell (RBC) folate concentra-
tions were associated with higher GDM risk, with a relative risk RR per 1 standard
deviation (1-SD) increase in RBC folate level equal to 1.16 (1.03 - 1.30) [20].
Folate and vitamin B12 are essential factors in the metabolism of homocysteine.
Interestingly, Guvenet al. in 2006 found no statistical difference between women
with GDM and normal controls concerning blood folate level, but blood homo-
cysteine levels were significantly higher among GDM women (9.0 ± 3.1 μmol/l)
compared to normal pregnant ladies (7.4 ± 1.6 μmol/l) [30].
Strengths and Limitations
This is the first systematic review to be conducted on such a topic. Several stu-
DOI: 10.4236/jdm.2021.111003 32 Journal of Diabetes Mellitus
S. Farah et al.
dies were contrived very recently to understand the correlation between FA
supplementation for pregnant women and GDM risk, making the whole idea a
hot topic in 2020, knowing that FA supplementation in specific doses is rec-
ommended starting preconception to decrease the risk of neural tube defects.
Hence, once more data are available and a clear conclusion can be drawn, FA
supplementation guidelines during pregnancy might be subject to change.
However, this systematic review has several limitations. First, just one rando-
mized controlled trial was included, while the rest of the studies were observa-
tional. Not all studies were able to specify dose and duration ranges. In addition,
FA supplementation from food could not be clearly assessed. Most of the obser-
vational studies were based on questionnaires or medical records, which impose
a high risk of bias. 8 out of 9 studies were conducted in China, making any
results driven from these studies only applicable on the Chinese population until
replicates are made on other populations. Finally, the diagnostic criteria for
GDM and the food frequency questionnaire used were different among the stu-
dies. This acted as a limitation for comparing the different results from similar
studies discussed in this review.
6. Conclusion
The inconsistent results from the different articles discussed in this review make
the formulation of a definitive conclusion on the relation between GDM and FA
supplementation in pregnancy difficult. Hence, larger studies, consisting of dif-
ferent ethnicities, and applying the same criteria for GDM diagnosis are needed
to be able to draw a conclusion. In addition, the dosage and duration of FA sup-
plementation should be assessed away from subjective questionnaires and re-
gardless of dietary FA intake, as studies have shown that FA intake from food
does not suffice the needs of pregnancy.
Contribution to Authorship
OA, SF and KJ conceived and designed the study. OA and SF performed the li-
terature search and data extraction. KJ checked the data. OA and SF performed
the data analysis. OA, SF and KJ wrote the article.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this
paper.
References
[1] American Diabetes Association (2018) Management of Diabetes in Pregnancy:
Standards of Medical Care in Diabetes. Diabetes Care, 41, S137-S143.
https://doi.org/10.2337/dc18-S013
[2] Deputy, N.P., Kim, S.Y., Conrey, E.J. and Bullard, K.M. (2018) Prevalence and
Changes in Preexisting Diabetes and Gestational Diabetes among Women Who
Had a Live Birth—United States, 2012-2016. Morbidity and Mortality Weekly Re-
DOI: 10.4236/jdm.2021.111003 33 Journal of Diabetes Mellitus
S. Farah et al.
port, 67, 1201-1207. https://doi.org/10.15585/mmwr.mm6743a2
[3] Ferrara, A. (2007) Increasing Prevalence of Gestational Diabetes Mellitus: A Public
Health Perspective. Diabetes Care, 30, S141-S146. https://doi.org/10.2337/dc07-s206
[4] Simmons, D. (2011) Diabetes and Obesity in Pregnancy. Best Practice & Research
Clinical Obstetrics & Gynaecology, 25, 25-36.
https://doi.org/10.1016/j.bpobgyn.2010.10.006
[5] Feig, D.S., Hwe, J., Shah, B.R., Booth, G.L., Bierman, A.S. and Lipscombe, L.L.
(2014) Trends in Incidence of Diabetes in Pregnancy and Serious Perinatal Out-
comes: A Large, Population-Based Study in Ontario, Canada, 1996-2010. Diabetes
Care, 37, 1590-1596. https://doi.org/10.2337/dc13-2717
[6] Yogev, Y., Xenakis, E.M.J. and Langer, O. (2020) The Association between Preec-
lampsia and the Severity of Gestational Diabetes: The Impact of Glycemic Control.
American Journal of Obstetrics and Gynecology, 191, 1655-1660.
https://pubmed.ncbi.nlm.nih.gov/15547538
[7] Ehrenberg, H.M., et al. (2004) The Influence of Obesity and Diabetes on the Risk of
Cesarean Delivery. American Journal of Obstetrics and Gynecology, 191, 969-974.
https://pubmed.ncbi.nlm.nih.gov/15467574
[8] Rosenstein, M.G., Cheng, Y.W., Snowden, J.M., Nicholson, J.M. and Caughey, A.B.
(2012) Risk of Stillbirth and Infant Death Stratified by Gestational Age. Obstetrics &
Gynecology, 120, 76-82. https://doi.org/10.1097/AOG.0b013e31825bd286
[9] Jiwani, A., Marseille, E., Lohse, N., Damm, P., Hod, M. and Kahn, J.G. (2012) Ges-
tational Diabetes Mellitus: Results from a Survey of Country Prevalence and Prac-
tices. Journal of Maternal-Fetal and Neonatal Medicine, 25, 600-610.
https://doi.org/10.3109/14767058.2011.587921
[10] Imbard, A., Benoist, J.-F. and Blom, H.J. (2013) Neural Tube Defects, Folic Acid
and Methylation. International Journal of Environmental Research and Public
Health, 10, 4352-4389. https://doi.org/10.3390/ijerph10094352
[11] Castaño, E., Piñuñuri, R., Hirsch, S. and Ronco, A.M. (2017) Folate and Pregnancy,
Current Concepts: It Is Required Folic Acid Supplementation? Revista Chilena de
Pediatría, 88, 199-206. https://doi.org/10.4067/S0370-41062017000200001
[12] Yajnik, C.S., Deshpande, S.S., Jackson, A.A., Refsum, H., Rao, S., Fisher, D.J., et al.
