Professional Documents
Culture Documents
Elements and Periodic Table Worksheet Yr7 Scss
Uploaded by
Barminga Kamuren0 ratings0% found this document useful (0 votes)
7 views1 pageCopyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
7 views1 pageElements and Periodic Table Worksheet Yr7 Scss
Uploaded by
Barminga KamurenCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
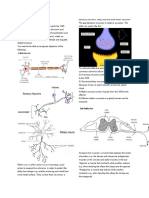
ELEMENTS, ATOMS AND COMPOUNDS
DEFINITION OF TERMS
Elements -It is a substance made up of only one
kind of an atom.
Compound - It is a substance made up of two
more different kinds of atoms chemically
combined.
molecule - two or more atoms of same or
different kinds bonded together chemically.
Atom - smallest units of an element that
maintains the elements properties.
DALTON’S ATOMIC MODEL/THEORY
The theory was first stated in 1803 by John
Dalton.
It has the following assumptions:
Elements consist of invisible small
particles(atoms). Qn. From the periodic table above, state the
All atoms of the same element are chemical symbol and chemical name of group
identical, different elements have different one elements.
types of atom. ….
Atoms can neither be created nor …………………………………………………………………………
destroyed. …………………………………………………………………………
……………………………………………………………………..
Qn. State charge of the subatomic particles Qn. State 5elements that are in period 3.
below. ….
…………………………………………………………………………
sub atomic particle charge …………………………………………………………………………
………………………………………………………………………
Electron
Qn. Give and explain 3 properties of metals.
Proton ….
…………………………………………………………………………
Nuetron …………………………………………………………………………
…………………………………………………………………………
…………………………………………………………………………
Qn. Using X to represent electrons, draw the ……………………………………………………………………..
structure of Lithium atom.
PERIODIC TABLE
The periodic table is used to arrange elements
from the lowest atomic number to the highest.
The element are arranged in groups and
periods.
Groups - are vertical arrangements of elements
as per the electrons on the outer most shell.
Period - horizontal arrangement of elements as
per the number of shells.
You might also like
- YR 10 ACC Refined End Term 3 ExamsDocument7 pagesYR 10 ACC Refined End Term 3 ExamsBarminga KamurenNo ratings yet
- Year 8 Exam ARTDocument4 pagesYear 8 Exam ARTBarminga KamurenNo ratings yet
- Halogenoalkanes Alcohol and Spectra Unit 2Document8 pagesHalogenoalkanes Alcohol and Spectra Unit 2Barminga KamurenNo ratings yet
- Law Unit 2 Year 12Document9 pagesLaw Unit 2 Year 12Barminga KamurenNo ratings yet
- Year 7 French End Term 3 2021Document6 pagesYear 7 French End Term 3 2021Barminga KamurenNo ratings yet
- Year 7 Exam ArtDocument4 pagesYear 7 Exam ArtBarminga KamurenNo ratings yet
- LLB 1Document9 pagesLLB 1Barminga KamurenNo ratings yet
- End of Term Year 7 Chem 1Document9 pagesEnd of Term Year 7 Chem 1Barminga KamurenNo ratings yet
- Formulae Equations and Amount EdexcelDocument17 pagesFormulae Equations and Amount EdexcelBarminga KamurenNo ratings yet
- End Term Exam Chemistry Year 13Document13 pagesEnd Term Exam Chemistry Year 13Barminga KamurenNo ratings yet
- Biology Exam YR 12Document9 pagesBiology Exam YR 12Barminga KamurenNo ratings yet
- End of Term Year 8 ChemDocument13 pagesEnd of Term Year 8 ChemBarminga KamurenNo ratings yet
- Chem Yr 10 End TermDocument11 pagesChem Yr 10 End TermBarminga KamurenNo ratings yet
- Genetic Engineering Igcse 9-1Document2 pagesGenetic Engineering Igcse 9-1Barminga KamurenNo ratings yet
- Ecology - Feeding Relationships SCSSDocument17 pagesEcology - Feeding Relationships SCSSBarminga KamurenNo ratings yet
