Professional Documents
Culture Documents
Nitriglycerine Patent US913653
Nitriglycerine Patent US913653
Uploaded by
Mekar Meina0 ratings0% found this document useful (0 votes)
3 views2 pagesCopyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
3 views2 pagesNitriglycerine Patent US913653
Nitriglycerine Patent US913653
Uploaded by
Mekar MeinaCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
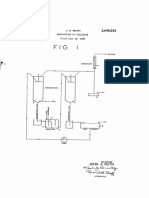
JNITED STATES PATENT OFFICE.
--------
: FRANZ AIGNER, OF POTSDAM, GERMANY., ASSIGNOR TO THE FIRM OF DYNAMIT-ACTIEN
GESELLSCHAFT woRMALS ALFRED NOBEL UND CO., OF HAMBUKG, GERMANY.
PROCESS OF MANUFACTURING NITRoGLYCERIN.
No. 913,653. Specification of Letters Patent.
Application filed February 28, 1905. Serial No. 247,777.
Patented Feb. 28, 1909.
To all whom it may concern: revived nitrating acid of tie same relative
Be it known ti at I, FRANZ AIGNER, a sub
uly
proportions of acids and water
ject of the Emperor of Austria-Hungary, re
siding at 10 Neue König. traige, iPotsdam, . . (HSO,:HNO, H.O. = 60:30:10),
Kingdom of Prussia, Geinian Empire, jave but composed of 450grams of waste acid
invented new and usefui Improvements in from a previous nitrating operation and 450
Processes of Manufacturing Nitroglycerin, of grams fresh acid. A similar favorable result
Willicii tie following is a specification. .
This invention relates to tie process of was obtained by a furter use of tire already .
once revived nitrating acid containing glyc
manufactuirng nitro-glycerin by causing a erin compounds: I obtained 225 grams pure 6 s
Imixture of nitric acid aid sulfuric acid to act Jaitro-glycerin by nitrating 100 grams glyc
on glycerin, and iias for its object to render egin witi,900 grams
the process more expeditious, considerably vived, of the same nitrating acid, twice re.
relative proportions of .
safer and ci:eaper... .. . .
Tite common process of manufacturing acids and water as quoted .
nitro-glycerin differs froin the usual process (IISO,:HNOHO = 60:30:10) 7
of manufacturing hitro-cellulose among but composed of 450 grams waste acid from
other things essentially in that the waste the third nitrating operation and 450 grams
acids from tie nitration of glycerin cannot be fresh acid.
20 revived but unust be denitrated. The reason
. . . .. . .."
The new technical effect obtained
of the said difference is that the nitrating revived waste nitrating acid containing glyc by using
acids of the nitro-glycerin manufacture are . erin compounds consists in an essential in
used up by the nitrating process in a larger creasing
degree than in tie manufacture of nitrocellu glycerin. ofThe the produced amount of nitro
said effect is shown in an 8. 3.
lose. From these reasons the waste acids of especially distinct
the nitro-glycerin manufacture that have ing acid is used. Idegree obtained if a 170
diluted
grainsnitrat
pure.
been separated from the nitroglycerin by nitro-glycerin by nitrating 100 grams glyc
settling etc. are with reference to tieir com erin with 1200 grams fresh nitrating acid of
position by far inferior to those of the nitro the composition
30 cellulose manufacture. .. .
S5
I have now found that the reviving of the HSO,------------------, 56.7%
waste acids of the nitro-glycerin manufac HNO.------------------ 28.3%
ture (which acids contain glycerin com HO-- - - - - - - - - - - - - - - - - - 15.0%
pounds) gives an unexpected advantage, be
35 cause revived waste acid gives an essentially I obtained however 218 grams pure nitro 9
higher amount of nitro-glycerin than the glycerin, i.e. surplus of 48 grams over the C.
original nitrating acid with tile same relative glycerin with 1200bygrams
quoted amount, nitrating 100 grams
once revived ni
proportions of sulfuric acid, nitric acid and trating acid of the same relative proportions
water in the same manner of operation.
40 reviving of the waste acid containing glyc
The of acids and water as quoted . .
erin compounds is effected by adding a fresh (HSO,:HNO,: H.O-56.7:28.3:15.0)
mixture of highly concentrated sulfuric and but composed of 750 grams waste acid from .