(2008) Vitamin B12 and Folate Concentrations during Pregnancy and Insulin Re-
sistance in the Offspring: The Pune Maternal Nutrition Study. Diabetologia, 51,
29-38. https://doi.org/10.1007/s00125-007-0793-y
[13] Cheng, G., Sha, T., Gao, X., He, Q., Wu, X., Tian, Q., et al. (2019) The Associations
between the Duration of Folic Acid Supplementation, Gestational Diabetes Mellitus,
and Adverse Birth Outcomes Based on a Birth Cohort. International Journal of En-
vironmental Research and Public Health, 16, 4511.
https://doi.org/10.3390/ijerph16224511
[14] Huang, L., Yu, X., Li, L., Chen, Y., Yang, Y., Yang, Y., et al. (2019) Duration of Pe-
riconceptional Folic Acid Supplementation and Risk of Gestational Diabetes Melli-
tus. Asia Pacific Journal of Clinical Nutrition, 28, 321-329.
[15] Krishnaveni, G.V., Hill, J.C., Veena, S.R., Bhat, D.S., Wills, A.K., Karat, C.L.S., et al.
(2009) Low Plasma Vitamin B12 in Pregnancy Is Associated with Gestational “Di-
abesity” and Later Diabetes. Diabetologia, 52, 2350-2358.
https://doi.org/10.1007/s00125-009-1499-0
[16] Lai, J.S., Pang, W.W., Cai, S., Lee, Y.S., Chan, J.K.Y., Shek, L.P.C., et al. (2018) High
Folate and Low Vitamin B12 Status during Pregnancy Is Associated with Gestation-
al Diabetes Mellitus. Clinical Nutrition, 37, 940-947.
DOI: 10.4236/jdm.2021.111003 34 Journal of Diabetes Mellitus
S. Farah et al.
https://doi.org/10.1016/j.clnu.2017.03.022
[17] Li, Q., Zhang, Y., Huang, L., Zhong, C., Chen, R., Zhou, X., et al. (2019) High-Dose
Folic Acid Supplement Use from Prepregnancy through Midpregnancy Is Asso-
ciated with Increased Risk of Gestational Diabetes Mellitus: A Prospective Cohort
Study. Diabetes Care, 42, e113-e115. https://doi.org/10.2337/dc18-2572
[18] Li, S., Hou, Y., Yan, X., Wang, Y., Shi, C., Wu, X., et al. (2019) Joint Effects of Folate
and Vitamin B12 Imbalance with Maternal Characteristics on Gestational Diabetes
Mellitus. Journal of Diabetes, 11, 744-751. https://doi.org/10.1111/1753-0407.12899
[19] Zhu, B., Ge, X., Huang, K., Mao, L., Yan, S., Xu, Y., et al. (2016) Folic Acid Supple-
ment Intake in Early Pregnancy Increases Risk of Gestational Diabetes Mellitus:
Evidence from a Prospective Cohort Study. Diabetes Care, 39, e36-e37.
https://doi.org/10.2337/dc15-2389
[20] Xie, K., Xu, P., Fu, Z., Gu, X., Li, H., Cui, X., et al. (2019) Association of Maternal
Folate Status in the Second Trimester of Pregnancy with the Risk of Gestational
Diabetes Mellitus. Food Science & Nutrition, 7, 3759-3765.
https://doi.org/10.1002/fsn3.1235
[21] Liu, P., Liu, Y., Ma, L., Yao, A.M., Chen, X.Y., Hou, Y.X., et al. (2020) Associations
between Gestational Diabetes Mellitus Risk and Folate Status in Early Pregnancy
and MTHFR C677T Polymorphisms in Chinese Women. Diabetes, Metabolic Syn-
drome and Obesity, 13, 1499-1507. https://doi.org/10.2147/DMSO.S250279
[22] Zhang, H., Dong, H., Ren, M., Liang, Q., Shen, X., Wang, Q., et al. (2020) Ambient Air
Pollution Exposure and Gestational Diabetes Mellitus in Guangzhou, China: A Pros-
pective Cohort Study. Science of the Total Environment, 699, Article ID: 134390.
https://doi.org/10.1016/j.scitotenv.2019.134390
[23] Li, X., Jiang, J., Xu, M., Xu, M., Yang, Y., Lu, W., et al. (2015) Individualized Sup-
plementation of Folic Acid According to Polymorphisms of Methylenetetrahydro-
folate Reductase (MTHFR), Methionine Synthase Reductase (MTRR) Reduced
Pregnant Complications. Gynecologic and Obstetric Investigation, 79, 107-112.
https://doi.org/10.1159/000367656
[24] Chen, Q., Feng, Y., Yang, H., Wu, W., Zhang, P., Wang, K., et al. (2019) A Vitamin
Pattern Diet Is Associated with Decreased Risk of Gestational Diabetes Mellitus in
Chinese Women: Results from a Case Control Study in Taiyuan, China. Journal of
Diabetes Research, 2019, Article ID: 5232308. https://doi.org/10.1155/2019/5232308
[25] Li, M., Li, S., Chavarro, J.E., Gaskins, A.J., Ley, S.H., Hinkle, S.N., et al. (2019) Pre-
pregnancy Habitual Intakes of Total, Supplemental, and Food Folate and Risk of Ges-
tational Diabetes Mellitus: A Prospective Cohort Study. Diabetes Care, 42, 1034-1041.
