Professional Documents
Culture Documents
Crirmi
Uploaded by
stanleyOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Crirmi
Uploaded by
stanleyCopyright:
Available Formats
J Appl Physiol
91: 1029–1034, 2001.
Inflammatory and mechanical factors of allergen-
induced bronchoconstriction in mild asthma and rhinitis
EMANUELE CRIMI, MANLIO MILANESE, SUSANNA ODDERA, CARLO MEREU,
GIOVANNI A. ROSSI, ANNAMARIA RICCIO, G. WALTER CANONICA, AND VITO BRUSASCO
Dipartimenti di Scienze Motorie e Riabilitative di Medicina Interna
e di Oncologia Biologica e Genetica, Università di Genova, 16132 Genova;
and Divisione di Pneumologia, Istituto G. Gaslini, 16130 Genova, Italy
Received 29 November 2000; accepted in final form 9 April 2001
Crimi, Emanuele, Manlio Milanese, Susanna Od- asthma than in rhinitis are, however, still largely un-
dera, Carlo Mereu, Giovanni A. Rossi, Annamaria explained.
Riccio, G. Walter Canonica, and Vito Brusasco. Inflam- In subjects with a similar type and degree of sys-
matory and mechanical factors of allergen-induced broncho- temic allergic sensitization, differences in bronchial
constriction in mild asthma and rhinitis. J Appl Physiol 91: responsiveness to allergen may be related to differ-
1029–1034, 2001.—We studied whether different bronchial
ences in bronchial inflammation and remodeling or
responses to allergen in asthma and rhinitis are associated
airway mechanics. It has been previously shown that
with different bronchial inflammation and remodeling or
airway mechanics. Nine subjects with mild asthma and eight the bronchi of rhinitic subjects are susceptible to de-
with rhinitis alone underwent methacholine and allergen velop an inflammatory response to allergen that is
inhalation challenges. The latter was preceded and followed indistinguishable from that of asthmatic bronchi (17).
by bronchoalveolar lavage and bronchial biopsy. The re- However, only bronchoalveolar lavage (BAL) was used,
sponse to methacholine was positive in all asthmatic but in which precluded any evaluation of bronchial wall in-
only two rhinitic subjects. The response to allergen was flammation or remodeling. Furthermore, possible dif-
positive in all asthmatic and most, i.e., five, rhinitic subjects. ferences of airway mechanics in response to allergen
No significant differences between groups were found in between asthmatic and rhinitic subjects were not in-
airway inflammatory cells or basement membrane thickness vestigated.
either at baseline or after allergen. The ability of deep inha- In the present study, we used both BAL and bron-
lation to dilate methacholine-constricted airways was greater chial biopsy (BB) to evaluate bronchial inflammation
in rhinitis than in asthma, but it was progressively reduced and remodeling in subjects with mild asthma (with or
in rhinitis during allergen challenge. We conclude that 1) without rhinitis) and subjects with rhinitis alone. Pos-
rhinitic subjects may develop similar airway inflammation
sible differences in airway mechanics between the two
and remodeling as the asthmatic subjects do and 2) the
difference in bronchial response to allergen between asthma
groups were investigated by comparing 1) the bron-
and rhinitis is associated with different airway mechanics. chial responsiveness to allergen and to methacholine
(MCh), and 2) the effects of deep inhalation (DI) on
airway hyperresponsiveness; basement membrane; bron- airway caliber (15) during allergen and MCh chal-
choalveolar lavage; bronchial biopsy; deep inhalation lenges.
METHODS
IT HAS BEEN RECENTLY SUGGESTED that asthma and rhini-
Subjects. The study was conducted on 17 male, nonsmok-
tis represent two different phenotypes of the same ing subjects, who were sensitized to house dust mite (HDM),
disease (9). In support of this hypothesis are the obser- as documented by a third-to-fourth radioallergosorbent test
vations that experimental exposure to allergen causes class (Pharmacia, Uppsala, Sweden) and a positive skin prick
bronchial narrowing (2, 6) and inflammation (17) in test. The latter was defined by a wheal response to HDM
subjects suffering from rhinitis alone, i.e., without extract (Lofarma, Milan, Italy) equal to or larger than that
asthma. An important difference between asthma and caused by 10 mg/ml histamine. Eight subjects (age 25 ⫾ 3 yr)
rhinitis alone is the much greater dose of allergen had a history of rhinitis and never experienced symptoms
that may be related to asthma or airway hyperresponsive-
necessary to cause significant bronchoconstriction in
ness, such as wheeze, waking up at night with shortness of
the latter (2), which may explain why some allergic breath or chest tightness, or cough or sputum on exposure to
subjects develop asthma symptoms on natural expo- house dust (4). Nine subjects (age 24 ⫾ 3 yr) had bronchial
sure to allergen, whereas others do not. The reasons for asthma, according to the definition of the American Thoracic
a greater bronchial responsiveness to allergen in Society (1). At the time of the study, all subjects were in a
Address for reprint requests and other correspondence: V. Bru- The costs of publication of this article were defrayed in part by the
sasco; Dipartimento di Scienze Motorie e Riabilitative; Università di payment of page charges. The article must therefore be hereby
Genova; Largo R. Benzi, 10:16132 Genova, Italy (E-mail: brusasco marked ‘‘advertisement’’ in accordance with 18 U.S.C. Section 1734
@dism.unige.it). solely to indicate this fact.
http://www.jap.org 8750-7587/01 $5.00 Copyright © 2001 the American Physiological Society 1029
Downloaded from www.physiology.org/journal/jappl by ${individualUser.givenNames} ${individualUser.surname} (185.050.250.187) on December 26, 2018.
