Professional Documents
Culture Documents
AKHSS Preliminary Paper XII
Uploaded by
ayaan khan0 ratings0% found this document useful (0 votes)
5 views8 pagespapers
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentpapers
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
5 views8 pagesAKHSS Preliminary Paper XII
Uploaded by
ayaan khanpapers
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 8
‘Aga Khan Higher Secondary School, Karaehi
+ Preliminary Exa
BIFK ~ XIT~ Chemistry ~ Paper 1
1 shift
‘Time: 30 Mioutes Max, Marks: 43,
SECTION ‘A°
(MULTIPLE CHOICE QUESTIONS). (M.C.Os3
Nore:
|) This section consists of 43 parts questions and all are to be answered. Each question carries one mark
1i)Donot copy the part questions in your answer script. Write only the answer in full against the proper
number ofthe question and its part.
1. Choase the correet answer for each from the given options.
i. Which of the following transition meta ions shows the strongest paramagnetic behavior?
ser Fett Fe? +20"
ji Which ofthe following structural formulae is NOT an alcohol?
R
iii For the given complex whieh option is comect?
HCH NI )3F*
‘Coordination number | ‘Type of figand
x é Bidentare
B 6 Tiexadentate
c 3 Bidentate
D. 3 Hexadentae
wn a < a
(i) Lithium isa good reducing agent de to its
>
‘small ionic radius * low ionization enerey
‘+ ow heat of sublimation ? * low density
\. The formula of the gas found in mashy places is
wh oth rout Cte
Vi An uincharged atom of an element X has a valence electron distribution of nS”. nP* The element X is
expected tobe
+ “tit nom meta + ametaod * an actnide
.
vii Whenacetyen is passed tough ht hie in presence of erganonickeit poly erizes to
+ Polythene Benrene *Protcin * Polyaceylene
Transition elements hve high melting an bling points duo he
+ Member of paired electrons, * member of unpaired electrons
+ Weak indiogen bonding Stony bing orees
is. lon ydries re formed by
+ Transition metals A Berets of high elect positivity
2 Metilos * Elements of high electoneatvty
% _ InAlumino-Thermite process Aluminium sets
«xidizing gent + semperature reducing agent
reducing agen + mire ining age
Xi_The destructive dition of ol produces:
7 cake and coal pas charcoal * phenol ¢ wood spit
@ _ Treeelocazaton of x eleston ina benzene ing is responsible for:
+ instability of aromatic ing 7 ity of aroma rng
© stration of beeen * amsataton of benzene
Xi Which ofthe following shows the ect ode of aly halide with magnesia?
+ ROC) R= BRT +R) R-C1)ReI
+ R-DR-cyR-Br PRD RBH R=
se. The inetionl group at highest priority in TUPAC naming in he sven compound i:
OHO
sono
4
a +0. +on “coon
xv. The compound in which each earbon show sp? hybridization is:
UF CHKCHSCHS *cinc=cH
2 ances *cinech—cr-ch
svi, The teacton of ety magnesium brome wth carbon doce resus in the Formation of
«Benoa acid * butane acid
A Forte acd * propionic cid
rit. -Buten3.yne has
‘+ sixerand four ® bonds ~Azeven 0 and three x bonds
+ ight and to x bonds * nine cand one x bond
Yet-tet-
Bs
boar
8 Ge ul
§ an 34
8 oy
svi j 3 and petiod3 of the modern period able
hen element is re
7 . Ast 25223 apt 3d pt 3 ‘
‘ 3s.Ad!83p) V0 + Ist2shaph3sh3p VA
@) _Anetement having low value oF ionization eneigy and low value of elecuon atin is ikely to
belong to
© Group ta A Group tla, * Group VA * Group VII
XX In the diagram of the Downs Cell shown, the parts labelled X, Y and Z ave
+ X= positive electiode, ¥ = steel sauze, Z = negative ek
+, X= negative electrode, Y = postive electrode, Z » stel gauze
X = postive electrode, Y = negative electrode, Z = steel gauze
‘+X =nepative electrode, ¥ = stee! gauze, Z = postive electrode
xxi, Name the metal which is protected from further corrosion by the oxide layer that forms on its surface
+ ion “Alun * Lithium * Sodium
‘xii, What isthe function ofthe steel gauzs inthe Downs Cell?
AL be
| }
i
stettGoure
‘+ Weacts asa catalyst during the process,
A Toprevent the sodium and chlorine coming into contact and reacting
‘+ Itis the negative electrode,
+ Itremoves any impurities from the sodium,
xxiii, Hydrogen resembles with carbon because of having:
‘= same number of electrons inthe valence shell similar physical state
A remarkable reducing properties * show same valency,
xxiv. Which statement about metals is FALSE
+ allthe metals are good conductor of electricity all he metals form acidic oxide
+ all the metals are good conductor ot heat + all the metals form positive ions
xxv. Tonic hydrides are formed by:
‘+ transition metals
+ metalloids
animal material only * Petrolium
plants material only plants and anions’ material
xxix. Which ofthe following isan electrphile? €* (AIMS. APES F
+ cho ec NHS rch
Xxx Which of the following is NOT a heteroeyelie compound
N
a + + Ve
2esxi, Which ofthe following is the IUPAC name ofthe given compound’?