https://doi.org/10.2337/dc18-2198
[26] Kozlowska, A., Jagielska, A.M., Okreglicka, K.M., Dabrowski, F., Kanecki, K.,
Nitsch-Osuch, A., et al. (2018) Dietary Vitamin and Mineral Intakes in a Sample of
Pregnant Women with Either Gestational Diabetes or Type 1 Diabetes Mellitus,
Assessed in Comparison with Polish Nutritional Guidelines. Ginekologia Polska,
89, 581-586. https://doi.org/10.5603/GP.a2018.0100
[27] Looman, M., Schoenaker, D.A.J.M., Soedamah-Muthu, S.S., Mishra, G.D., Geelen,
A. and Feskens, E.J.M. (2019) Pre-Pregnancy Dietary Micronutrient Adequacy Is
Associated with Lower Risk of Developing Gestational Diabetes in Australian
Women. Nutrition Research, 62, 32-40. https://doi.org/10.1016/j.nutres.2018.11.006
[28] Barzilay, E., Moon, A., Plumptre, L., Masih, S.P., Sohn, K.-J., Visentin, C.E., et al.
(2018) Fetal One-Carbon Nutrient Concentrations May Be Affected by Gestational
Diabetes. Nutrition Research, 55, 57-64.
DOI: 10.4236/jdm.2021.111003 35 Journal of Diabetes Mellitus
S. Farah et al.
https://doi.org/10.1016/j.nutres.2018.04.010
[29] Berglund, S.K., García-Valdés, L., Torres-Espinola, F.J., Segura, M.T., Martínez-Zaldívar,
C., Aguilar, M.J., et al. (2016) Maternal, Fetal and Perinatal Alterations Associated
with Obesity, Overweight and Gestational Diabetes: An Observational Cohort Study
(PREOBE). BMC Public Health, 16, 207. https://doi.org/10.1186/s12889-016-2809-3
[30] Guven, M.A., Kilinc, M., Batukan, C., Ekerbicer, H.C. and Aksu, T. (2006) Elevated
Second Trimester Serum Homocysteine Levels in Women with Gestational Diabetes
Mellitus. Archives of Gynecology and Obstetrics, 274, 333-337.
https://doi.org/10.1007/s00404-006-0191-6
DOI: 10.4236/jdm.2021.111003 36 Journal of Diabetes Mellitus
S. Farah et al.
Appendix S1: Study Design
Design and methods used for this systematic review complied with the Preferred
Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.
Eligibility criteria were based on the SPIDER guidelines. It is important to note that
this is a systematic review that imposes no extra risk on patients as it just collected
what was already published in the literature without further patient recruitment.
Studies eligible for this review were those targeting the association of GDM
development and folic acid supplementation, including pregnant women who
developed GDM and pregnant women who were on folic acid supplementation
and developed GDM. Both interventional (randomized controlled trials) and
observational studies (cohort, cross sectional, case control) were included. Qua-
litative, quantitative and mixed-methods research were searched for. The search
employed topic-based strategies designed for each database in June 2020. There
was no language or geographical restrictions. Databases searched were Pubmed,
the Cochrane Library, Medline and the Lebanese American University (LAU)
online database. The search strategy included the study population and the phe-
nomenon of interest using terms and keywords derived from scoping search and
expertise in the subject field: pregnant, women, pregnancy, association, folic ac-
id, folate, tetrahydrofolate, methenyltetrahydrofolate, folinic acid, folacine, pte-
royglutamic acid, vitamin B9, complications, gestational diabetes mellitus.
Records were managed through a specific software while two reviewers (OA
and SF) searched information sources independently and assessed identified
studies for inclusion, facilitated by grading each eligibility criterion as eligible,
not eligible or might be eligible. The full text of a study was reviewed and the
study considered potentially relevant, following discussion between the two re-
viewers, when it could not be clearly excluded on the basis of its title or abstract.
Full text was obtained for abstracts with insufficient information or in a situa-
tion of disagreement. A study was included when both reviewers assessed it as
satisfying the inclusion criteria. A third reviewer (KJ) mediated in the event of
disagreement following discussion.
Using a standardized form, two reviewers (SF/OA) extracted the data inde-
pendently. A third reviewer (KJ) checked the data for consistency and clarity.
Data extracted included the following: sample characteristics, sample size and
outcomes. Risk of bias for each included trial was independently assessed by the
same initial reviewers. The third reviewer mediated in situations of disagree-
ment. Cohen’s κ was used to assess agreement between reviewers. All tools and
processes were piloted prior to use. Risk of bias was assessed using the Newcas-
tle-Ottawa Scale (NOS).
Data was presented in a tabulated form to allow for semi qualitative compari-
son of study sample, association and outcome. All results were reported in the
context of overall study quality. It is unlikely that a meta-analysis could have
been possible based on the findings that most of the retrieved studies are obser-
vational. The strength of the overall body of evidence was assessed using Grad-
ing of Recommendations, Assessment, Development and Evaluation (GRADE).
DOI: 10.4236/jdm.2021.111003 37 Journal of Diabetes Mellitus
S. Farah et al.
Table S1. Included studies.
First Author’s Year/Country/
Sample size Inclusion/Exclusion Criteria Results
Name Study design
Inclusion criteria: women aged 18 Higher folate and potassium level in
9556: 1464 (15.3%) years or older with gestational age of the vitamin supplements increased
2019/China/ GDM cases and 8092 20 weeks or more and without the risk of GDM.
Chen, Q. mental illness
Case-Control (84.7%) non-GDM Increased FA level aggravates the
controls Exclusion criteria: previous DM, effects of vitamin B12 deficiency and
Gestational age < 24 exacerbates insulin resistance.