1030 BRONCHIAL RESPONSE TO ALLERGEN IN RHINITIS AND ASTHMA
stable clinical condition, with a 1-s forced expiratory volume tract to which a value of 1,000 AU had been assigned. One
(FEV1) ⬎70% of predicted (16), and none of them was taking arbitrary unit corresponded to 7.5 IU of the World Health
inhaled or oral steroids, cromolyn, antihistamine, or regular Organization International Union of Immunological Societ-
bronchodilators. Asthmatic subjects were using short-acting ies Dermatophagoides pteronyssinus International Standard
2-agonists on demand, which were avoided for 12 h before (National Institute for Biological Standards and Control code
studies. The study was approved by the institutional ethics 82/518). The mean particle size of the allergen powder deter-
committee, and subjects gave written, informed consent. mined by laser particle sizer (Analysette 22, Fritsch, Idar,
Protocol. All subjects first underwent a MCh inhalation Oberstain, Germany), with ethanol as dispersing fluid, was
challenge followed, 24 h later, by BAL and BB. After 1 wk at 2.5 m, with 99.9% of the particles ⬍ 18.2 m, 70% ⬍ 3.9 m,
least, they underwent a HDM inhalation challenge followed, 50% ⬍ 2.5 m, 30% ⬍ 1.5 m, and 10% ⬍ 0.7 m. Micronized
24 h later, by BAL and BB. Six asthmatic and six rhinitic allergen in the form of inert lactose powder was contained in
subjects attended the laboratory on two further occasions to rigid gelatin capsules (40 ⫾ 2 mg of powder each). The
have MCh and HDM challenges repeated to determine the following doses were made available by the manufacturing
effects of DI. firm: 5, 10, 25, 50, 100, 200, and 400 AU. The powder was
Lung function measurements. A Vmax 6200 system (Sen- administered through a turboinhaler device (Schiapparelli-
sorMedics, Yorba Linda, CA) was used for baseline pulmo- Searle, Torino, Italy) with a series of slow inspiratory capac-
nary function tests and MCh challenge. Portable spirometers ity maneuvers repeated until the capsule was empty. A
(Micro Medical, Rochester, Kent, UK) were used to monitor control measurement of FEV1 was obtained 15 min after
lung function during the allergen challenge, with the same inhalation of lactose. The challenge was then started from
subject using the same instrument at the hospital and at the lowest dose of allergen (5 AU) and stopped when the
home. On each occasion, the highest of three FEV1 measure- FEV1, measured 15 min after the dose, was decreased below
ments within 100 ml was retained. The effects of DI during 80% of control value. If such a decrease of FEV1 did not occur
allergen and MCh challenges were determined by comparing with the dose of 790 AU, an additional 400 AU were admin-
expiratory flows at 40% of control forced vital capacity (FVC) istered, and the challenge stopped at the cumulative dose of
during forced expiratory maneuvers started from full infla- 1,190 AU. In rhinitic subjects, the first three doses (5, 10, and
tion (maximal flow) and from ⬃60% of FVC (partial flow) 25 AU) were inhaled consecutively as a unique dose of 40 AU.
using dedicated software (Vmax 6200, SensorMedics). Three Cumulative PD20 was calculated by interpolation of the dose-
acceptable sets of partial and maximal flow-volume curves response curve. In subjects who did not reach a 20% reduc-
were obtained at control, and one at each step of the chal- tion of FEV1, the last allergen dose was retained as PD20 for
lenges. Each set consisted of a forceful expiration from ⬃60% statistical analysis. FEV1 measurements were then taken
of FVC to residual volume, followed by a fast inhalation to hourly for 24 h, except when the subject was asleep. A
total lung capacity and, without breath hold, a forceful expi- decrease of FEV1ⱖ 20% of control value recorded at any time
ration to residual volume. The bronchodilator effect of DI was within 24 h after allergen inhalation was considered as
inferred from the slope (MP slope) and the intercept (MP evidence of a positive response.
intercept) of the regression line of maximal (M) vs. partial (P) Bronchoscopic procedure. After premedication with atro-
flows measured at all steps of the challenges (15). In this pine (0.5 mg im) and prometazine hydrochloride (50 mg im),
analysis, a slope ⬍1 indicates that, for any given decrease of lidocaine (2% solution) and adrenaline (0.1:1,000 solution)
partial flow, the maximal flow decreases less, thus suggest- were instilled into the nostrils. A fiber-optic bronchoscope
ing a bronchodilator effect of DI. The coefficient of variation (Olympus BF, type 1T20) was then passed through the nose
(CV) of MP slope and MP intercept measurements was de- or the mouth without endotracheal tube and, after local
termined in a separate group of stable asthmatic subjects to anesthesia of pharynx and airways, wedged into a subseg-
be 14 and 20%, respectively (unpublished data). mental bronchus of the right middle lobe. Sterile saline (5
MCh challenge. Solutions of MCh were prepared by adding aliquots of 20 ml each) was instilled. The BAL fluid was
distilled water to dry powder MCh chloride (Laboratorio collected by applying a negative pressure (50–120 mmHg) at
Farmaceutico Lofarma). Aerosols were delivered by an SM-1 the proximal port of the bronchoscope. Immediately after
Rosenthal breath-activated dosimeter (SensorMedics) driven BAL, four biopsy specimens were taken from the right upper
by compressed air (30 psi) with 1-s actuations. The aerosol lobe distal bifurcation.
output at the mouth was 10 l per actuation. Aerosols were BAL analysis. After filtration through two layers of sterile
inhaled during quiet tidal breathing. After 20 inhalations of gauze, the fluid was centrifuged at 500 rpm for 5 min. The
saline as a control, the subjects inhaled double-increasing cell pellet was washed once and resuspended in Hanks’
doses of MCh from 0.02 mg until FEV1, measured 1 min after balanced salt solution, without Ca2⫹ and Mg2⫹, at a concen-
each dose, decreased below 80% of control value or the max- tration of 106 cells/ml. A small sample of the cell suspension
imum dose of 1.2 mg was achieved. The double increments of was centrifuged (Cytospin, Shandon Southern Instruments,
dose were obtained by using two MCh concentrations (1 and Sewickley, PA) at 500 rpm for 5 min, spinning ⬃100,000 cells
10 mg/ml) with appropriate numbers of breaths. A 3-min onto a glass slide. Cells were air dried and stained (Diff-Quik;
interval was allowed between dose increments. The noncu- Merz & Dade, Dudingen, Switzerland) for a differential cell
mulative dose causing a 20% reduction of FEV1 from control count of 300 cells per slide, by light microscopy. Epithelial
(PD20) was calculated by interpolation between two adjacent cells were not included in the differential count, and their
points of the log dose-response curve. In subjects who did not absolute values are reported separately. Cell-free superna-
reach a 20% reduction of FEV1, the last MCh dose was tants were assayed for eosinophil cationic protein (ECP) by
retained as PD20 for statistical analysis. PD20 values ⬍1.2 fluoroimmunoassay (Pharmacia UniCAP System Fluoroen-
mg were considered positive for airway hyperresponsiveness. zymeimmunoassay, Pharmacia Diagnostic) and for tumor
HDM challenge. A powder-allergen method was used (13). necrosis factor-␣ (TNF-␣) content by enzyme immunoassay
The HDM extract consisted of a Dermatophagoides pteronys- (Cytelisa, Cytimmune Science, College Park, MD) following
sinus and farinae dry mix (50:50) predosed in arbitrary units the manufacturer’s instruction. The sensitivity of ECP and
(AU) by radioallergosorbent test inhibition technique with an TNF-␣ assay is 2 ng/ml and 4.8 pg/ml, respectively. The
internal (Laboratorio Farmaceutico Lofarma) reference ex- albumin concentration in the supernatant was determined
J Appl Physiol • VOL 91 • SEPTEMBER 2001 • www.jap.org
Downloaded from www.physiology.org/journal/jappl by ${individualUser.givenNames} ${individualUser.surname} (185.050.250.187) on December 26, 2018.