95
nitric acid to the waste acid. . in a suitable
proportion. .. . the first nitration and 450 grams fresh
45 In nitrating 100 grams of glycerin with 900 nitrating acid. A twice revived waste acid
grams of freshly prepared nitrating acid of of the same percentage gave 221 grams pure l 00
nitro-glycerin, i. e. a surplus of 51 grams
the composition when compared with the result of the fresh
50
HSO,---------------...-- 60% nitrating acid. One can obtain the said
HNO-. - - - - - - ------------ 30% . .advantage of an increased yield of pure
- sO------- -------------- 10% nitro-glycerin by using revived nitrating 105
I have for instance obtained 201 grams pure water,even
acid with acids containing 20 per cent.
nitroglycerin. I obtained, however, 228 tures whichapproaching
i.e. to those acid mix
cannot have any nitrating
grams pure nitroglycerin in nitrating 100 action.
55 grams glycerin with 900 grams of a once The difference in the nitrating action 110
2. 913,653
between a revived nitrating acid and a fresh yield of trinitroglycerin can be obtained than
acid has been quite unknown in the manu inventor hasthealsowaste
by causing acid to stand. The
stated the reason for the
facture of nitro-cellulose. The possibility increasing of the yield of trinitroglycerin by 50
by using revived acids of obtaining essen using a waste acid. In the nitrating process
tially higher amounts of nitroglycerin than
by using fresh acids enables the operator to carried out in the ordinary way, besides
trinitroglycerin
vary the degree of concentration of nitrating glycerin) relatively (i. e. the common nitro
acids in a relatively wide range and espe nitrated nitroglycerins large amounts of lower
cially to use less concentrated nitrating monoglycerin) will be (dinitroglycerin generally
and 55
formed.
0 acids, the use of which was formerly un The common trinitroglycerin now is very
economical in the prior process without re
viving. The concentration of the usual slightly soluble inrelatively
acids andcompletely.
separates there
nitrating acid for manufacturing nitro-glyc mononitroglycerin and dinitroglycerin The
fore quickly and
are, 60
erin differed hitherto only by 5 per cent. It however, very soluble in acids and can there
15 has been considered hitherto by all experts fore not separate, even by very long stand
as nearly unavoidable to use only acids of ing. Mononitroglycerin and dinitroglycerin
the highest concentration in order to pre are converted into trinitroglycerin, if one
vent a reduction of the yield of nitro causes
glycerin to below 200 grams of pure nitro On thisfresh the
nitrating acids to act on them. 65
new process is based.
20 glycerin, the average yield being 205 grams What I claim as my invention, and desire
R" nitro-glycerin from 100 grams glycerin. secure by Letters Patent, is
y the possibility given by the present in to The process of nitrating glycerin to ob
vention of using acids with 10 and even 15
per cent. water one obtains the further ad from successively tain a maximum yield of tri-nitro-glycerin 70
treated portions of glyc
2. vantage of a greater security for the nitrat erin, which consists in nitrating a quantity
ing operation. For instance I obtain an of glycerin with a suitable quantity of a
essentially gentle nitration of glycerin with fresh mixture of concentrated nitric and
out the formation of froth by using a nitrat sulfuric acids, separating the resultant tri 75
ing acid of the composition nitro-glycerin from the partially exhausted
30 (HSO:HNO:HO = 60:30:10) acids, containing less highly nitrated de
according to my method instead of the usual rivatives tity of
of glycerin, adding a suitable quan
fresh concentrated nitric and sulfuric
mixture. acids to restore the acid mixture to sub 80
HSO,------------------- 60% stantially its original percentage content of
35 HNO - - - - - - - - - - - - - - - - - - - - 35% nitric acid, sulfuric acid and water, and
HO--------------------- 5% ! nitrating
said acid
a second portion of glycerin with
mixture, substantially as described.
The greater yield of trinitroglycerin (; b Jn testimony whereof
tained by using the waste acid instead of name to this specificationIinhave the
signed my
presence of
40 fresh acids alone is not caused by nitro two subscribing witnesses.
glycerin suspended in the spent acid. By FRANV, Al(N.E.R.
causing waste acid from nitrating glycerin
stand for a sufficient time, one can only ob Witnesses:
to
tain from it1.1process,
to 1.4%however,
trinitroglycerin. By Wol, DEMAR IIAUPT,
IEN Y IIASPER.