Inclusion criteria: infants had health
care records in the CHMIS; mothers
and infants’ guardians agreed to
participate and signed the written
informed consents; and mothers took FA FA supplementation for ≥3 months
2019/China/ 950 (GDM cases supplements at a dose of 400 mcg/day before pregnancy was associated
Cheng, G.
Cohort were 10.2%) before, and during, pregnancy. with an increased risk of GDM
Exclusion criteria: mothers had a (aRR: 1.72; 95% CI: 1.17 - 2.53)
history of mental illness or brain
disease, diabetes before pregnancy;
multiple pregnancy; congenital
abnormalities in the newborn
After adjusting for confounding
factors, the aOR of GDM comparing
2019/China/ 326: 33 (10.1%)
Huang, L. N/A taking folic acid for >90 days with
Cohort had GDM
taking folic acid for ≤60 days was
3.45 (95% CI: 1.01, 11.8)
Exclusion criteria: those with diabetes, FA supplement use >800 mg/day
2019/China/ multiple pregnancies, abortions, from pre-pregnancy through
Li, Q. 4,353: 8.6% had GDM
Cohort without reliable OGTT or folic mid-pregnancy was associated
acid supplementation values with the development of GDM
Exclusion criteria: women with one
of the following First trimester exposure to ai polluted
with SO2 increased the risk of GDM,
2019/China/ 5165: 604 (11.7%) Conditions: pre-existing type 1 and
Zhang, H. especially if between the 4th and 10th
Cohort had GDM type 2 DM or GDM, thyroid disease, weeks of gestation, and if using
hypertension, asthma and systemic FA supplements.
lupus erythematosus.
Higher risk of GDM was found
Inclusion criteria: Women who had
2019/China/ 1938: 249 (12.8%) with daily FA supplementation
Zhu, B. either used FA supplements or never
Cohort had GDM during the first trimester
used any vitamin supplements
(aOR = 2.25 [95% CI 1.35 - 3.76])
DOI: 10.4236/jdm.2021.111003 38 Journal of Diabetes Mellitus
S. Farah et al.
Table S2. Excluded studies.
Author’s name Study type/Country/Year Reason for exclusion
This study focused on multivitamins, including folate. It did not focus in any section
Kozlowska, A [26] Prospetive/Poland/2018
on folate as an independent factor that can impact gestational diabetes risk
This study did not measure folate intake. Instead, it used serum folate level
Li, S [18] Cross-sectional/China/2019
to relate to the risk of developing gestational diabetes
This study measured serum concentrations of one-carbon
Barzilay, E [28] Cohort/Canada/2018
nutrients, not folate intake
This study tried to link serum concentrations of folate along with other
Lai, JS [16] Cohort/Singapore/2018
vitamins to the risk of gestational diabetes
This study measured folate uptake by primary cultured cytotrophoblasts isolated
Araujo, JR Case-control/Portugal/2012 from human placentas of patients with gestational diabetes and compared
them to controls
This review did not relate folic acid supplementation to the development of
Reece, EA Review/USA/2012 gestational diabetes, but it discussed the protective impact of folate on the
risk of neural tube defects
DOI: 10.4236/jdm.2021.111003 39 Journal of Diabetes Mellitus
You might also like
- Prediabetes: A Fundamental Text: Pathophysiology, Complications, Management & ReversalFrom EverandPrediabetes: A Fundamental Text: Pathophysiology, Complications, Management & ReversalNo ratings yet
- Gestational Diabetes Mellitus Update and Review of Literature 2161 038X.S2 008 PDFDocument6 pagesGestational Diabetes Mellitus Update and Review of Literature 2161 038X.S2 008 PDFBuluan Mps McadNo ratings yet
- Complementary and Alternative Medical Lab Testing Part 10: ObstetricsFrom EverandComplementary and Alternative Medical Lab Testing Part 10: ObstetricsNo ratings yet
- Prenatal Diagnosis - 2020 - Murray - Short and Long Term Outcomes of Gestational Diabetes and Its Treatment On FetalDocument7 pagesPrenatal Diagnosis - 2020 - Murray - Short and Long Term Outcomes of Gestational Diabetes and Its Treatment On FetalVicente Fasce OlmosNo ratings yet
- Towards A Global Consensus On GDM Diagnosis Light at The End of The TunnelDocument5 pagesTowards A Global Consensus On GDM Diagnosis Light at The End of The TunnelYuliana NaviaNo ratings yet
- 2021 Diagnosis and Management of Gestational Diabetes Mellitus An Overview of National and International GuidelinesDocument15 pages2021 Diagnosis and Management of Gestational Diabetes Mellitus An Overview of National and International GuidelinesJuan Pablo Patiño SánchezNo ratings yet
- Gestationaldiabetes Mellitus: Emily D. Szmuilowicz,, Jami L. Josefson,, Boyd E. MetzgerDocument15 pagesGestationaldiabetes Mellitus: Emily D. Szmuilowicz,, Jami L. Josefson,, Boyd E. Metzgerjose ricardo escalante perezNo ratings yet