BRONCHIAL RESPONSE TO ALLERGEN IN RHINITIS AND ASTHMA 1031
Table 1. Bronchoalveolar lavage data
Rhinitis (n ⫽ 8) Asthma (n ⫽ 9)
Pre-HDM Post-HDM Pre-HDM Post-HDM
Cells ⫻ 10 6
2 (1.2–4.1) 3.65 (2.08–8)* 1.17 (0.94–2.5) 3.55 (3.07–8.1)
Epithelial cells ⫻ 104 17.2 (7.5–30) 10.3 (8.5–19) 11.8 (3.45–25) 9.5 (2.85–25.3)
Macrophages, % 92.8 (89.1–96.4) 84.6 (81.1–89.7)* 90.5 (87.5–93.4) 84.2 (71.8–90)*
Lymphocytes, % 4.3 (1.5–6.5) 1.4 (0.5–10.3) 2.7 (2–8.3) 4 (2.1–12.1)
Neutrophils, % 0.75 (0–1) 2 (0.5–2.6) 1.1 (0–1.3) 1.3 (0.9–2.5)
Eosinophils, % 1.3 (0.7–5.5) 8.7 (5.2–3.6)* 4.4 (1.8–5.1) 6.6 (3.5–8.8)
Albumin, mg/100 ml 8.1 (5.2–11.8) 6.8 (5.6–11.9) 7 (6–8.3) 8.6 (6.5–10)
ECP, pg/ml 3.0 (1.4–16.5) 25.8 (7.5–47.4)* 3.1 (2–8.8) 16.1 (8.2–23)*
TNF-␣, pg/ml 121 (56.5–180) 80 (59–99) 81 (78–140) 70 (61–78)
IgE, U/ml
Total (n ⫽ 5) 0.58 (0.51–1.02) 0.83 (0.77–1.12)
Specific (n ⫽ 5) 0.44 (0.35–0.85) 0.8 (0.41–1.22)
Values are medians with lower and upper quartiles in parentheses; n, no. of subjects. HDM, house dust mite; ECP, eosinophil cationic
protein; TNF-␣, tumor necrosis factor-␣. * P ⬍ 0.05 vs. pre-HDM.
by nephelometry. Total and specific IgE to dermatophagoides m), until 40 readings were obtained. The intraobserver
were measured by reverse enzyme allergosorbent test (Labo- mean CV for three replicate measurements was 3%. The
ratorio Farmaceutico, Lofarma), which is based on the cap- percentage of RBM covered by epithelium was also calcu-
ture of both total and specific IgE by a specific antihuman IgE lated.
antibody and the subsequent reaction with streptoavidin- Statistical analysis. Unpaired t-test was used to compare
peroxidase and chromogenic substrate (8). The optical den- anthropometric and lung function data between groups.
sity was read on a human IgE reference curve titrated refer- Fisher’s exact test was used to compare categorical variables.
ring to the Second World Health Organization IgE standard Two-factor, repeated-measure ANOVA with least significant
75/502. For both total and specific IgE, the lower and upper difference post hoc test was used to compare MP slopes and
detection limits were 0.2 and 100 U/ml, respectively. MP intercepts between and within groups. Mann-Whitney
BB analysis. Biopsy specimens were fixed in 10% formal- U-test was used for comparisons of BAL and BB data be-
dehyde at 4°C for 4 h, embedded in paraffin, cut at 5 m with tween groups before and after allergen challenge. Wilcoxon
a rotative microtome, and stained with hematoxylin and matched-rank test was used to compare data before and after
eosin and toluidine blue. Eight too small and incorrectly allergen challenge. P ⬍ 0.05 was considered statistically
oriented biopsies were discarded, but for each subject at least significant. Data are presented as means ⫾ SD or medians
one biopsy was available for analysis. Microscopic examina- with interquartile ranges.
tion was performed by one independent observer, who was
unaware of the aim of the study. For cell counts (eosinophils RESULTS
and mast cells), bronchial mucosa was examined to a depth of
100 m from the basement membrane over adjacent non- BAL data. See Table 1. Neither at baseline nor after
overlapping high-power fields (at ⫻500 magnification with HDM challenge were the differences in inflammatory
the aid of an eyepiece graticule) until all of the available area cell counts and epithelial cells statistically significant
was covered. For each quantification, three sections were between asthma and rhinitis (P ⬎ 0.2, for all compar-
examined, and cells were expressed as number per square isons). Total cell number and percentages of eosino-
millimeter of submucosa. Mast cells were also examined at phils were increased after HDM challenge significantly
⫻1,000 magnification to detect ongoing degranulation (gran- in rhinitis and nonsignificantly in asthma, whereas the
ules surrounding the cell or crossing the cell membrane). The percentage of macrophages decreased in both groups.
intraobserver mean CV for three repeated measurements
was 9% for eosinophils and 8% for mast cells. The thickness
The ECP and TNF-␣ contents of supernatant were not
of reticular basement membrane (RBM) was measured on significantly different at baseline (P ⬎ 0.2), and ECP
hematoxylin- and eosin-stained preparations by light micro- increased similarly in the two groups after HDM chal-
scope image analysis (Q 500, Leica, Cambridge, UK) at ⫻400 lenge. Total and specific IgE, detectable in five rhinitic
magnification from the base of bronchial epithelium to the and five asthmatic subjects, did not show significant
outer limit of the reticular lamina, at regular intervals (20 differences between groups (P ⬎ 0.3). Also the albumin
Table 2. Bronchial biopsy data
Rhinitis (n ⫽ 8) Asthma (n ⫽ 9)
Pre-HDM Post-HDM Pre-HDM Post-HDM
Eosinophils, mm⫺2 20.5 (8.5–61) 68.5 (31–110)* 17 (8–35) 17 (15–24)
Mast cells, mm⫺2 28.5 (28–42.5) 42.5 (21.5–61.5) 29 (10–38) 38 (19–57)
Degranulating mast cells, % 33 (23.5–67.5) 51 (33–74) 33 (0–66) 62 (40–75)*
RBM thickness, m 10 (7.4–15.6) 11.2 (9.1–11.6) 12 (8.8–14.1) 10.6 (6.8–16)
Epithelium-covered RBM, % 85 (68.5–100) 87.5 (76–96) 78 (62–100) 75 (36–80)
Values are medians with lower and upper quartiles in parentheses; n, no. of subjects. * P ⬍ 0.05 vs. preallergen. RBM, reticular basement
membrane.
J Appl Physiol • VOL 91 • SEPTEMBER 2001 • www.jap.org
Downloaded from www.physiology.org/journal/jappl by ${individualUser.givenNames} ${individualUser.surname} (185.050.250.187) on December 26, 2018.
1032 BRONCHIAL RESPONSE TO ALLERGEN IN RHINITIS AND ASTHMA
content of BAL supernatant was not significantly dif-
ferent between groups (P ⬎ 0.5).