45 the presel a far greater
You might also like
- McCombie-EtNO2 From Et2SO4Document2 pagesMcCombie-EtNO2 From Et2SO4Tilen SeverNo ratings yet
- Buku Teks Digital KSSM - Additional Science Form 4Document207 pagesBuku Teks Digital KSSM - Additional Science Form 4Mohammad SaifulNo ratings yet
- US457002Document2 pagesUS457002AdhiNo ratings yet
- Piric Acid PatentDocument2 pagesPiric Acid PatentOmar valdesNo ratings yet
- Beer Production 02Document8 pagesBeer Production 02Denny SamuelNo ratings yet
- United States Patent: Benzene From Pyrolysis Naphtha Produced by High-TemperaDocument8 pagesUnited States Patent: Benzene From Pyrolysis Naphtha Produced by High-TemperalandagoNo ratings yet
- US3510538 PatentDocument3 pagesUS3510538 PatentrgNo ratings yet
- UNITED STATES Pitjlllillitl'll @FFICE.: Sees-IsDocument3 pagesUNITED STATES Pitjlllillitl'll @FFICE.: Sees-IsWira Pratiwi PinemNo ratings yet
- United States Patent Office: 1. 2 This Serves Not Only To Free Nitrourea From The Acid SoluDocument3 pagesUnited States Patent Office: 1. 2 This Serves Not Only To Free Nitrourea From The Acid SoluSmokeNo ratings yet
- PPR635 KraftLigninExtractionDocument24 pagesPPR635 KraftLigninExtractionOvelly AmamehiNo ratings yet
- United States Patent OfficeDocument3 pagesUnited States Patent OfficefhafizfrNo ratings yet
- Patent For ...Document2 pagesPatent For ...TriNurRahmaNo ratings yet
- Uniteolstates Patent Office: "133.2535.lftii?iiii'tfiiiw 7 'Document2 pagesUniteolstates Patent Office: "133.2535.lftii?iiii'tfiiiw 7 'Juan Camilo HenaoNo ratings yet
- United States Patent Office: Patented May 4, 1954Document4 pagesUnited States Patent Office: Patented May 4, 1954Stefano Martin Lizarbe WongNo ratings yet
- United States Patent Office: Patented Nov. 7, 1950Document2 pagesUnited States Patent Office: Patented Nov. 7, 1950bayuminecraftNo ratings yet
- United States Patent (19) (11) Patent Number: 5,990,074: Gross Et Al. (45) Date of Patent: Nov. 23, 1999Document5 pagesUnited States Patent (19) (11) Patent Number: 5,990,074: Gross Et Al. (45) Date of Patent: Nov. 23, 1999AmeerRashidNo ratings yet
- ' United States Patent Office : Ljatented Nov. 7, 195.0Document2 pages' United States Patent Office : Ljatented Nov. 7, 195.0Agape Ruth BaliloNo ratings yet
- United States Patent Office: Patented Sept. 23, 1952Document2 pagesUnited States Patent Office: Patented Sept. 23, 1952ZDENKO SEBASTIAN CHAMERY CUEVASNo ratings yet
- United States Patent Office: Patented Nov. 13, 1956Document2 pagesUnited States Patent Office: Patented Nov. 13, 1956karmilaNo ratings yet
- Us 3625879 PatentDocument8 pagesUs 3625879 PatentJuPe Juniawan PrakosoNo ratings yet
- United States PatentDocument4 pagesUnited States Patentwiam wiamNo ratings yet
- The Catalytic Polymerization of Butylenes - S H McallisterDocument4 pagesThe Catalytic Polymerization of Butylenes - S H McallisterJasonChristianNo ratings yet
- United States Patent Office: Patented Oct. 7, 1958Document4 pagesUnited States Patent Office: Patented Oct. 7, 1958udinPet0tNo ratings yet
- United States: Patent OfficeDocument3 pagesUnited States: Patent OfficeIRIENE DELFITA TKIMNo ratings yet
- Us 3689541Document6 pagesUs 3689541Santiago BorgesNo ratings yet
- pNO DPADocument4 pagespNO DPATri DoNo ratings yet
- US3049543Document3 pagesUS3049543Julia Acevedo FuentesNo ratings yet