- Screening and Diagnosis GDMDocument3 pagesScreening and Diagnosis GDMAkarapon SugandhanandaNo ratings yet
- Local Versus International Criteria in Predicting Gestational Diabetes Mellitus Related Pregnancy OutcomesDocument10 pagesLocal Versus International Criteria in Predicting Gestational Diabetes Mellitus Related Pregnancy OutcomesCj CCNo ratings yet
- The Pathophysiology of Gestational Diabetes MellitusDocument35 pagesThe Pathophysiology of Gestational Diabetes MellituscalliemozartNo ratings yet
- Screening For Biomarkers Predictive of Gestational Diabetes MellitusDocument9 pagesScreening For Biomarkers Predictive of Gestational Diabetes MellitusTomNo ratings yet
- 190 - Gestational Diabetes Mellitus AgogDocument16 pages190 - Gestational Diabetes Mellitus Agogjorge vergara100% (2)
- Repub 103040-OaDocument8 pagesRepub 103040-OaEmma Lyn SantosNo ratings yet
- Gestational Diabetes and Intra Uterine Fetaldeath Complication in A Tertiary Health FacilityDocument5 pagesGestational Diabetes and Intra Uterine Fetaldeath Complication in A Tertiary Health Facilityade lydia br.siregarNo ratings yet
- Diagnosis of Gestational DiabetesDocument7 pagesDiagnosis of Gestational DiabetesAl Hasyr SarminNo ratings yet
- JurnalDocument3 pagesJurnalARINANo ratings yet
- WWW Ncbi NLM Nih GovDocument10 pagesWWW Ncbi NLM Nih GovJumrah KhumairahNo ratings yet
- Prevalence of Diabetes Mellitus Amongst Antenatal Clinic Attendees at Booking in A Teaching Hospital in Rivers State, NigeriaDocument3 pagesPrevalence of Diabetes Mellitus Amongst Antenatal Clinic Attendees at Booking in A Teaching Hospital in Rivers State, NigeriaInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- HHS Public Access: Gestational Diabetes MellitusDocument19 pagesHHS Public Access: Gestational Diabetes MellitusAnne MarieNo ratings yet
- Diagnosis and Treatment of Gestational Diabetes: Scientific Impact Paper No. 23Document6 pagesDiagnosis and Treatment of Gestational Diabetes: Scientific Impact Paper No. 23Ane DamayantiNo ratings yet
- The Role of First-Trimester Hba1C in The Early Detection of Gestational DiabetesDocument7 pagesThe Role of First-Trimester Hba1C in The Early Detection of Gestational DiabetesNéfer TitiNo ratings yet
- Gestational Diabetes Mellitus Acog CopDocument8 pagesGestational Diabetes Mellitus Acog CopAndrea Sandoval OteroNo ratings yet
- Effect of Treatment of Gestational Diabetes Mellitus: A Systematic Review and Meta-AnalysisDocument9 pagesEffect of Treatment of Gestational Diabetes Mellitus: A Systematic Review and Meta-AnalysisAngnes Dera MustikaNo ratings yet
- Pre-Existing Diabetes Mellitus and Adverse PDFDocument5 pagesPre-Existing Diabetes Mellitus and Adverse PDFMetebNo ratings yet
- Diabetes Gestacional 2022 NejmDocument12 pagesDiabetes Gestacional 2022 NejmGabrielaNo ratings yet
- ACOG Practice Bulletin No137 PDFDocument11 pagesACOG Practice Bulletin No137 PDFRodrigo Pérez CuelloNo ratings yet
- Diabetes Gestacional Una Actualizacion 2022Document31 pagesDiabetes Gestacional Una Actualizacion 2022CARLOS MACHADONo ratings yet
- 17 MIJATOVIC 2020 Nqaa137Document9 pages17 MIJATOVIC 2020 Nqaa137iriartenela14No ratings yet
- 11 ORATILE. P. ROBERTO OLMOSTrat. Con Dieta y EjercicoDocument8 pages11 ORATILE. P. ROBERTO OLMOSTrat. Con Dieta y Ejercicoiriartenela14No ratings yet
- 17 Diagnostic Strategies For Gestational Diabetes MellitusreviewDocument10 pages17 Diagnostic Strategies For Gestational Diabetes MellitusreviewdianaNo ratings yet
- Dean OfficeDocument73 pagesDean Officearief19No ratings yet
- Researcharticle Open AccessDocument13 pagesResearcharticle Open AccessAngnes Dera MustikaNo ratings yet
- Gestational Diabetes DissertationDocument4 pagesGestational Diabetes DissertationPayToDoMyPaperUK100% (1)
- Diabetes Acog 2017Document15 pagesDiabetes Acog 2017Holger Vicente Guerrero Guerrero100% (1)
- Screening and DiagnosisDocument25 pagesScreening and DiagnosisM AbhiNo ratings yet
- 2021 Bastidas Uriel de La Hoz Romero Pre Proof Perinatal Outcomes Diagnosis DM Systematic Review Meta-AnalysisDocument8 pages2021 Bastidas Uriel de La Hoz Romero Pre Proof Perinatal Outcomes Diagnosis DM Systematic Review Meta-AnalysisMiguelEstebanBaqueroAcuñaNo ratings yet
- ACOG Tech BullitinDocument20 pagesACOG Tech BullitinJosephNo ratings yet
- Medi 102 E33898Document7 pagesMedi 102 E33898ade lydia br.siregarNo ratings yet
- Guideline No. 393-Diabetes in Pregnancy: Sogc Clinical Practice GuidelineDocument13 pagesGuideline No. 393-Diabetes in Pregnancy: Sogc Clinical Practice GuidelineAmel ZaoumaNo ratings yet
- Gestational Diabetes - On Broadening The DiagnosisDocument2 pagesGestational Diabetes - On Broadening The DiagnosisWillians ReyesNo ratings yet