- Inheritance and Variation Worksheet Year 9 SCSSDocument1 pageInheritance and Variation Worksheet Year 9 SCSSBarminga KamurenNo ratings yet
- Coordination and Response Plants and AnimalsDocument6 pagesCoordination and Response Plants and AnimalsBarminga KamurenNo ratings yet
- Separation of Mixtures WorksheetDocument1 pageSeparation of Mixtures WorksheetBarminga KamurenNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Financial Accounting 2 SummaryDocument10 pagesFinancial Accounting 2 SummaryChoong Xin WeiNo ratings yet
- Making Effective Powerpoint Presentations: October 2014Document18 pagesMaking Effective Powerpoint Presentations: October 2014Mariam TchkoidzeNo ratings yet
- Fss Presentation Slide GoDocument13 pagesFss Presentation Slide GoReinoso GreiskaNo ratings yet
- Nutrition and CKDDocument20 pagesNutrition and CKDElisa SalakayNo ratings yet
- Technical Specification For 33KV VCB BoardDocument7 pagesTechnical Specification For 33KV VCB BoardDipankar ChatterjeeNo ratings yet
- SimovertDocument41 pagesSimovertRamez YassaNo ratings yet
- Science7 - q1 - Mod3 - Distinguishing Mixtures From Substances - v5Document25 pagesScience7 - q1 - Mod3 - Distinguishing Mixtures From Substances - v5Bella BalendresNo ratings yet
- (500eboard) Version Coding Model 140 As of MY 1995Document1 page(500eboard) Version Coding Model 140 As of MY 1995Saimir SaliajNo ratings yet
- Golf Croquet Refereeing Manual - Croquet AustraliaDocument78 pagesGolf Croquet Refereeing Manual - Croquet AustraliaSenorSushi100% (1)
- Azimuth Steueung - EngDocument13 pagesAzimuth Steueung - EnglacothNo ratings yet
- DN Cross Cutting IssuesDocument22 pagesDN Cross Cutting Issuesfatmama7031No ratings yet
- B122 - Tma03Document7 pagesB122 - Tma03Martin SantambrogioNo ratings yet
- My Personal Code of Ethics1Document1 pageMy Personal Code of Ethics1Princess Angel LucanasNo ratings yet
- 2021-03 Trophy LagerDocument11 pages2021-03 Trophy LagerAderayo OnipedeNo ratings yet
- Problem Set-02Document2 pagesProblem Set-02linn.pa.pa.khaing.2020.2021.fbNo ratings yet
- Soosan Crane Training: (Principles)Document119 pagesSoosan Crane Training: (Principles)Boumediene CHIKHAOUINo ratings yet
- Köppen Climate Classification - Wikipedia, The Free EncyclopediaDocument15 pagesKöppen Climate Classification - Wikipedia, The Free EncyclopediaAndreea Tataru StanciNo ratings yet
- Proceeding of Rasce 2015Document245 pagesProceeding of Rasce 2015Alex ChristopherNo ratings yet
- 7400 IC SeriesDocument16 pages7400 IC SeriesRaj ZalariaNo ratings yet
- Excon2019 ShowPreview02122019 PDFDocument492 pagesExcon2019 ShowPreview02122019 PDFSanjay KherNo ratings yet
- Mixed Up MonstersDocument33 pagesMixed Up MonstersjaneNo ratings yet
- Project ManagementDocument11 pagesProject ManagementBonaventure NzeyimanaNo ratings yet
- Deal Report Feb 14 - Apr 14Document26 pagesDeal Report Feb 14 - Apr 14BonviNo ratings yet
- Chapter 1 ClassnotesDocument35 pagesChapter 1 ClassnotesAllison CasoNo ratings yet
- Lecture 4 ENGR 243 DynamicsDocument45 pagesLecture 4 ENGR 243 DynamicsRobby RebolledoNo ratings yet
- Dynamics of Machinery PDFDocument18 pagesDynamics of Machinery PDFThomas VictorNo ratings yet
- Module 1: Overview of Applied Behaviour Analysis (ABA)Document37 pagesModule 1: Overview of Applied Behaviour Analysis (ABA)PriyaNo ratings yet
- Global Geo Reviewer MidtermDocument29 pagesGlobal Geo Reviewer Midtermbusinesslangto5No ratings yet
- User S Manual AURORA 1.2K - 2.2KDocument288 pagesUser S Manual AURORA 1.2K - 2.2KEprom ServisNo ratings yet
- All You Need To Know About Egg YolkDocument7 pagesAll You Need To Know About Egg YolkGolden Era BookwormNo ratings yet