BB data. See Table 2. There were no significant
differences in inflammatory cell numbers between rhi-
nitis and asthma at baseline (P ⬎ 0.5 for all compari-
sons), but the number of eosinophils increased signifi-
cantly in rhinitis, and the percentage of degranulating
mast cells increased in asthma after HDM. Neither
RBM thickness nor the percentage of RBM covered by
intact epithelium were significantly different between
groups (P ⬎ 0.7 for both comparisons), although the
latter tended (P ⫽ 0.06) to be less in asthma after
HDM.
Lung function data. See Table 3. Both FEV1 and its
ratio to FVC (FEV1/FVC) were less in asthma than in
rhinitis, suggesting mild airway obstruction at base-
line in the former. Individual baseline FEV1 values on
the allergen challenge days were always within ⫾8% of
those on the MCh challenge days, without significant
differences between or within groups. Both allergen
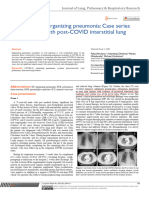
PD20 and MCh PD20 were significantly less in asthma Fig. 1. Mean regression lines of maximal vs. partial flows during
than in rhinitis. The response to MCh was positive in challenges, calculated by solving the linear model y ⫽ a ⫹ bx, where
all asthmatic subjects but in only two rhinitic subjects; a is mean intercept and b is mean slope from Table 3. The extreme
x values for each line are the mean values of partial flow at control
this difference was statistically significant (P ⬍ 0.01). and at challenge end point. Solid lines indicate methacholine chal-
The response to HDM was positive in all asthmatic lenge; dashed lines indicate house dust mite challenge. A, asthmatic
subjects and in five out of eight rhinitic subjects, a subjects; R, rhinitic subjects. See Table 3 for statistical differences.
difference that was not statistically significant (P ⬎ Note the higher maximal flows for the same partial flows in R than
0.08). Of the two rhinitic subjects with positive re- in A during MCh challenge, suggesting a greater bronchodilator
effect of deep inhalation. Note also the increase in slope in R during
sponse to MCh, one (PD20 ⫽ 1,200 g) did respond to allergen challenge, indicating a progressive reduction of the bron-
HDM, whereas the other (PD20 ⫽ 487 g) did not. The chodilator effect of deep inhalation with increasing allergen dose.
late-phase bronchial response to HDM tended to be
more severe in asthma than in rhinitis (maximum late
FEV1 fall: 32 ⫾ 16 vs. 18 ⫾ 11%, P ⫽ 0.05), although asthma when bronchoconstriction was induced by
five asthmatic subjects were given bronchodilator MCh, as indicated by the significantly (P ⬍ 0.05)
treatment to relieve symptoms. higher MP intercept, but decreased progressively when
In both groups, DI had a bronchodilator effect during bronchoconstriction was induced by HDM, as indicated
induced bronchoconstriction, as indicated by the MP by the significantly (P ⬍ 0.05) steeper MP slope.
slope values that were significantly (P ⬍ 0.01) less than DISCUSSION
unity (Fig. 1). This effect was greater in rhinitis than in
The new findings of this study are that 1) the re-
sponse to MCh better separated asthmatic from rhi-
Table 3. Lung function data nitic subjects than did the response to HDM, 2) the
bronchodilator effect of DI was greater in rhinitis than
Rhinitis (n ⫽ 8) Asthma (n ⫽ 9)
in asthma during MCh challenge, but it progressively
FEV1, % predicted 104 ⫾ 8 91 ⫾ 10† decreased in rhinitis during HDM challenge, and 3)
FEV1/FVC, % 86 ⫾ 6 77 ⫾ 7* bronchial responsiveness to HDM was greater in
Log MCh-PD20, mg 3.031 ⫾ 0.139 2.078 ⫾ 0.400‡
Log HDM-PD20, AU 2.940 ⫾ 0.184 2.446 ⫾ 0.551* asthma than in rhinitis, even if baseline airway inflam-
MCh positive/negative, no. 2/6 9/0† mation and remodeling were similar. In addition, we
HDM positive/negative, no. 5/3 9/0 confirm and extend previous studies (17), showing that
MP-MCh the bronchi of rhinitic subjects developed an inflamma-
Slope, units 0.54 ⫾ 0.18 0.66 ⫾ 0.17
Intercept, l/s 1.76 ⫾ 0.58 1.18 ⫾ 0.39*
tory response to inhaled allergen that was similar to
MP-HDM that of asthmatic subjects.
Slope, units 0.79 ⫾ 0.08§ 0.53 ⫾ 0.11* Comments on methodology. This study has some
Intercept, l/s 1.16 ⫾ 0.66§ 1.15 ⫾ 0.32 limitations. First, inflammatory cell data were ob-
FEV1, 1-s forced expiratory volume; FVC, forced vital capacity; tained only 24 h after the HDM inhalation. Therefore,
FEV1/FVC, ratio of FEV1 to FVC; MCh, methacholine; PD20, provoc- differences in the timing of inflammatory cell influx
ative dose causing a FEV1 decrease of 20% from control; AU, arbi- into the airways cannot be evaluated. However, enu-
trary units. Slopes and intercepts are from linear regressions of meration of blood eosinophils in seven subjects (4 with
maximal (M) vs. partial (P) flows recorded at all steps of MCh and
HDM challenges. Significant difference vs. rhinitis: * P ⬍ 0.05; asthma and 3 with rhinitis alone) showed a nonsignifi-
† P ⬍ 0.01; ‡ P ⬍ 0.001. Significant difference vs. MP-MCh, § P ⬍ cantly different decrease 3 h after HDM inhalation
0.05. (57 ⫾ 9% in asthmatic subjects vs. 70 ⫾ 30% in rhinitic
J Appl Physiol • VOL 91 • SEPTEMBER 2001 • www.jap.org
Downloaded from www.physiology.org/journal/jappl by ${individualUser.givenNames} ${individualUser.surname} (185.050.250.187) on December 26, 2018.