- Correlations Between Parameters of Glycaemic Variability and Foetal Growth Neonatal Hypoglycaemia and Hyperbilirubinemia in Women With Gestational diabetesPLoS ONEDocument14 pagesCorrelations Between Parameters of Glycaemic Variability and Foetal Growth Neonatal Hypoglycaemia and Hyperbilirubinemia in Women With Gestational diabetesPLoS ONEVicente Fasce OlmosNo ratings yet
- Scientific Advisory Committee Opinion Paper 23: 1. BackgroundDocument5 pagesScientific Advisory Committee Opinion Paper 23: 1. BackgroundNatia DemetradzeNo ratings yet
- Diabetes Gestacional.Document7 pagesDiabetes Gestacional.Isaac GarciaNo ratings yet
- Same 4Document15 pagesSame 4elnadi.grafistNo ratings yet
- Asociacion Funcion Renal Materna y Resultados Embarazo DM 1 y 2.seah.2020Document8 pagesAsociacion Funcion Renal Materna y Resultados Embarazo DM 1 y 2.seah.2020Andrés Gaviria CNo ratings yet
- 10 1097aog 0000000000002159Document15 pages10 1097aog 0000000000002159Kathleen Cherrepano AguirreNo ratings yet
- Literature Review of Gestational Diabetes MellitusDocument7 pagesLiterature Review of Gestational Diabetes Mellitusc5rc7ppr100% (1)
- Who PDFDocument23 pagesWho PDFMayra PereiraNo ratings yet
- Placental Metabolic Derangements and Fetal Complications in Gestational DiabetesDocument11 pagesPlacental Metabolic Derangements and Fetal Complications in Gestational Diabetesbalachandiran manoharanNo ratings yet
- Diabetes Mellitus in Pregnancy: November 2015Document7 pagesDiabetes Mellitus in Pregnancy: November 2015eka putri nur azizahNo ratings yet
- Biomarkers For Macrosomia Prediction in Pregnancies Affected by DiabetesDocument25 pagesBiomarkers For Macrosomia Prediction in Pregnancies Affected by DiabetesBianca CaterinalisendraNo ratings yet
- March 2019 1551446825 124Document3 pagesMarch 2019 1551446825 124Anjali Rahul AjmeriNo ratings yet
- Aisyah GDM ArticleDocument18 pagesAisyah GDM ArticleBesari Md DaudNo ratings yet
- Assignment On Course During CCGDM.Document6 pagesAssignment On Course During CCGDM.Biman MondalNo ratings yet
- The Hyperglycemia and Adverse Pregnancy Outcome Study: (HAPO)Document9 pagesThe Hyperglycemia and Adverse Pregnancy Outcome Study: (HAPO)Estefania GutierrezNo ratings yet
- Optimizing Postpartum Care For The Patient With Gestational Diabetes MellitusDocument8 pagesOptimizing Postpartum Care For The Patient With Gestational Diabetes MellitussaryindrianyNo ratings yet
- Annotated BibliographyDocument20 pagesAnnotated Bibliographyapi-347153077No ratings yet
- Journal GAJOMSDocument6 pagesJournal GAJOMSmade dharmaNo ratings yet
- Ref 3Document10 pagesRef 3asshagab04No ratings yet
- Zacharzewski 2019Document6 pagesZacharzewski 2019Ario DaniantoNo ratings yet
- Abstract Submission Format ORLHN - GHIDocument1 pageAbstract Submission Format ORLHN - GHIArio DaniantoNo ratings yet
- Immune Response Covid19Document9 pagesImmune Response Covid19Ario DaniantoNo ratings yet
- SimMom SS v9Document2 pagesSimMom SS v9Ario DaniantoNo ratings yet
- Maternal Physiological Changes in Pregnancyand Pathophysiology of COVID-19Document5 pagesMaternal Physiological Changes in Pregnancyand Pathophysiology of COVID-19Ario DaniantoNo ratings yet
- Twin-To-Twin Transfusion Syndrome (TTTS) Pathogenesis, Diagnostics, Classification and Treatment OptionsDocument11 pagesTwin-To-Twin Transfusion Syndrome (TTTS) Pathogenesis, Diagnostics, Classification and Treatment OptionsArio DaniantoNo ratings yet
- COVID-19 During Pregnancy and PostpartumDocument28 pagesCOVID-19 During Pregnancy and PostpartumArio DaniantoNo ratings yet
- CHCCCS011 Meet Personal Support Needs Resource InterCare1Document146 pagesCHCCCS011 Meet Personal Support Needs Resource InterCare1komalbajaj100% (1)
- As A PHNDocument5 pagesAs A PHNJULIANNE BAYHONNo ratings yet
- AkapulkoDocument3 pagesAkapulkowacnagNo ratings yet
- Diresul Navy RDT-GF LiqDocument18 pagesDiresul Navy RDT-GF Liqabdlahmid_bahriNo ratings yet
- LMMU September 2022 ADVERT - 1Document9 pagesLMMU September 2022 ADVERT - 1FredroNo ratings yet
- Felver Et Al., 2015 Yoga in Public School Imroves Adolescent Mood and AffectDocument10 pagesFelver Et Al., 2015 Yoga in Public School Imroves Adolescent Mood and AffectDANIEL MAURICIO RIOBO MOLANONo ratings yet
- Q 2 TLE HK 9 10 Module 1 1Document16 pagesQ 2 TLE HK 9 10 Module 1 1Neil Abay100% (1)
- Growth Considerations in Stability of Orthodontic Treatment: Chapter OutlineDocument4 pagesGrowth Considerations in Stability of Orthodontic Treatment: Chapter OutlineHabeeb HatemNo ratings yet
- A Review of Garment Ventilation Strategies For Structural Firefighter Protective ClothingDocument16 pagesA Review of Garment Ventilation Strategies For Structural Firefighter Protective Clothingaqsa imran100% (1)