HO aC CH= HCH,
4. mesy -1-petene 4-yne 7: meth 2ipeinene tyne
‘+ Smetiyl-l-penhne ene ¢ Smal 2-pentyne 4 ene
rox, Matkovnikovs addition of Isnt applicable to
+ Propene * butene * Lpentene A utene
ssl, The essential component of ferlie is
+ Ammonia niogen 4 ety aines + atk nies
cet Cel
soni, Ethylene and acetylene can be distinguished by sing:
+ Bromine in CCl A Toles reagent
& Bacyers reagent * Phenyhydrazne
rixy, Predict the two most likly mechanins which occur when 2-Jodohexane is heat ingthanol pO?
+ SN2and SNI * El and E2 + SN2 and E2 ‘Ei and SNI
aux ake is obtained from
“Phenol and formaldehyde * Adipic acid and hexamethylene diamine
Dimethyl terephthalate and eliylene glycol * Neoprene
sexxvii Polymers which soften on heating and harden when cooled are
+ Crosslinked polymers * Copolymers * Thermosetting polymers Thermoplastics
Sxwvil) Tonic hyries eset with water and produce in sotto
+ ow vw “n to
Xxx, Which of the fllowing group VIA hydrides isa neutral hydride
1S. iho *hSe *tbTe
XL The following given structure contain:
2H
‘+ 6 sigma and 5 pi bonds SA 7 sigma ond 3 pi bonds
+ 12 sigma and 6 pi bonds +12 sigma and 3 pi bonds
as follows,
Xl, Annueleophilic substitution reaction is summarized in the form of ts rate equ
Rate MR XPOW | Swe
Which ofthe following ltement it BALSE fo the yen reaction?
‘+ The overall reaction is 2™ order. 7 OW attacks on Re in final step
Thera determining step ie biolecular * Olle atocks RX before RX bois broken
Alii The correct mame of Nas{Co(Nttne is S020" iorvanrine sabilteste (D
«Disadium pert chloroammine cob) * Sodium penta chlorine amine cobalt 1
| 2% Sodium pentachloro ammine cobaltate (HI) * Cobalt ammine penta chloro Sodium (111)
Which ofthe ftlowig is teria alcoho!”
+ CHACHOH) Cals + HO.CH-C(CtI»-CHs
Ma {NOs}. ¢ 2CH) xy
SfH)+tHNOs —
+ Tho
1OUNs + 49 —a YMG (NOsha + Nad
9 4
‘Aga Klnan Higher Secondary School, Karachi
vi shirt
‘Time: 2 Hours Max. Marks: 85
SECTION ‘BY
(SHORT ~ ANSWER QUESTIONS) (25 MARKS)
NOTE: Attempt FIVE questions from this section in all, selecting atleast TWO questions from inorganic
chemistry and TWO from organie chemistry. All questions carry equal marks,
2 INORGANIC CHEMISTRY
\A™ Hydrogen closely resembles halogens with respect ls properties, but its not a member ofthe
hhalogen family. Justify the statement (write any four points)
HCeone) + HNO3(cone) >
Cut HNOSUil) >
HS +Ch >
Sit HNO: >
@
@
8) Describe the produetion of Chlorine gas by Nelson's cell method. Draw a neat and labeled diagram
of the cell to support your answer.
) Referring to the following diagram answer the following questions
A
B
co
‘© Label the diferent parts in sequence of alphabets
‘© Name the process and Write in detail the processes occurring at “A, D and F
‘Also write equations of reactions involved.
G
@
“
KG NNEC CHE MINTY
4) Wore m detail molec orbial stucture of benzene Also draw all the stuctutes to support your
angwer io}
'b) Wate the IUPAC nantes of the following structures a
© HOOC-CHb-CHy-CHy Cl COOHt
© CHO-CH(OH)-CHEOH)-CHLON
Cle CHCL-CO-CH:-CHO.
© CHSCH-C(OCI))-CH-CO.CI,
48) Define enzymes, discuss enzymatic ati ity and factors which influence rate oFenzyme acon (3)
8) Give chemical equations with sutable conditions forthe preparation of following compounds: (4)
+ Ethene from vicinal dichloride
Mustard gas fom ethane
# Oxime ftom methanol
+ lodoform from ketone
6) +2HA03 ——> Si0r +20, + 204]
mids + (1) —» ND, + tho 7?