BRONCHIAL RESPONSE TO ALLERGEN IN RHINITIS AND ASTHMA 1033
subjects), suggesting a similar early recruitment of itive response to HDM. By contrast, the majority of
eosinophils from blood in both groups. Second, inflam- rhinitic subjects exhibited a bronchial responsiveness
matory cells lying in the bronchial mucosa deeper than to MCh within the normal range but a positive re-
100 m below the basement membrane were not eval- sponse to HDM. It should be noted that falsely positive
uated, and differences cannot be excluded. Third, no responses in rhinitis are unlikely to have occurred
relationship between inflammatory response and HDM owing to the rather large cutoff value chosen, i.e., a
dose could be determined, as it would have been ethi- 20% decrease of FEV1. MCh is a bronchoconstrictor
cally unacceptable to have repeated HDM challenges stimulus acting directly on airway smooth muscle. Al-
and bronchoscopies. Fourth, in this study, biopsy spec- lergen is an indirect stimulus acting via the release of
imens were taken at a single site and assumed to be a number of mediators, which may cause airway nar-
representative of airway remodeling throughout the rowing by other mechanisms in addition to airway
lung. This assumption seems to be justified by the smooth muscle contraction. A greater response to MCh
findings of Bradley et al. (3) and Jeffery et al. (11). in asthma than in rhinitis is, therefore, suggestive of a
Finally, only subjects with mild asthma were studied, greater airway smooth muscle responsiveness. The ob-
and the findings cannot be extrapolated to more severe served effects of DI on airway caliber are in line with
disease. this interpretation. The magnitude of the bronchodila-
Comments on results. In asthma, the degree of aller- tor effect of DI is believed to be directly related to
gic sensitization and the degree of bronchial respon- airway hysteresivity and to the amplitude of stretching
siveness are independent determinants of both early exerted by lung parenchyma (7). A direct contractile
and late bronchoconstrictor responses to allergen (5). stimulus to airway smooth muscle should result in an
In the present study, neither blood nor BAL IgE levels increase in hysteresivity only, thus fostering the bron-
were significantly different between asthmatic and rhi- chodilator effect of DI. An indirect bronchoconstrictor
nitic subjects. For the same degree of systemic allergic stimulus may also cause airway wall edema, thus re-
sensitization, as revealed by serum-specific IgE level ducing the magnitude of airway stretching and blunt-
and/or skin test response, the bronchoconstrictor re- ing the bronchodilator effect of DI. The greater MP
sponse to allergen may be expected to be determined by intercept during MCh challenge in rhinitis than in
local factors, such as baseline bronchial inflammation, asthma suggests that the ability to distend contracted
allergen-induced bronchial inflammation, and mechan- airway smooth muscle was greater in the former. Fur-
ical response. thermore, the similarity between the effects of DI dur-
In the present study, the degree of baseline bronchial ing MCh and HDM challenges in asthmatic subjects
inflammation was not greater in asthma than in rhi- suggests airway smooth muscle contraction as the ma-
nitis, but the dose of HDM required to cause a bron- jor determinant of response to HDM in this group. By
choconstrictor response (PD20) was lower in the former. contrast, the increased MP slope during HDM in rhi-
This suggests that the bronchoconstrictor response to nitis suggests a progressive reduction of the broncho-
allergen is not critically dependent on the presence of dilator effect of DI with the increasing allergen dose.
inflammatory cells or mediators in the airways before This suggests a significant contribution of other mech-
challenge. The thickness of RBM was above the re- anisms, such as airway wall edema and vascular leak-
ported normal values (19) but with no difference be- age, possibly uncoupling airways from lung paren-
tween groups, suggesting similar degrees of airway chyma (12). The tendency for a greater eosinophil
wall remodeling (10). The functional consequences of influx into rhinitic than asthmatic bronchi is consistent
RMB thickening are complex and are discussed in a with a somehow greater inflammatory response in the
companion paper (14). former, likely related to the much greater dose of
As in a previous study (17), the inflammatory re- allergen received. Another possibility is that the differ-
sponse to allergen was similar in asthma and rhinitis, ence in the ability of DI to dilate constricted airways
but the HDM dose given to rhinitic subjects was between rhinitic and asthmatic subjects is due to a
greater than that given to asthmatic subjects. Possible relative difference in epithelial function, as suggested
reasons for a similar inflammatory response to differ- by a tendency for less RBM surface being covered by
ent doses of allergen in subjects with similar allergic intact epithelium in the latter.
sensitization may be due to the compartmentalization In conclusion, the results of this study suggest that
of the inflammatory-immune response with differences different mechanical responses, possibly related to air-
in IgE receptor density on metachromatic cells, vascu- way smooth muscle responsiveness (18), may contrib-
lar leakage, or release of chemotactic substances from ute to the different airway response to allergen, and
resident cells, including smooth muscle cells. Whatever perhaps in symptoms, between asthma and rhinitis,
the underlying mechanism, these data suggest, al- although different inflammatory responses cannot be
though they do not prove, a different bronchial inflam- excluded.
matory responsiveness to allergen between asthma
and rhinitis. The authors gratefully acknowledge Dr. Riccardo Pellegrino for
An important, new finding of this study is that the valuable comments on the manuscript.
This study was supported in part by grants from Ministero
two groups differed more in respect to bronchial re- dell’Università e della Ricerca Scientifica e Tecnologica (Rome, Italy)
sponsiveness to MCh than to HDM. All asthmatic (to V. Brusasco and G. W. Canonica) and by Associazione per la
subjects were hyperresponsive to MCh and had a pos- Ricerca delle Malattie Immunologiche ed Allergiche (Genoa, Italy).
J Appl Physiol • VOL 91 • SEPTEMBER 2001 • www.jap.org
Downloaded from www.physiology.org/journal/jappl by ${individualUser.givenNames} ${individualUser.surname} (185.050.250.187) on December 26, 2018.
1034 BRONCHIAL RESPONSE TO ALLERGEN IN RHINITIS AND ASTHMA
REFERENCES mensions in asthma. Am J Respir Crit Care Med 161: A110,
2000.
1. American Thoracic Society. Standards for the diagnosis and
11. Jeffery PK, Godfred RW, Ädelroth E, Nelson F, Rogers A,
care of patients with chronic obstructive pulmonary disease
and Johansson S-A. Effects of treatment on airway inflamma-
(COPD) and asthma. Am Rev Respir Dis 136: 225–244, 1987.
2. Bonavia M, Crimi E, Quaglia A, and Brusasco V. Compari- tion and thickening of basement membrane reticular collagen in
son of early and late asthmatic response between patients with asthma. Am Rev Respir Dis 145: 890–899, 1992.
allergic rhinitis and mild asthma. Eur Respir J 9: 905–909, 1996. 12. Macklem PT. A theoretical analysis of the effect of airway
3. Bradley BL, Azzawi M, Jacobson M, Assoufi B, Collins JV, smooth muscle load on airway narrowing. Am J Respir Crit Care
Irani A-M, Schwartz LB, Durham SR, Jeffery PK, and Kay Med 153: 83–89, 1996.
AB. Eosinophils, T-lymphocytes, mast cells, neutrophils, and 13. Melillo G, Bonini S, Cocco G, Davies RJ, De Monchy JGR,
macrophages in bronchial biopsy specimens from atopic subjects Frolund L, and Pelikan Z. EAACI provocation tests with
with asthma: comparison with biopsy specimens from atopic allergens. Allergy 52, Suppl 35: 1–35, 1997.
subjects and relationship to bronchial hyperresponsiveness. J 14. Milanese M, Crimi E, Scordamaglia A, Riccio A, Pellegrino
Allergy Clin Immunol 88: 661–674, 1991. R, Canonica GW, and Brusasco V. On the functional conse-
4. Burney PGJ, Chinn S, Britton JR, Tattersfield AE, and quences of bronchial basement membrane thickening. J Appl
Papacosta AO. What symptoms predict the bronchial response Physiol 91: 1035–1040, 2001.
to histamine? Evaluation in a community survey of the Bron- 15. Pellegrino R, Wilson O, Jenouri G, and Rodarte JR. Lung
chial Symptoms Questionnaire (1984) of the International Union mechanics during induced bronchoconstriction. J Appl Physiol
Against Tubercolosis and Lung Disease. Int J Epidemiol 18: 81: 964–975, 1996.