- Disaster Understanding 2021Document11 pagesDisaster Understanding 2021miasoejosoNo ratings yet
- Safety Data Sheet: Section 1. IdentificationDocument14 pagesSafety Data Sheet: Section 1. IdentificationEDUARDONo ratings yet
- Pediatric Iga Nephropathy in Europe: Review ArticleDocument7 pagesPediatric Iga Nephropathy in Europe: Review ArticleAmabelle PanganibanNo ratings yet
- Factors Affecting Adherence To Infant Immunization University of Thephilippines ManilaDocument1 pageFactors Affecting Adherence To Infant Immunization University of Thephilippines Manilaheidi leeNo ratings yet
- Me So TherapyDocument53 pagesMe So TherapySophie KneynsbergNo ratings yet
- Brukner CSM5e Vol2 ReferencesDocument99 pagesBrukner CSM5e Vol2 Referencesrenae_vardNo ratings yet
- Marine Pollution Bulletin: Hemal Chowdhury, Tamal Chowdhury, Sadiq M. SaitDocument7 pagesMarine Pollution Bulletin: Hemal Chowdhury, Tamal Chowdhury, Sadiq M. SaitHama BabaNo ratings yet
- Script For Culminating Activity Day 2 EDITEDDocument6 pagesScript For Culminating Activity Day 2 EDITEDKhenoEnrileNo ratings yet
- Negotiations Between Possibilities and R PDFDocument23 pagesNegotiations Between Possibilities and R PDFAkaashi KeijiNo ratings yet
- Gender and Equality Towards Policy Enhancement A Phenomenological InquiryDocument8 pagesGender and Equality Towards Policy Enhancement A Phenomenological InquiryIOER International Multidisciplinary Research Journal ( IIMRJ)No ratings yet
- Annual Medical Examination Masterlist 2022 1Document6 pagesAnnual Medical Examination Masterlist 2022 1Alfie BurbosNo ratings yet
- Rubrics For Preparing AppetizerDocument2 pagesRubrics For Preparing AppetizerBonifacio PinoNo ratings yet
- Sagar Bhandare Updated CV - 2021Document4 pagesSagar Bhandare Updated CV - 2021garima kathuriaNo ratings yet
- Estimation of The Metabolic Equivalent (Met) of An Exercise Protocol Based On Indirect CalorimetryDocument5 pagesEstimation of The Metabolic Equivalent (Met) of An Exercise Protocol Based On Indirect CalorimetryDyah SafitriNo ratings yet
- Whipple Procedure: Procedure at A GlanceDocument4 pagesWhipple Procedure: Procedure at A GlanceAze Andrea PutraNo ratings yet
- Grade 2 Sses ScienceDocument8 pagesGrade 2 Sses Sciencenicole macabante100% (1)
- 2 Risk Assessment Template - Situation 1Document10 pages2 Risk Assessment Template - Situation 1Menol KulathungaNo ratings yet
- Neonatal Sepsis: Dr. Sayali Dhodapkar SSAC, KalolDocument16 pagesNeonatal Sepsis: Dr. Sayali Dhodapkar SSAC, KalolSayali Prashant DhodapkarNo ratings yet
- The Optimum Temporo-Mandibular Joint Condyle Position in Clinical Practice.Document26 pagesThe Optimum Temporo-Mandibular Joint Condyle Position in Clinical Practice.Jose L LlanosNo ratings yet
- Vascular Problems in DiabetesDocument27 pagesVascular Problems in Diabetesdangermou5eNo ratings yet
- 4th - 9th March Academic ScheduleDocument3 pages4th - 9th March Academic Schedulehexit73528No ratings yet
- The Obesity Code: Unlocking the Secrets of Weight LossFrom EverandThe Obesity Code: Unlocking the Secrets of Weight LossRating: 4 out of 5 stars4/5 (6)
- Secrets From the Eating Lab: The Science of Weight Loss, the Myth of Willpower, and Why You Should Never Diet AgainFrom EverandSecrets From the Eating Lab: The Science of Weight Loss, the Myth of Willpower, and Why You Should Never Diet AgainRating: 3.5 out of 5 stars3.5/5 (38)
- Rapid Weight Loss Hypnosis: Deep Sleep Your Way to Rapid Weight Loss, Healing Your Body and Self Esteem with Guided Meditations and Positive AffirmationsFrom EverandRapid Weight Loss Hypnosis: Deep Sleep Your Way to Rapid Weight Loss, Healing Your Body and Self Esteem with Guided Meditations and Positive AffirmationsRating: 5 out of 5 stars5/5 (7)
- Foods That Cause You to Lose Weight: The Negative Calorie EffectFrom EverandFoods That Cause You to Lose Weight: The Negative Calorie EffectRating: 3 out of 5 stars3/5 (5)
- Metabolism Revolution: Lose 14 Pounds in 14 Days and Keep It Off for LifeFrom EverandMetabolism Revolution: Lose 14 Pounds in 14 Days and Keep It Off for LifeNo ratings yet
- The Diabetes Code: Prevent and Reverse Type 2 Diabetes NaturallyFrom EverandThe Diabetes Code: Prevent and Reverse Type 2 Diabetes NaturallyRating: 4.5 out of 5 stars4.5/5 (6)
- Instant Loss On a Budget: Super-Affordable Recipes for the Health-Conscious CookFrom EverandInstant Loss On a Budget: Super-Affordable Recipes for the Health-Conscious CookRating: 3.5 out of 5 stars3.5/5 (2)
- The Diet Trap Solution: Train Your Brain to Lose Weight and Keep It Off for GoodFrom EverandThe Diet Trap Solution: Train Your Brain to Lose Weight and Keep It Off for GoodNo ratings yet
