Professional Documents
Culture Documents
Bullous Pemphigoid: Author: Professor Philippe Bernard
Bullous Pemphigoid: Author: Professor Philippe Bernard
Uploaded by
Je'ii PadillaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Bullous Pemphigoid: Author: Professor Philippe Bernard
Bullous Pemphigoid: Author: Professor Philippe Bernard
Uploaded by
Je'ii PadillaCopyright:
Available Formats
Bullous pemphigoid
Author: Professor Philippe Bernard
Service de Dermatologie, Hôpital Robert-Debré, Avenue du Général-Koenig, 51092 Reims
cedex, France. pbernard@chu-reims.fr
Section Editor: Dr Enrico Bertini
Creation date: June 2001
Updated: November 2003, April 2006
Abstract
Key words
Disease name and synonyms
Definition
Epidemiology
Diagnostic criteria
Differential diagnosis
Clinical description
Etiology
Management including treatment
References
Abstract
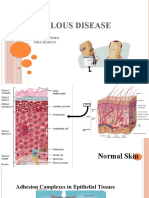
Bullous pemphigoid (BP) is an autoimmune subepidermal bullous dermatosis defined
immunologically by the existence of autoantibodies directed against 2 structural proteins found
in the hemidesmosomes of the dermal-epidermal junction. These proteins, called BP antigen 1
(BPAG1 or AgBP230), and BPAG2 (or AgBP180 or collagen XVII) have respective molecular
masses of 230 and 180 kDa. The disease is characterized clinically by tight bullae, with clear
content, often large, developing primarily on the edge of erythematous plaques. Intense itching
is common. BP is the most common of the autoimmune bullous dermatoses, with an annual
incidence of more than 400 new cases in France. It affects primarily the elderly. However, it has
also been described in children. It occurs within the first year after birth and presents as bullous
lesions on erythematous skin or on normal acral skin. Very rare familial cases have been
reported. Studies conducted in France demonstrated that the prognosis of survival of BP
patients was very poor, with a death rate exceeding 30% after 1 year of treatment. Prognosis of
infantile BP is favourable. Systemic corticotherapy (prednisone: 1 mg/kg/day) remains the
standard treatment for many authors whereas, the treatment of choice for localized, pauci-
bullous and/or slightly evolving forms of pemphigoid is topical corticotherapy with class I
dermatocorticoids.
Key words
erythematous plaques, bullae, auto-immune disease, corticotherapy
Bernard P. Bullous pemphigoïd. Orphanet Encyclopedia, May 2006.
http://www.orpha.net/data/patho/GB/uk-BullousPemphigoid.pdf 1
Disease name and synonyms
• Bullous pemphigoid (BP),
• Pemphigoid,
• Lever's pemphigoid.
Definition
BP is an autoimmune subepidermal bullous dermatosis defined immunologically by the
existence of autoantibodies directed against 2 structural proteins found in the
hemidesmosomes of the dermal-epidermal junction. These proteins, called BP antigen 1
(BPAG1) or AgBP230, and BPAG2 (or AgBP180 or collagen XVII) have respective molecular
masses of 230 and 180 kDa [8, 14]. The autoantibodies are localized in vivo along the
epidermal basement membrane. BP is mostly found in the elderly. However, cases have also
been reported in children.
Epidemiology
BP is the most common of the autoimmune bullous dermatoses, representing 70% of these
diseases, with an annual incidence of more than 400 new cases in France [2]. It affects primarily
the elderly (mean age in France: between 75 and 80 years). Very rare familial cases have been
reported.
BP is very rare in children. About 50 cases have been reported [5].
Diagnostic criteria
In typical cases, the diagnosis of BP can be made based on 3 presumptive elements:
• clinical features (bullae developing on erythematous skin predominantly located on the
flexor sides of the limbs);
• histological aspect (subepidermal cleavage);
• direct immunofluorescence (IF) of the affected skin (linear IgG and/or C3 deposits all
along the epidermal basement membrane).
A recent French study validated the following clinical criteria for the diagnosis of BP:
• age over 70 years;
• absence of mucosal involvement;
• absence of atrophic scars;
• absence of preferential involvement of the head, neck and the upper half of the trunk
[15].
The presence of 3 of these 4 criteria allows the diagnosis of BP with a probability of better than
90% for an autoimmune, subepidermal bullous dermatosis with linear IgG and/or C3 deposits
seen on direct IF.