- United States Patent (191: Merk Er AlDocument4 pagesUnited States Patent (191: Merk Er AlPlatiitha Winchester WilliamsNo ratings yet
- PA-PAC Eutectic MixturesDocument4 pagesPA-PAC Eutectic MixturesRajeshNo ratings yet
- United States Patent Office.: Be It Known That I, HAROLD HIBBERT, A Formula in The Following MannerDocument2 pagesUnited States Patent Office.: Be It Known That I, HAROLD HIBBERT, A Formula in The Following MannerTeleson MarquesNo ratings yet
- PETNDocument5 pagesPETNDaniel Ivan CortiNo ratings yet
- US3816523Document5 pagesUS3816523Argo Rizky Kusuma 2007110716No ratings yet
- United States Patent Office: Patented Jan. 19, 1943Document6 pagesUnited States Patent Office: Patented Jan. 19, 1943ahmad taufikNo ratings yet
- Metodo de Purificação Do EsterDocument2 pagesMetodo de Purificação Do Esterbeatriz cristina de mirandaNo ratings yet
- Acrylol SynthesisDocument2 pagesAcrylol Synthesis8612106535No ratings yet
- US2468923Document3 pagesUS2468923rajesh kothariNo ratings yet
- US3903185Document6 pagesUS3903185Muhammad Akbar FahleviNo ratings yet
- Polymerization, A Gasoline: New SourceDocument5 pagesPolymerization, A Gasoline: New Sourcehadi H.HussenNo ratings yet
- Benzyl Cyanide: α-TolunitrileDocument3 pagesBenzyl Cyanide: α-TolunitrileEric M NevarezNo ratings yet
- Purification of Benzoic Acid by Sublimation andDocument4 pagesPurification of Benzoic Acid by Sublimation andKat Visco100% (2)
- US2789119Document3 pagesUS2789119Feride Elif ErtürkNo ratings yet
- US2960514Document4 pagesUS2960514PRASSAN SHAHNo ratings yet
- United States Patent: Bartholomé Et AlDocument8 pagesUnited States Patent: Bartholomé Et AlWidya Isti AriantiNo ratings yet
- US3373187Document2 pagesUS3373187Haruo YamashitaNo ratings yet
- 2,4-Dimethyl-3,5-Dicarbethoxypyrrole: 2,4-Pyrroledicarboxylic Acid, 3,5-Dimethyl-, Diethyl EsterDocument3 pages2,4-Dimethyl-3,5-Dicarbethoxypyrrole: 2,4-Pyrroledicarboxylic Acid, 3,5-Dimethyl-, Diethyl EsterlibretasviejasNo ratings yet
- 1933 Process For Manufacturing Barium Sulphate of Definite Granule SizeDocument2 pages1933 Process For Manufacturing Barium Sulphate of Definite Granule SizeDeluxe pNo ratings yet
- US2846376Document4 pagesUS2846376Manoj BNo ratings yet
- SPE 164768 Experimental Investigation of Low Salinity Hot Water Injection To Enhance The Recovery of Heavy Oil ReservoirsDocument10 pagesSPE 164768 Experimental Investigation of Low Salinity Hot Water Injection To Enhance The Recovery of Heavy Oil ReservoirsMohanned KhairyNo ratings yet
- Process For The Manufacture of Citric Acid Esters of Partial Fatty Acid GlyceridesDocument3 pagesProcess For The Manufacture of Citric Acid Esters of Partial Fatty Acid GlyceridesHarry CortezNo ratings yet
- Biotech Bioengineering - 20 April 1987 - Bar - An Unusual Pattern of Product Inhibition Batch Acetic Acid FermentationDocument3 pagesBiotech Bioengineering - 20 April 1987 - Bar - An Unusual Pattern of Product Inhibition Batch Acetic Acid FermentationEvelin RamirezNo ratings yet
- The Oil: CrackingDocument2 pagesThe Oil: CrackingFinka Pertama PutriNo ratings yet
- 4 5 SubramaniumDocument28 pages4 5 SubramaniumDharamNo ratings yet
- Salt Purification - 3Document4 pagesSalt Purification - 3Ruchita PoilkarNo ratings yet
- Poeb86 Nravi2Document8 pagesPoeb86 Nravi2Navin COYGNo ratings yet