+ ONO; ——> Bi0.4 “Nd. + 2D
an \
By, oe ay
o=¢- CH=CH Ch - 0H CHy- (Het ehh gro
y oH u
Q-CH3 6
(His -CH, ~~ CHL -& CH
e !
oe tH;
You might also like
- Frequently Asked Questions (Faqs) : Undergraduate AdmissionsDocument5 pagesFrequently Asked Questions (Faqs) : Undergraduate Admissionsayaan khanNo ratings yet
- Atomic Commit and Concurrency Control: COS 418: Distributed Systems Wyatt LloydDocument40 pagesAtomic Commit and Concurrency Control: COS 418: Distributed Systems Wyatt Lloydayaan khanNo ratings yet
- Guideline For: Undergraduate AdmissionsDocument2 pagesGuideline For: Undergraduate Admissionsayaan khanNo ratings yet
- Ieee ICDCS90Document8 pagesIeee ICDCS90ayaan khanNo ratings yet
- Silo - Tips - Stat X 0 X 1Document5 pagesSilo - Tips - Stat X 0 X 1ayaan khanNo ratings yet
- Are Online Exams An Invitation To Cheat?: The Journal of Economic Education February 2008Document20 pagesAre Online Exams An Invitation To Cheat?: The Journal of Economic Education February 2008ayaan khanNo ratings yet
- Research On Abnormal Behavior Detection of Online Examination Based On Image InformationDocument4 pagesResearch On Abnormal Behavior Detection of Online Examination Based On Image Informationayaan khanNo ratings yet
- Geo Strategic and Policy of PakistanDocument4 pagesGeo Strategic and Policy of Pakistanayaan khan100% (1)
- Handout 3 (Week 3)Document32 pagesHandout 3 (Week 3)ayaan khanNo ratings yet
- Oop Week 3Document4 pagesOop Week 3ayaan khanNo ratings yet
- Week # 4 (Pointers) : Task1Document3 pagesWeek # 4 (Pointers) : Task1ayaan khanNo ratings yet
- Lab 11 and 12 Objectives: Lab Task Object Oriented Programming (C++ and C#) NED University of Engineering and TechnologyDocument9 pagesLab 11 and 12 Objectives: Lab Task Object Oriented Programming (C++ and C#) NED University of Engineering and Technologyayaan khanNo ratings yet
- Week 8 (Object Oriented Programming) Inheritance and Method OveridingDocument2 pagesWeek 8 (Object Oriented Programming) Inheritance and Method Overidingayaan khanNo ratings yet
- Week #6 Objective: For Students To Get Some Practice Of:: TheoryDocument5 pagesWeek #6 Objective: For Students To Get Some Practice Of:: Theoryayaan khanNo ratings yet
- C++ PracticeDocument3 pagesC++ Practiceayaan khanNo ratings yet
- Ned University of Engineering & Technology: BS English Linguistics Programme in 2015Document1 pageNed University of Engineering & Technology: BS English Linguistics Programme in 2015ayaan khanNo ratings yet
- Economy of PakistanDocument16 pagesEconomy of Pakistanayaan khanNo ratings yet
- Lab Task 14Document2 pagesLab Task 14ayaan khanNo ratings yet
- Lab 7Document3 pagesLab 7ayaan khanNo ratings yet
- Background-Color Color Display Justify-Content Height: #316dc3 White Flex Space-EvenlyDocument5 pagesBackground-Color Color Display Justify-Content Height: #316dc3 White Flex Space-Evenlyayaan khanNo ratings yet
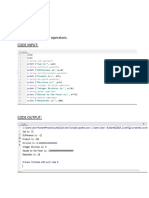
- Lab Task-2 Program#1: Practicing With Math Operators. Code InputDocument16 pagesLab Task-2 Program#1: Practicing With Math Operators. Code Inputayaan khanNo ratings yet
- Assignment No 1Document8 pagesAssignment No 1ayaan khanNo ratings yet
- Lab Task 12 RandomDocument2 pagesLab Task 12 Randomayaan khanNo ratings yet
- News BackupDocument1 pageNews Backupayaan khanNo ratings yet
- HTML Lang Charset Name Content Rel Href Rel Href Href Rel Href Rel Href RelDocument7 pagesHTML Lang Charset Name Content Rel Href Rel Href Href Rel Href Rel Href Relayaan khanNo ratings yet
- Lab Task No#1: Problem#1 Write An Algorithm and Draw The Flowchart For Finding The Average of Two NumbersDocument19 pagesLab Task No#1: Problem#1 Write An Algorithm and Draw The Flowchart For Finding The Average of Two Numbersayaan khanNo ratings yet
- Program 1: Lab Task #5Document12 pagesProgram 1: Lab Task #5ayaan khanNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)