The diagnosis is confirmed by:
• indirect IF detection of serum antibodies, of IgG class, directed against the epidermal
basement membrane of normal human skin separated by molar NaCl, that bind to the
roof of the cleavage zone [4];
Bernard P. Bullous pemphigoïd. Orphanet Encyclopedia, May 2006.
http://www.orpha.net/data/patho/GB/uk-BullousPemphigoid.pdf 2
• the characterization of specific circulating autoantibodies by immunoblotting, detectable
in ~ 80% of pemphigoid patients; they react with at least one of the 2 target antigens of
the hemidesmosome: AgBP230 and/or AgBP180 [8, 14];
• immunoelectron microscopy of a skin biopsy showing immune deposits (IgG, C3) in the
upper part of the lamina lucida [4].
Serological techniques to detect antibodies to BP230 and, especially, BP180 by enzyme-linked
immunosorbent assay (ELISA) have been developed recently. This latter method detects anti-
BP180 antibodies in more than 90% of the sera from patients with BP, particularly antibodies
reacting with the NC16a domain of AgBP180. The epitopes seem to be numerous, particularly
on the extracellular domain of AgBP180. These highly sensitive techniques are still
experimental.
Differential diagnosis
In atypical cases, i.e. those with predominant mucosal involvement, with unusual topography or
scarring of bullous lesions, other autoimmune subepidermal bullous dermatoses with linear IgG
and/or C3 deposits (cicatricial pemphigoid, acquired bullous epidermolysis) can be eliminated
by more sophisticated immunological techniques (immunoelectron microscopy, indirect IF on
skin separated by molar NaCl, immunoblotting, immunoprecipitation).
Clinical description
The disease is characterized clinically by tight bullae, with clear content, often large, developing
primarily on the edge of erythematous plaques [10]. Intense itching is common. The lesions are
symmetrical with a predilection for the flexor sides of the limbs, the anterior-internal face of the
thighs and abdomen. Mucosal lesions are rare, predominantly affecting the buccal mucosa (10-
20% of the cases). Nikolsky's sign is absent. The disease progresses by successive bouts, the
bullae healing without scarring. Affect on the patient's general status is variable; it depends on
the extent of the lesions, and the severity and duration of itching.
Numerous clinical variants have been described like, for example, those with vesicular
lesions suggestive of dermatitis herpetiformis, those with persistent lesions of the major folds,
those with lesions resembling prurigo nodularis or erythroderma, or localized forms.
Diverse immune disorders have been described as isolated cases in association with BP
(rheumatoid arthritis, systemic lupus erythematosus, primary biliary cirrhosis, pemphigus
vulgaris, etc.). Although sometimes included within the framework of a "multiple autoimmune
disease", this kind of association is often coincidental. In contrast, the frequency of diabetes and
psoriasis was shown to be significantly higher during the course of BP in two case-control
studies.
The BP-cancer association has been explored in many studies. At present, it has been
concluded that BP patients have no significantly increased risk of cancer compared to patients
of comparable age.
Childhood BP occurs within the first year of life. It presents as bullous lesions on
erythematous skin or on normal acral skin. In 2002, a classification of childhood BP was
suggested by Fisler et al. [7] into two subtypes:
• Infantile BP
• Childhood localized vulval BP
Bernard P. Bullous pemphigoïd. Orphanet Encyclopedia, May 2006.
http://www.orpha.net/data/patho/GB/uk-BullousPemphigoid.pdf 3
Childhood localized vulval BP is self-limited and non scarring. Prognosis of infantile BP is also
favourable. [7]
Etiology
BP is a specific autoimmune disease whose antigen targets are part of the normal human
dermal-epidermal junction [13]. The mechanisms leading in the elderly subject to rupture of
tolerance of hemidesmosomal proteins are still unknown. Within the hemidesmosome, the
molecules recognized by the autoantibodies are 2 structural proteins normally present in the
dermal-epidermal junction: AgBP230, strictly intracellular, and AgBP180, a transmembrane
protein with a large extracellular domain. The pathogenic character of the antibodies to
AgBP180, which has an extracellular domain directly accessible to the action of the
autoantibodies, has been formally demonstrated in an animal (mouse) model [11]. In contrast,
no definitive proof has yet been obtained supporting the direct pathogenicity of anti-AgBP230
autoantibodies directed against a strictly intracytoplasmic hemidesmosomal protein and thus, in
principle, inaccessible to the direct action of the autoantibodies.