- Us 2731492Document3 pagesUs 2731492ayuniNo ratings yet
- Sept. 25, 1951 L. A. Stenge 2,568,901: Eles. 44tagedDocument4 pagesSept. 25, 1951 L. A. Stenge 2,568,901: Eles. 44tagedwakanda foreverNo ratings yet
- US2373717Document2 pagesUS2373717Ruchita PoilkarNo ratings yet
- US3383416-Process For Preparing AminophenolDocument3 pagesUS3383416-Process For Preparing AminophenolconsultshreejiNo ratings yet
- Us 4772757Document8 pagesUs 4772757Naomi HerreraNo ratings yet
- The Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresFrom EverandThe Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresRating: 5 out of 5 stars5/5 (1)
- Production of Sorbitol Via Catalytic Transfer HydrDocument12 pagesProduction of Sorbitol Via Catalytic Transfer HydrnicoNo ratings yet
- Swain P PDFDocument23 pagesSwain P PDFnicoNo ratings yet
- Ghanta Ku 0099D 12191 DATA 1Document259 pagesGhanta Ku 0099D 12191 DATA 1nicoNo ratings yet
- Swain P PDFDocument23 pagesSwain P PDFnicoNo ratings yet
- US3923967Document9 pagesUS3923967nicoNo ratings yet
- Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release StudiesDocument21 pagesPoly-Lactic Acid: Production, Applications, Nanocomposites, and Release StudiesnicoNo ratings yet
- Simulation of The Acrylic Acid Production Process Through Catalytic Oxidation of Gaseous Propylene Using Chemcad ® SimulatorDocument10 pagesSimulation of The Acrylic Acid Production Process Through Catalytic Oxidation of Gaseous Propylene Using Chemcad ® SimulatornicoNo ratings yet
- European Patent Specification: ThereinDocument7 pagesEuropean Patent Specification: ThereinnicoNo ratings yet
- Poly (Lactic Acid) - Mass Production, Processing, Industrial Applications, and End of LifeDocument35 pagesPoly (Lactic Acid) - Mass Production, Processing, Industrial Applications, and End of LifenicoNo ratings yet
- Acetone-Butanol Fermentation PR: EngineeringDocument6 pagesAcetone-Butanol Fermentation PR: EngineeringnicoNo ratings yet
- Newly Developed Techniques On Polycondensation, Ring-Opening Polymerization and Polymer Modification: Focus On Poly (Lactic Acid)Document15 pagesNewly Developed Techniques On Polycondensation, Ring-Opening Polymerization and Polymer Modification: Focus On Poly (Lactic Acid)nicoNo ratings yet
- US2439791 Patente SaccharoperacetobutilicumDocument4 pagesUS2439791 Patente SaccharoperacetobutilicumnicoNo ratings yet
- Butanol Production Using Clostridium Beijerinckii BA101 Hyper-Butanol Producing Mutant Strain and Recovery by PervaporationDocument12 pagesButanol Production Using Clostridium Beijerinckii BA101 Hyper-Butanol Producing Mutant Strain and Recovery by PervaporationnicoNo ratings yet
- Clostidium PropagacionDocument2 pagesClostidium PropagacionnicoNo ratings yet
- APR GeneralDocument53 pagesAPR GeneralAlex McMinnNo ratings yet
- 03 Stoichiometry With AnswersDocument19 pages03 Stoichiometry With Answersapi-287405319100% (1)
- Levels of Biological OrganizationsDocument23 pagesLevels of Biological OrganizationsYuu KieNo ratings yet
- Chestnut Oak Shells Activated CarbonDocument41 pagesChestnut Oak Shells Activated CarbonVũ Văn NguyênNo ratings yet
- EMB - Carboxypeptidase YDocument2 pagesEMB - Carboxypeptidase YJim Well MartinNo ratings yet
- Lewis Acid-Activated Reactions of Silyl Ketenes For The PreparationDocument8 pagesLewis Acid-Activated Reactions of Silyl Ketenes For The PreparationJonathan MendozaNo ratings yet