- Ultrametabolism: The Simple Plan for Automatic Weight LossFrom EverandUltrametabolism: The Simple Plan for Automatic Weight LossRating: 4.5 out of 5 stars4.5/5 (28)
- The Beck Diet Solution Weight Loss Workbook: The 6-Week Plan to Train Your Brain to Think Like a Thin PersonFrom EverandThe Beck Diet Solution Weight Loss Workbook: The 6-Week Plan to Train Your Brain to Think Like a Thin PersonRating: 3.5 out of 5 stars3.5/5 (33)
- Glucose Revolution: The Life-Changing Power of Balancing Your Blood SugarFrom EverandGlucose Revolution: The Life-Changing Power of Balancing Your Blood SugarRating: 5 out of 5 stars5/5 (352)
- Body Love Every Day: Choose Your Life-Changing 21-Day Path to Food FreedomFrom EverandBody Love Every Day: Choose Your Life-Changing 21-Day Path to Food FreedomRating: 4 out of 5 stars4/5 (1)
- Eat Complete: The 21 Nutrients That Fuel Brainpower, Boost Weight Loss, and Transform Your HealthFrom EverandEat Complete: The 21 Nutrients That Fuel Brainpower, Boost Weight Loss, and Transform Your HealthRating: 2 out of 5 stars2/5 (1)
- Keto Friendly Recipes: Easy Keto For Busy PeopleFrom EverandKeto Friendly Recipes: Easy Keto For Busy PeopleRating: 3.5 out of 5 stars3.5/5 (2)
- The Complete Beck Diet for Life: The 5-Stage Program for Permanent Weight LossFrom EverandThe Complete Beck Diet for Life: The 5-Stage Program for Permanent Weight LossRating: 3.5 out of 5 stars3.5/5 (6)
- The Whole Body Reset: Your Weight-Loss Plan for a Flat Belly, Optimum Health & a Body You'll Love at Midlife and BeyondFrom EverandThe Whole Body Reset: Your Weight-Loss Plan for a Flat Belly, Optimum Health & a Body You'll Love at Midlife and BeyondRating: 4.5 out of 5 stars4.5/5 (28)
- Allen Carr's Easy Way for Women to Lose Weight: The original Easyway methodFrom EverandAllen Carr's Easy Way for Women to Lose Weight: The original Easyway methodRating: 4.5 out of 5 stars4.5/5 (18)
- Glucose Goddess Method: A 4-Week Guide to Cutting Cravings, Getting Your Energy Back, and Feeling AmazingFrom EverandGlucose Goddess Method: A 4-Week Guide to Cutting Cravings, Getting Your Energy Back, and Feeling AmazingRating: 5 out of 5 stars5/5 (61)
- The Stark Naked 21-Day Metabolic Reset: Effortless Weight Loss, Rejuvenating Sleep, Limitless Energy, More MojoFrom EverandThe Stark Naked 21-Day Metabolic Reset: Effortless Weight Loss, Rejuvenating Sleep, Limitless Energy, More MojoNo ratings yet
- Rapid Weight Loss Hypnosis for Women: Self-Hypnosis, Affirmations, and Guided Meditations to Burn Fat, Gastric Band, Eating Habits, Sugar Cravings, Mindfulness, and More.From EverandRapid Weight Loss Hypnosis for Women: Self-Hypnosis, Affirmations, and Guided Meditations to Burn Fat, Gastric Band, Eating Habits, Sugar Cravings, Mindfulness, and More.Rating: 5 out of 5 stars5/5 (36)
- Body Confidence: Venice Nutrition's 3 Step System That Unlocks Your Body's Full PotentialFrom EverandBody Confidence: Venice Nutrition's 3 Step System That Unlocks Your Body's Full PotentialRating: 4 out of 5 stars4/5 (2)
- The Volumetrics Eating Plan: Techniques and Recipes for Feeling Full on Fewer CaloriesFrom EverandThe Volumetrics Eating Plan: Techniques and Recipes for Feeling Full on Fewer CaloriesNo ratings yet
- Dr. Nowzaradan's Diet Plan: The Scales Don't Lie, People Do! The Only 1200 kcal Diet from Dr. NOW to Lose Weight Fast. 30-Day Diet PlanFrom EverandDr. Nowzaradan's Diet Plan: The Scales Don't Lie, People Do! The Only 1200 kcal Diet from Dr. NOW to Lose Weight Fast. 30-Day Diet PlanNo ratings yet
- The Glucose Goddess Method: The 4-Week Guide to Cutting Cravings, Getting Your Energy Back, and Feeling AmazingFrom EverandThe Glucose Goddess Method: The 4-Week Guide to Cutting Cravings, Getting Your Energy Back, and Feeling AmazingRating: 4.5 out of 5 stars4.5/5 (6)
- The Ultimate Hypnosis For Weight Loss: Bundle 5 in 1, Weight loss Hypnosis, Hypnotic Gastric Band HypnosisFrom EverandThe Ultimate Hypnosis For Weight Loss: Bundle 5 in 1, Weight loss Hypnosis, Hypnotic Gastric Band HypnosisRating: 4.5 out of 5 stars4.5/5 (23)
- The Atkins Essentials: A Two-Week Program to Jump-start Your Low Carb LifestyleFrom EverandThe Atkins Essentials: A Two-Week Program to Jump-start Your Low Carb LifestyleNo ratings yet
- Autophagy: Introduction to Autophagy, Learn How to Get Into Autophagy for Anti-aging, Weight Loss and Naturally Heal Your BodyFrom EverandAutophagy: Introduction to Autophagy, Learn How to Get Into Autophagy for Anti-aging, Weight Loss and Naturally Heal Your BodyRating: 4.5 out of 5 stars4.5/5 (59)
- Think Yourself Thin: A 30-Day Guide to Permanent Weight LossFrom EverandThink Yourself Thin: A 30-Day Guide to Permanent Weight LossRating: 4.5 out of 5 stars4.5/5 (22)