Isolated observations have suggested that the disease could sometimes be induced by
PUVAtherapy (psoralen + UV-A irradiation, i.e., photochemotherapy) or by certain drugs
(spironolactone, bumetanide, fluoxetine, etc.). An epidemiological case-control study suggested
a potential triggering role for spironolactone and, to a lesser degree, neuroleptics [1].
Etiology of childhood BP is also unknown. However, drug intake and vaccination have been
incriminated in some cases [5].
Management including treatment
Studies conducted in France demonstrated that the prognosis of survival of BP patients was
very poor, with a death rate exceeding 30% after 1 year of treatment [3, 12]. Although the
pejorative impact of the presence of circulating anti-AgBP180 antibodies was suggested [3], age
and Karnofsky score of 40 or less have been shown to significantly affect prognosis [12]. It is
likely that co-morbidities and practice patterns (use of immunosuppressive drugs and/or
systemic corticosteroids) also influence overall morbidity and mortality. Nevertheless, the cure
can be achieved, in the absence of complications, in a time interval ranging from 1 to 5 years.
For the severe forms of extensive and progressive pemphigoid, the first-line therapy consists of
corticosteroid monotherapy. Systemic corticotherapy (prednisone: 1 mg/kg/day) remains the
standard treatment for many authors, notably the English and Americans [6].
In France, topical corticotherapy tends to replace systemic steroids, provided that high doses
of dermocorticoids are used as induction therapy (clobetasol propionate: 30-40 g/day).
Regardless of the corticotherapy prescribed, the induction dose is maintained for about 1 month
and until the post bullous lesions are completely healed During the first month of therapy, the
weekly evaluation of treatment efficacy is based on the number of new bullae per day. Then, the
corticosteroid dose is progressively tapered over a period of 4-6 months until a maintenance
dose (5-7 mg/day of prednisone or 20-30 g/week of clobetasol propionate) is reached. This
dose is maintained for several months before complete withdrawal of steroids. In the case of
corticoresistance or corticodependence, adjuvant immunosuppressive therapy (azathioprine:
100-150 mg/day) can be prescribed. In the case of initial corticoresistance in a patient with very
severe pemphigoid, plasma exchanges can be tried.
Bernard P. Bullous pemphigoïd. Orphanet Encyclopedia, May 2006.
http://www.orpha.net/data/patho/GB/uk-BullousPemphigoid.pdf 4
The treatment of choice for localized, pauci-bullous and/or slightly evolving forms of
pemphigoid is topical corticotherapy with class I dermatocorticoids. [9]
Treatment of the 2 infantile forms defined by Fisler et al. with systemic or topical
corticosteroids is all the more effective as it is started early, before the disease becomes
widespread [7]:
A clinical trial on BP is currently carried out in France. The aim is to evaluate the criteria for
stopping corticotherapy.
References
1) Bastuji-Garin S, Joly P, Picard-Dahan C, Bernard P, Vaillant L, Pauwels C, Salagnac V, Lok
C, Roujeau JC. Drugs associated with bullous pemphigoid : a case-control study. Arch Dermatol
1996; 132:272-6.
2) Bernard P, Vaillant , Labeille B, Bedane C, Arbeille B, Denoeux JP, Lorette G, Bonnetblanc
JM, Prost C. Incidence and distribution of subepidermal bullous skin disorders in three French
regions. Arch Dermatol 1995; 131: 48-52.
3) Bernard P, Bedane C, Bonnetblanc JM. Anti-BP180 autoantibodies as a marker of poor
prognosis in bullous pemphigoid: a cohort analysis of 94 elderly patients. Br J Dermatol 1997;
136:694-8.
4) Bernard P, Bedane C. Bullous and cicatricial pemphigoid in Atlas of immunpathology of the
skin, Kanitakis, Vassilieva & Woodley Ed, Chapman & Hill, London 1998.
5) Erbagci Z. Childhood bullous pemphigoid following hepatitis B immunization. J Dermatol.
2002; 29:781-5.
6) Fine JD. Management of autoimmune bullous disases. N Engl J Med 1995; 333:1475-84.
7) Fisler RE, Saeb M, Liang MG, Howard RM, McKee PH. Childhood bullous pemphigoid: a
clinicopathologic study and review of the literature. Am J Dermatopathol. 2003; 25:183-9.