- Unit-1: by Kiran Walia, CDAC, NoidaDocument20 pagesUnit-1: by Kiran Walia, CDAC, NoidaAdityaNo ratings yet
- Experimental Determination of Hansen Solubility Parameters For Select POSS and Polymer Compounds As A Guide To POSS Polymer Interaction PotentialsDocument6 pagesExperimental Determination of Hansen Solubility Parameters For Select POSS and Polymer Compounds As A Guide To POSS Polymer Interaction Potentialsmarco_ravelo_10No ratings yet
- Adobe Scan Mar 01, 2021Document15 pagesAdobe Scan Mar 01, 2021Mayank MishraNo ratings yet
- Electrochemistry With AnswersDocument27 pagesElectrochemistry With AnswersKris CruzNo ratings yet
- Cambridge Igcse Chemistry Revision GuideDocument50 pagesCambridge Igcse Chemistry Revision GuideReta SahawnehNo ratings yet
- Mosh Moah Pao Synthetics Considerations enDocument7 pagesMosh Moah Pao Synthetics Considerations enDANIEL ZORRONo ratings yet
- Test-3 (State Board) - (Chem) - Paper - 19.02.2022Document4 pagesTest-3 (State Board) - (Chem) - Paper - 19.02.2022Ammar AnsariNo ratings yet
- Rev. A English 09 / 2020: SpecificationDocument11 pagesRev. A English 09 / 2020: SpecificationNuno ciprianoNo ratings yet
- Metal Adatoms On Graphene and Hexagonal Boron Nitride: Towards The Rational Design of Self-Assembly TemplatesDocument5 pagesMetal Adatoms On Graphene and Hexagonal Boron Nitride: Towards The Rational Design of Self-Assembly Templatesanca irinaNo ratings yet
- Presentation ON Minerals: Submitted By:Shubham Gupta Submitted To:Mr. Rajesh Bijalwan SirDocument8 pagesPresentation ON Minerals: Submitted By:Shubham Gupta Submitted To:Mr. Rajesh Bijalwan SirTitiksha NegiNo ratings yet
- Characterization of Fayalite From Copper Slags: January 2010Document11 pagesCharacterization of Fayalite From Copper Slags: January 2010EREED saasaNo ratings yet
- Quartzene in Coatings - Technical BulletinDocument12 pagesQuartzene in Coatings - Technical BulletinrasasiNo ratings yet
- High Production Volume Status of Chemicals On The 2019Document20 pagesHigh Production Volume Status of Chemicals On The 2019Marcos ROSSINo ratings yet
- Progress in Materials Science: Lenka Kunc Ická, Radim Kocich, Terry C. LoweDocument49 pagesProgress in Materials Science: Lenka Kunc Ická, Radim Kocich, Terry C. LoweDaniel FridmanNo ratings yet
- CHEMISTRY FOR CIVIL ENGINEERS Supplementary Academic Educational MaterialDocument107 pagesCHEMISTRY FOR CIVIL ENGINEERS Supplementary Academic Educational MaterialErnestoTresNo ratings yet
- Calculating Moles To Grams PDFDocument6 pagesCalculating Moles To Grams PDFjaymarmandiaNo ratings yet
- Design and Analysis of Catalytic ConvertDocument13 pagesDesign and Analysis of Catalytic ConvertMi Ra DarilagNo ratings yet
- IECR (Vol. 59, No. 20, P. 9459-9468, 2020) - Acs - Iecr.0c01061Document10 pagesIECR (Vol. 59, No. 20, P. 9459-9468, 2020) - Acs - Iecr.0c01061Dika CodNo ratings yet
- TOTAL PROTEIN LiquicolorDocument1 pageTOTAL PROTEIN LiquicolorMaher100% (1)
- Shapes of MoleculesDocument17 pagesShapes of Moleculesbasaallen566No ratings yet
- 9E Quick QuizDocument3 pages9E Quick QuizZain AliNo ratings yet
- Acid Recap 3E4 28 JuneDocument2 pagesAcid Recap 3E4 28 JuneChen Soon Cheng (Unityss)No ratings yet
- Materials: Classification and CategoriesDocument1 pageMaterials: Classification and Categoriesmuhd.qasimNo ratings yet