165–173, 1989. 16. Quanjer PH, Tammelin GJ, Cotes JE, Pedersen OF, Peslin
5. Crimi E, Brusasco V, Losurdo E, and Crimi P. Predictive R, and Yernault JC. Lung volumes and forced ventilatory
accuracy of late asthmatic reaction to dermatophagoides ptero- flows. Report Working Party “Standardization of Lung Function
nyssinus. J Allergy Clin Immunol 78: 908–913, 1986. Tests” European Coal and Steel Community. Eur Respir J 6,
6. Fish JE, Rosenthal RR, Lichtenstein LM, and Norman PS.
Suppl 16: 5–40, 1993.
Quantitative inhalation bronchial challenge in ragweed hay fe-
17. Shaver JR, O’Connor JJ, Pollice M, Cho SK, Kane GC,
ver patients: a comparison with ragweed-allergic asthmatics. Am
Fish JE, and Peters SP. Pulmonary inflammation after seg-
Rev Respir Dis 113: 579–586, 1976.
7. Fredberg JJ, Inouye D, Miller B, Nathan M, Jafari S, mental ragweed challenge in allergic asthmatic and nonasth-
Raboudi SH, Butler JP, and Shore SA. Airway smooth mus- matic subjects. Am J Respir Crit Care Med 152: 1189–1197,
cle, tidal stretches, and dynamically determined contractile 1995.
states. Am J Respir Crit Care Med 156: 1752–1759, 1997. 18. Solway J and Fredberg JJ. Perhaps airway smooth muscle
8. Giannarini L and Maggi E. Decrease of allergen-specific T-cell dysfunction contributes to asthmatic bronchial hyperresponsive-
response induced by local nasal immunotherapy. Clin Exp Al- ness after all. Am J Respir Cell Mol Biol 17: 144–146, 1997.
lergy 28: 404–412, 1998. 19. Vignola AM, Chanez P, Campbell AM, Souques F, Lebel B,
9. Grossman J. One airway one disease. Chest 111: 11–16, 1996. Enander I, and Bousquet J. Airway inflammation in mild
10. James AL, Pearce-Pinto G, Elliot J, and Carroll N. The intermittent and in persistent asthma. Am J Respir Crit Care
relationship between basement membrane and airway wall di- Med 157: 403–409, 1998.
J Appl Physiol • VOL 91 • SEPTEMBER 2001 • www.jap.org
Downloaded from www.physiology.org/journal/jappl by ${individualUser.givenNames} ${individualUser.surname} (185.050.250.187) on December 26, 2018.
You might also like
- Allergic Vs Nonallergic Asthma: What Makes The Difference?: Original ArticleDocument7 pagesAllergic Vs Nonallergic Asthma: What Makes The Difference?: Original ArticleJosueNo ratings yet
- Arizmendi 2011Document10 pagesArizmendi 2011TifanyNabilahNo ratings yet
- 1993 - Balmes - The Role of Ozone Exposure in The Epidemiology of AsthmaDocument6 pages1993 - Balmes - The Role of Ozone Exposure in The Epidemiology of Asthmapond_1993No ratings yet
- Journal of Otolaryngology and Rhinology Jor 1 009Document8 pagesJournal of Otolaryngology and Rhinology Jor 1 009Rizki Putri AndiniNo ratings yet
- 1 s2.0 S0954611113000371 MainDocument11 pages1 s2.0 S0954611113000371 Mainani putkaradzeNo ratings yet
- Eosinophilic Bronchitis Is An Important Cause of Chronic CoughDocument5 pagesEosinophilic Bronchitis Is An Important Cause of Chronic CoughNurfitrianti ArfahNo ratings yet
- Oxygen TerapyDocument6 pagesOxygen TerapyCamila AgudeloNo ratings yet
- Wilson Et Al 2012 Allergic Sensitization Through The Airway Primes th17 Dependent Neutrophilia and AirwayDocument11 pagesWilson Et Al 2012 Allergic Sensitization Through The Airway Primes th17 Dependent Neutrophilia and AirwaytempeuedanNo ratings yet
- Relationship Between Asthma and Rhinitis: Epidemiologic, Pathophysiologic, and Therapeutic AspectsDocument7 pagesRelationship Between Asthma and Rhinitis: Epidemiologic, Pathophysiologic, and Therapeutic AspectsStanley SuhermanNo ratings yet
- PNIF EhnhageDocument105 pagesPNIF EhnhageCaio GonçalvesNo ratings yet
- Short Term Effect of An Active Heat and Moisture Exchanger During Invasive VentilationDocument7 pagesShort Term Effect of An Active Heat and Moisture Exchanger During Invasive VentilationekaNo ratings yet
- Asthma in elderly patientsDocument6 pagesAsthma in elderly patientsbrakim23No ratings yet
- Mechanisms of Asthma: William W. Busse, MD, and Lanny J. Rosenwasser, MDDocument6 pagesMechanisms of Asthma: William W. Busse, MD, and Lanny J. Rosenwasser, MDaulia kamal ansari panggabeanNo ratings yet
- Chest GuidelineDocument13 pagesChest Guidelineyujin KimNo ratings yet
- Buku Modul Kuliah KewarganegaraanDocument4 pagesBuku Modul Kuliah KewarganegaraanYunita KimNo ratings yet
- The Effect of Intranasal Carbon Dioxide On The Acute Response To Nasal Challenge With AllergenDocument8 pagesThe Effect of Intranasal Carbon Dioxide On The Acute Response To Nasal Challenge With AllergenNiniek Putri SujiwaNo ratings yet
- 1982 - Platts-Mills Et Al. - Reduction of Bronchial Hyperreactivity During Prolonged Allergen AvoidanceDocument4 pages1982 - Platts-Mills Et Al. - Reduction of Bronchial Hyperreactivity During Prolonged Allergen Avoidancepond_1993No ratings yet
- Comparison of Asthma Phenotypes in OVAinduced Mice Challenged Via Inhaled andDocument11 pagesComparison of Asthma Phenotypes in OVAinduced Mice Challenged Via Inhaled andmafe.z.solarte87No ratings yet
- Lung Tissue Resistance in Diffuse Interstitial Pulmonary FibrosisDocument9 pagesLung Tissue Resistance in Diffuse Interstitial Pulmonary FibrosisEndhy KurniawanNo ratings yet
- Airway Inflammation and Remodelling in Asthma - Cause and Effect?Document13 pagesAirway Inflammation and Remodelling in Asthma - Cause and Effect?Pauroosh KaushalNo ratings yet