8) Guidice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous
pemphigoid autoantigen BP180. J Invest Dermatol 1992; 99:243-50
9) Joly P, Roujeau JC, Benichou J, Picard C, Dreno B, Delaporte E, Vaillant L, D'Incan M,
Plantin P, Bedane C, Young P, Bernard P; Bullous Diseases French Study Group. A
comparison of oral and topical corticosteroids in patients with bullous pemphigoid. N Engl J Med
2002; 31;346:321-7.
10) Lever WF. Pemphigus and pemphigoid : a review of the advances made since 1964. J Am
Acad Dermatol 1979; 1:2-30.
11) Liu Z, Diaz LA, Troy JL et al. A passive transfer model of the organ-specific auto-immune
disease: bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen
BP180. J Clin Invest 1993; 92:2480-8.
12) Joly P, Benichou J, Lok C et al. Prediction of survival for patients with bullous pemphigoid.
Arch Dermatol 2005;141:691-8.
13) Salmon-Ehr V, Bernard P. Physiopathologie des dermatoses bulleuses auto-immunes de la
jonction dermo-épidermique. Ann Dermatol Vénéréol 1998; 125:817-23.
14) Tanaka T, Parry DA, Klaus-Kovtun V, Steinert PM, Stanley JR. Comparison of molecularly
cloned bullous pemphigoid antigen to desmoplakin I confirms that they define a new family of
cell adhesion junction plaque proteins. J Cell Biol 1991; 266:12555-9
15) Vaillant L, Bernard P, Joly P, Prost C, Labeille B, Bedane C, Arbeille B, Thomine E,
Bertrand P, Lok C, Roujeau JC. Evaluation of clinical criteria for diagnosis of bullous
pemphigoid. Arch Dermatol 1998; 134:1075-80.
Bernard P. Bullous pemphigoïd. Orphanet Encyclopedia, May 2006.
http://www.orpha.net/data/patho/GB/uk-BullousPemphigoid.pdf 5
You might also like
- Parasitology TablesDocument9 pagesParasitology Tables2013SecB92% (26)
- Fitzpatricks Dermatology in General Medicine 8ed 1Document10 pagesFitzpatricks Dermatology in General Medicine 8ed 1ANTINNo ratings yet
- Class 9 Biology Chapter 16 Diseases Causes and ControlDocument6 pagesClass 9 Biology Chapter 16 Diseases Causes and ControlwanroyNo ratings yet
- "Art With Impact Case Study 2021"的副本Document9 pages"Art With Impact Case Study 2021"的副本Longtan JingNo ratings yet
- 13.autoimmune Basement AbreuVelezAMDocument16 pages13.autoimmune Basement AbreuVelezAMJulie Payne-KingNo ratings yet
- Bullous PemphigoidDocument13 pagesBullous PemphigoidSharifah HanimNo ratings yet
- Bullous Pemphigoid: Clinical Practice Guidelines: ReviewDocument19 pagesBullous Pemphigoid: Clinical Practice Guidelines: Reviewsri karuniaNo ratings yet
- Etiology: Psoriasis Lichen Planus Diabetes Mellitus Rheumatoid Arthritis Ulcerative Colitis Multiple SclerosisDocument4 pagesEtiology: Psoriasis Lichen Planus Diabetes Mellitus Rheumatoid Arthritis Ulcerative Colitis Multiple SclerosisnataschaNo ratings yet
- Pemphigus vulgarisPPTDocument23 pagesPemphigus vulgarisPPTSiti HanisaNo ratings yet
- Bernard2017 PDFDocument16 pagesBernard2017 PDFPande Agung MahariskiNo ratings yet
- Bullous Pemphigoid: Etiology, Pathogenesis, and Inducing Factors: Facts and ControversiesDocument9 pagesBullous Pemphigoid: Etiology, Pathogenesis, and Inducing Factors: Facts and ControversiesRiefka Ananda ZulfaNo ratings yet
- Direct Immmuno Flourescence Study in Auto Immune Bullous DisordersDocument3 pagesDirect Immmuno Flourescence Study in Auto Immune Bullous DisordersInternational Organization of Scientific Research (IOSR)No ratings yet