- Clinical and Imaging Evaluation of COVID-19-Related Olfactory DysfunctionDocument8 pagesClinical and Imaging Evaluation of COVID-19-Related Olfactory DysfunctionLudmila ArantesNo ratings yet
- H (HBOT) (AD) : Olish Yperbaric EsearchDocument6 pagesH (HBOT) (AD) : Olish Yperbaric EsearchVera MHNo ratings yet
- The Relationship Between Duration of Asthma and Pulmonary Function Test Parameters in Patients With Mild To Moderate AsthmaDocument5 pagesThe Relationship Between Duration of Asthma and Pulmonary Function Test Parameters in Patients With Mild To Moderate AsthmaInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Pulmonary Function in Patients Surviving To COVID 19 PneumoniaDocument5 pagesPulmonary Function in Patients Surviving To COVID 19 PneumoniaRahmanu ReztaputraNo ratings yet
- GERN Et Al-2000Document6 pagesGERN Et Al-2000ferNo ratings yet
- Impact of Immunoglobulin E and Airway Obstruction On BronchiectasisDocument7 pagesImpact of Immunoglobulin E and Airway Obstruction On BronchiectasisRichie Irvanto CiandraNo ratings yet
- Gosset Et Al. - 1991 - Increased Secretion of Tumor Necrosis Factor Alpha and Interleukin-6 by Alveolar Macrophages Consecutive To The DDocument11 pagesGosset Et Al. - 1991 - Increased Secretion of Tumor Necrosis Factor Alpha and Interleukin-6 by Alveolar Macrophages Consecutive To The DMaría Reynel TarazonaNo ratings yet
- Inflamación Por Inhaladores de Corticosteroides en La Faringe de AsmáticosDocument7 pagesInflamación Por Inhaladores de Corticosteroides en La Faringe de AsmáticosEstefani Romero ArriagadaNo ratings yet
- Tonsil Volume and Allergic Rhinitis in ChildrenDocument6 pagesTonsil Volume and Allergic Rhinitis in ChildrenAsri Nikita SariNo ratings yet
- Articulo de Alergia A Polipropileno en Implante CoclearDocument9 pagesArticulo de Alergia A Polipropileno en Implante CoclearFreddy RojasNo ratings yet
- Post Covid-19 Organizing Pneumonia Case Series For 6 Patients With post-COVID Interstitial Lung DiseaseDocument4 pagesPost Covid-19 Organizing Pneumonia Case Series For 6 Patients With post-COVID Interstitial Lung DiseaseKhangNo ratings yet
- Induced Sputum IL-8 Gene Expression, Neutrophil Influx and MMP-9 in Allergic Bronchopulmonary AspergillosisDocument7 pagesInduced Sputum IL-8 Gene Expression, Neutrophil Influx and MMP-9 in Allergic Bronchopulmonary Aspergillosispaijo09No ratings yet
- Airway Remodelling in AsthmaDocument6 pagesAirway Remodelling in AsthmaJocilene Dantas Torres NascimentoNo ratings yet
- NasalDocument5 pagesNasalYuliana LatifNo ratings yet
- VRS Vs AsmaDocument9 pagesVRS Vs AsmaPablo ParicahuaNo ratings yet
- Borz One 2001Document6 pagesBorz One 2001Rodrigo Mañuico RicceNo ratings yet
- The Toxicant Induction of Irritant Asthma, Rhinitis, and Related ConditionsFrom EverandThe Toxicant Induction of Irritant Asthma, Rhinitis, and Related ConditionsNo ratings yet
- Exhaled Nitric Oxide Among Bulgarian Children With Asthma ExacerbationDocument7 pagesExhaled Nitric Oxide Among Bulgarian Children With Asthma ExacerbationinventionjournalsNo ratings yet
- 1 s2.0 S0091674921001706 MainDocument13 pages1 s2.0 S0091674921001706 MainEducation serviceNo ratings yet
- Aeroallergens, Air Pollutants, and Chronic Rhinitis and RhinosinusitisDocument7 pagesAeroallergens, Air Pollutants, and Chronic Rhinitis and RhinosinusitisRidho RifhansyahNo ratings yet
- Utility of The Sputum Cytology Applying MGG and Pap Stains in Monitoring Sudanese Patients Complaining of Bronchial AsthmaDocument6 pagesUtility of The Sputum Cytology Applying MGG and Pap Stains in Monitoring Sudanese Patients Complaining of Bronchial AsthmaDr. Asaad Mohammed Ahmed BabkerNo ratings yet
- 4 PDFDocument9 pages4 PDFAdan MorenoNo ratings yet
- Endotoxinas 1Document9 pagesEndotoxinas 1Carlos Israel Esparza AndradeNo ratings yet
- Mechanical Power and Development of VentilatorDocument9 pagesMechanical Power and Development of VentilatorJesus FrancoNo ratings yet
- Intranasal CorticosteroidDocument9 pagesIntranasal CorticosteroidIRANo ratings yet
- Bronchial Hyperresponsiveness To Mannitol, Airway Inflammation and Asthma Control Test in Atopic Asthmatic ChildrenDocument8 pagesBronchial Hyperresponsiveness To Mannitol, Airway Inflammation and Asthma Control Test in Atopic Asthmatic ChildrenCalistaParamithaNo ratings yet
- ARTICULO 50. Educational Case PneumoconiosisDocument6 pagesARTICULO 50. Educational Case PneumoconiosisMaría Paula Niño CristanchoNo ratings yet
- 803 FullDocument8 pages803 FullamandachamiehNo ratings yet
- Gattinoni2006 Reclutamiento Pulmonar en Pacientes Con SdraDocument12 pagesGattinoni2006 Reclutamiento Pulmonar en Pacientes Con SdraRenatoNo ratings yet
- Cap AsmaDocument10 pagesCap AsmaAuliaNo ratings yet
- RETRACTED The First Tissue Engineered Airway TranDocument7 pagesRETRACTED The First Tissue Engineered Airway TranJoni SiahaanNo ratings yet
- Fast Facts: Asthma: Improve patient self-management and drug use, achieve asthma controlFrom EverandFast Facts: Asthma: Improve patient self-management and drug use, achieve asthma controlNo ratings yet
- Alternaria in Patients With Allergic Rhinitis.Document8 pagesAlternaria in Patients With Allergic Rhinitis.Dasi HipNo ratings yet
- 1 PDFDocument9 pages1 PDFFlorencia IrenaNo ratings yet
- Dafpus Baru 1Document3 pagesDafpus Baru 1stanleyNo ratings yet
- Article Asthma From CINAHLDocument10 pagesArticle Asthma From CINAHLmalyn1218No ratings yet