- 2020 - Pemphigus Vulgaris and Bullous Pemphigoid Update On Diagnosis and TreatmentDocument12 pages2020 - Pemphigus Vulgaris and Bullous Pemphigoid Update On Diagnosis and TreatmentnancyerlenNo ratings yet
- Bullous Pemphigoid: EtiologyDocument22 pagesBullous Pemphigoid: EtiologyDanielNo ratings yet
- New Diagnosis Criteria For Common Variable Immunodeficiency Exploration Systematic Review and Current PerspectivesDocument8 pagesNew Diagnosis Criteria For Common Variable Immunodeficiency Exploration Systematic Review and Current PerspectivesInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Pi Is 0011853213000542Document20 pagesPi Is 0011853213000542Jose Antonio RamosNo ratings yet
- 2 5456181012360856193Document70 pages2 5456181012360856193Maha KhalidNo ratings yet
- Blistering DisordersDocument36 pagesBlistering Disordersallenshanique1999No ratings yet
- Ecthyma Gangrenosum - StatPearls - NCBI BookshelfDocument5 pagesEcthyma Gangrenosum - StatPearls - NCBI BookshelfSyafira Laila NurulitaNo ratings yet
- Diagnosing New Autoimmune Blistering Skin Diseases of Dogs and CatsDocument5 pagesDiagnosing New Autoimmune Blistering Skin Diseases of Dogs and CatsLauNo ratings yet
- Bullous Pemphigoid: Primary Care Diagnostic InstituteDocument1 pageBullous Pemphigoid: Primary Care Diagnostic InstituteMuhammad Alief FahrenNo ratings yet
- Bullous Pemphigoid: PathogenesisDocument5 pagesBullous Pemphigoid: PathogenesisLukman GhozaliNo ratings yet
- PemphigusDocument32 pagesPemphigusAlondra CastilloNo ratings yet
- Vesiculobullous DiseasesDocument40 pagesVesiculobullous Diseasessgoeldoc_550661200100% (1)
- Photo QuizDocument21 pagesPhoto Quizabas_maytham1021100% (4)
- Seminar: Enno Schmidt, Detlef ZillikensDocument13 pagesSeminar: Enno Schmidt, Detlef ZillikensUssiy RachmanNo ratings yet
- Pyoderma Gangrenosum: Etiology and PathogenesisDocument13 pagesPyoderma Gangrenosum: Etiology and PathogenesisIga Nurwani RidwanNo ratings yet
- Epiderm BulosaDocument10 pagesEpiderm BulosaAnonymous hTivgzixVNNo ratings yet
- Diagnosis of Autoimmune Blistering DiseasesDocument14 pagesDiagnosis of Autoimmune Blistering DiseasesElisa CarreraNo ratings yet
- Chapter 53:: Paraneoplastic Pemphigus:: Grant J. Anhalt & Daniel MimouniDocument15 pagesChapter 53:: Paraneoplastic Pemphigus:: Grant J. Anhalt & Daniel MimouniFiraNo ratings yet
- Pemphigoid Gestationis: Case Report and Review of LitteratureDocument5 pagesPemphigoid Gestationis: Case Report and Review of LitteratureIJAR JOURNALNo ratings yet
- Yang 2019Document11 pagesYang 2019RaissaNo ratings yet
- Jurnal DR - ResatiDocument34 pagesJurnal DR - ResatiIwanAtassonggeNo ratings yet
- Artikel Kelainan Kulit - Pioderma DLLDocument251 pagesArtikel Kelainan Kulit - Pioderma DLLRosa SeptianaNo ratings yet
- Ulcerated Lesions 5555555Document11 pagesUlcerated Lesions 5555555ربيد احمد مثنى يحيى كلية طب الاسنان - جامعة عدنNo ratings yet
- Childhood Erythrodermic Lichen Planus Pemphigoides After Nonavalent Human Papillomavirus VaccinationDocument3 pagesChildhood Erythrodermic Lichen Planus Pemphigoides After Nonavalent Human Papillomavirus Vaccinationnurul hidayatiNo ratings yet
- 13 DH-OrtizBDocument4 pages13 DH-OrtizBD U N I A A N I M ENo ratings yet
- Childhood PsioriasisDocument6 pagesChildhood PsioriasisYuliana DaisongNo ratings yet
- Pemphigus: by Demetris Ioannides, MD Associate Professor of DermatologyDocument43 pagesPemphigus: by Demetris Ioannides, MD Associate Professor of DermatologyYulita PurbaNo ratings yet
- Japanese Guidelines For The Management of Pemphigoid (Including Epidermolysis Bullosa Acquisita)Document34 pagesJapanese Guidelines For The Management of Pemphigoid (Including Epidermolysis Bullosa Acquisita)Rizka ZulaikhaNo ratings yet
- ImmunoBullous DisordersDocument18 pagesImmunoBullous DisordersNali peterNo ratings yet
- Nonbullous Pemphigoid: Prodrome of Bullous Pemphigoid or A Distinct Pemphigoid Variant?Document7 pagesNonbullous Pemphigoid: Prodrome of Bullous Pemphigoid or A Distinct Pemphigoid Variant?FatimaNo ratings yet
- Bullous Disease: Farah Alsheikh Dima ArabiyatDocument58 pagesBullous Disease: Farah Alsheikh Dima ArabiyatAhmad AltarefeNo ratings yet
- Review On Pathogenesis of Pemphigus: Dr. P. T. Chan Social Hygiene Service (Dermatology), Department of Health, Hong KongDocument7 pagesReview On Pathogenesis of Pemphigus: Dr. P. T. Chan Social Hygiene Service (Dermatology), Department of Health, Hong KongMeuthia AlamsyahNo ratings yet
- Bullous Skin Disorders: Assist Prof. DR - Ali Elethawi Specialist Dermatologist C.A.B.D, F .I .C.M.SDocument47 pagesBullous Skin Disorders: Assist Prof. DR - Ali Elethawi Specialist Dermatologist C.A.B.D, F .I .C.M.SBurcu KimNo ratings yet
- Oral Mucous Membrane Pemphigoid: Updates in Diagnosis and ManagementDocument4 pagesOral Mucous Membrane Pemphigoid: Updates in Diagnosis and ManagementtitusmulambaNo ratings yet
- Guillain-Barré Syndrome (GBS) : Anand B. Pithadia, Nimisha KakadiaDocument13 pagesGuillain-Barré Syndrome (GBS) : Anand B. Pithadia, Nimisha KakadiaKahfi Rakhmadian KiraNo ratings yet
- Bullos DiseaseDocument4 pagesBullos DiseaseFiras HamidehNo ratings yet
- Guillain-Barre SyndromeDocument42 pagesGuillain-Barre Syndromesuraj rajpurohitNo ratings yet
- Inmunoterapia Del Síndrome de Guillain-BarréDocument45 pagesInmunoterapia Del Síndrome de Guillain-Barréfrancisco bacaNo ratings yet
- BULLOUS PEMPHIGOID-anto-1Document21 pagesBULLOUS PEMPHIGOID-anto-1Nur RifqahNo ratings yet
- Lecture 5/ Medical Microbiology / 2ed Class StreptococcusDocument9 pagesLecture 5/ Medical Microbiology / 2ed Class Streptococcusهدى قحطان جليلNo ratings yet
- 1 s2.0 S1578219020300792 MainDocument2 pages1 s2.0 S1578219020300792 MainFeyzullah TokgözNo ratings yet
- Ecthyma Gangrenosum That Revealed An Agammaglobulinemia: A Case ReportDocument4 pagesEcthyma Gangrenosum That Revealed An Agammaglobulinemia: A Case ReportIJAR JOURNALNo ratings yet
- Ecthyma Gangrenosum That Revealed An Agammaglobulinemia: A Case ReportDocument4 pagesEcthyma Gangrenosum That Revealed An Agammaglobulinemia: A Case Reportalafiyasaifee30No ratings yet
- 2 Gram Negative Bacterial InfectionDocument89 pages2 Gram Negative Bacterial InfectionCoy NuñezNo ratings yet
- Bullous Pemphigoid Profile and Outcome in A Series of 100 Cases in SingaporeDocument4 pagesBullous Pemphigoid Profile and Outcome in A Series of 100 Cases in SingaporeJual Beli PromosiNo ratings yet
- Case Report: Subcorneal Pustular Dermatosis An Immnohisto-Pathological PerspectiveDocument4 pagesCase Report: Subcorneal Pustular Dermatosis An Immnohisto-Pathological PerspectiveLewishoppusNo ratings yet
- Diagnosisandclinical Featuresofpemphigus Vulgaris: Supriya S. Venugopal,, Dédée F. MurrellDocument1 pageDiagnosisandclinical Featuresofpemphigus Vulgaris: Supriya S. Venugopal,, Dédée F. MurrellDwiKamaswariNo ratings yet