- Cea 13169 PDFDocument15 pagesCea 13169 PDFrisanataliasiburianNo ratings yet
- Pi Is 1323893016301745Document27 pagesPi Is 1323893016301745Afria TikaNo ratings yet
- Abpa Vs Ia 13223 - 2016 - Article - 170Document5 pagesAbpa Vs Ia 13223 - 2016 - Article - 170sricharangodNo ratings yet
- 8 Nitrit Oksida Yang Dihembuskan Pada Penyakit Paru Obstruktif KronisDocument5 pages8 Nitrit Oksida Yang Dihembuskan Pada Penyakit Paru Obstruktif KronisWita Citra DewiNo ratings yet
- RiccaDocument4 pagesRiccastanleyNo ratings yet
- ValeroDocument6 pagesValerostanleyNo ratings yet
- OkanoDocument8 pagesOkanostanleyNo ratings yet
- Updated rhinitis diagnosis and management guidelinesDocument84 pagesUpdated rhinitis diagnosis and management guidelinesdarkreign86No ratings yet
- GavaertDocument8 pagesGavaertstanleyNo ratings yet
- PDFDocument43 pagesPDFTammy StephanieNo ratings yet
- LohiaDocument11 pagesLohiastanleyNo ratings yet
- SLIDE MANAGEMENT (Teks Baca Nasional)Document1 pageSLIDE MANAGEMENT (Teks Baca Nasional)stanleyNo ratings yet
- Dafpus Baru 4Document10 pagesDafpus Baru 4stanleyNo ratings yet
- Dafpus Baru 5Document11 pagesDafpus Baru 5stanleyNo ratings yet
- Dafpus Baru 3Document12 pagesDafpus Baru 3stanleyNo ratings yet
- Role of FEF As An Early Marker of Bronchial Impairment in Patients With Seasonal Allergic RhinitisDocument7 pagesRole of FEF As An Early Marker of Bronchial Impairment in Patients With Seasonal Allergic RhinitisstanleyNo ratings yet
- Dafpus Baru 1Document3 pagesDafpus Baru 1stanleyNo ratings yet
- Dafpus Baru 1Document3 pagesDafpus Baru 1stanleyNo ratings yet
- Dafpus Baru 2Document10 pagesDafpus Baru 2stanleyNo ratings yet
- Dafpus Baru 4Document10 pagesDafpus Baru 4stanleyNo ratings yet
- Dafpus Baru 2Document10 pagesDafpus Baru 2stanleyNo ratings yet
- Dafpus Baru 5Document11 pagesDafpus Baru 5stanleyNo ratings yet
- Dafpus Baru 3Document12 pagesDafpus Baru 3stanleyNo ratings yet
- Dafpus Baru 1Document3 pagesDafpus Baru 1stanleyNo ratings yet
- Prevalence of GERD in Dyspepsia PatientsDocument6 pagesPrevalence of GERD in Dyspepsia PatientsAbrar TaraNo ratings yet
- Mercury in The MistDocument2 pagesMercury in The MistAnand BhagwaniNo ratings yet
- Concept PaperDocument3 pagesConcept Papergladylou siocoNo ratings yet
- Meat Inspection Certificate For Export of Pork/Pork Products To MalaysiaDocument2 pagesMeat Inspection Certificate For Export of Pork/Pork Products To MalaysiaLingSiewyingNo ratings yet
- Chapter 1 2 3Document77 pagesChapter 1 2 3Ferissa MohammadNo ratings yet
- Lec 4 Principles of Health Financing PDFDocument6 pagesLec 4 Principles of Health Financing PDFRem Alfelor100% (1)
- Caldorol (Ibuprofen IV)Document14 pagesCaldorol (Ibuprofen IV)Agus SusantoNo ratings yet
- Leni Robredo CredentialsDocument2 pagesLeni Robredo CredentialsJhelliene Rose V. De CastroNo ratings yet
- Travel Guide to Thailand in 2021Document14 pagesTravel Guide to Thailand in 2021Russell KhanNo ratings yet
- ResistanceDocument8 pagesResistanceAndi Soraya WalyddainiNo ratings yet
- Food Safety Plan For CateringDocument80 pagesFood Safety Plan For CateringNghia Khang100% (1)
- Solution Focused TherapyDocument8 pagesSolution Focused Therapyrimpa29No ratings yet
- IELTS Essay SamplesDocument5 pagesIELTS Essay SamplesRebaz Jamal AhmedNo ratings yet
- Asq 3 42 MDocument8 pagesAsq 3 42 MMohammed AlsuwaidiNo ratings yet
- Cairo: Pilbeam's Mechanical Ventilation, 6th EditionDocument7 pagesCairo: Pilbeam's Mechanical Ventilation, 6th EditionLesly Peinado Torres100% (1)
- UKHO Survey Specification Acoustic V3 Jul18Document102 pagesUKHO Survey Specification Acoustic V3 Jul18Iqbal FauzanNo ratings yet
- Autoimmune HepatitisDocument3 pagesAutoimmune HepatitisMohammed FaragNo ratings yet
- Taping Techniques: Moazzam Hussain Khan M.P.T. Sports Medicine New DelhiDocument38 pagesTaping Techniques: Moazzam Hussain Khan M.P.T. Sports Medicine New DelhiMeena SharmaNo ratings yet
- Veteran Resource Guide For Congressional District 9Document27 pagesVeteran Resource Guide For Congressional District 9RepSinemaNo ratings yet
- Otto Chemie PVT LTD: Material Safety Data SheetDocument4 pagesOtto Chemie PVT LTD: Material Safety Data Sheetenes duhanNo ratings yet
- CPT 2021 guide for identifying medical proceduresDocument7 pagesCPT 2021 guide for identifying medical proceduresChester FernandezNo ratings yet
- NCP LymphomaDocument3 pagesNCP Lymphomamahmoud fuqahaNo ratings yet
- Water Treatment PDFDocument87 pagesWater Treatment PDFJubin KumarNo ratings yet
- Couchiching FN Toll BoothDocument8 pagesCouchiching FN Toll BoothpegspirateNo ratings yet
- Acute Respiratory Distress Syndrome: Jason D. Sciarretta, M.D. Critical Care Conference October 13, 2010Document24 pagesAcute Respiratory Distress Syndrome: Jason D. Sciarretta, M.D. Critical Care Conference October 13, 2010JelenaNo ratings yet
- Evaluation and Treatment of Aphasia Among The Elderly With StrokeDocument11 pagesEvaluation and Treatment of Aphasia Among The Elderly With StrokemgpastorNo ratings yet
- DocumentDocument12 pagesDocumentJon Saiman0% (1)
- Cuplocks Scaffolding JSA UtilityDocument4 pagesCuplocks Scaffolding JSA UtilitysoubhagyaNo ratings yet
- Product SelectionDocument5 pagesProduct Selectionemmanuelgk100No ratings yet
- Grizz Phys Tier 2 PDFDocument19 pagesGrizz Phys Tier 2 PDFJ VivianNo ratings yet