- Biomedicines 10 01197 v2Document10 pagesBiomedicines 10 01197 v2Silvia Montejo FareloNo ratings yet
- Micro ChAP 15Document34 pagesMicro ChAP 15Farah ZahidNo ratings yet
- Bollous MCQDocument21 pagesBollous MCQalh bashar100% (1)
- Family Case PresentationDocument61 pagesFamily Case PresentationMay Chelle ErazoNo ratings yet
- GR 11 Modyul 5 7 Personal Dev.Document13 pagesGR 11 Modyul 5 7 Personal Dev.Louielyn MagalangNo ratings yet
- Haemoglobin J Iron and Total Iron-Binding CapacityDocument21 pagesHaemoglobin J Iron and Total Iron-Binding CapacityK WNo ratings yet
- A Practical Approach To Gynecologic OncologyDocument244 pagesA Practical Approach To Gynecologic Oncologysalah subbah100% (1)
- HF ArticleDocument14 pagesHF ArticleDR. Shobhit RajNo ratings yet
- FloTrac Sensor Clinical UtilityDocument21 pagesFloTrac Sensor Clinical UtilityAnestesia 2017 UDECNo ratings yet
- Vtal Signs LectureDocument89 pagesVtal Signs LectureHazel Romerosa AmatosNo ratings yet
- Budwig Cancer Guide PDFDocument97 pagesBudwig Cancer Guide PDFDiana MartincowskiNo ratings yet
- Fostamatinib For The Treatment of Warm Antibody Autoimmune Hemolytic Anemia Phase 2Document9 pagesFostamatinib For The Treatment of Warm Antibody Autoimmune Hemolytic Anemia Phase 2Maria Eugenia VelisNo ratings yet
- BIO103 Course Syllabus - IQACDocument14 pagesBIO103 Course Syllabus - IQACBarnardoNo ratings yet
- 1 - Partial Pulpotomy With Two Bioactive Cements in Permanent Teeth of 6 - To 18-Year-Old Patients WithDocument11 pages1 - Partial Pulpotomy With Two Bioactive Cements in Permanent Teeth of 6 - To 18-Year-Old Patients WithAbdul Rahman AlmishhdanyNo ratings yet
- Manajemen Cairan Pada Luka BakarDocument38 pagesManajemen Cairan Pada Luka Bakarwati suwartaNo ratings yet
- Literature Review On Lipid ProfileDocument7 pagesLiterature Review On Lipid Profileea7gjrm5100% (1)
- Diabetic KetoacidosisDocument35 pagesDiabetic KetoacidosisTatik AgilaNo ratings yet
- NCP For Acute Coronary SyndromeDocument3 pagesNCP For Acute Coronary Syndromesarahtot75% (4)
- Mehrdad Mohammadpour MD Resume: OphthalmologyDocument15 pagesMehrdad Mohammadpour MD Resume: OphthalmologyHamed NazariNo ratings yet
- Systemic Methotrexate Treatment in Childhood Psoriasis: Further Experience in 24 Children From IndiaDocument5 pagesSystemic Methotrexate Treatment in Childhood Psoriasis: Further Experience in 24 Children From IndiaNana AdistyNo ratings yet
- Annotated Bibliography: Recovery MonthDocument3 pagesAnnotated Bibliography: Recovery MonthAnmari SablanNo ratings yet
- 1 - Cairo Uni - Final Revision MCQ PedoDocument43 pages1 - Cairo Uni - Final Revision MCQ PedoYugi El-yaddakNo ratings yet
- 2020 Immediate Care of The Newborn 3Document56 pages2020 Immediate Care of The Newborn 3Ellah PerenioNo ratings yet
- BOT - EXPERIMENT NO.2 PLANT HORMONE - Group5Document14 pagesBOT - EXPERIMENT NO.2 PLANT HORMONE - Group5Zionne TanafrancaNo ratings yet
- Mumps VirusDocument13 pagesMumps VirusLara MasriNo ratings yet
- Aes 05 37Document13 pagesAes 05 37Marco Antonio MiraveteNo ratings yet
- Course in The Ward 2Document17 pagesCourse in The Ward 2ABBEYGALE JOYHN GALANNo ratings yet
- Treatment For Spider Veins On LegsDocument3 pagesTreatment For Spider Veins On LegsZackNo ratings yet
- Safety Summary For SceneScope SCDocument2 pagesSafety Summary For SceneScope SCjuk expertNo ratings yet
- Micro-G EnvironmentDocument11 pagesMicro-G Environmentالقيصر صالحNo ratings yet