Professional Documents
Culture Documents
Melting Point Procedure
Melting Point Procedure
Uploaded by
oyama0 ratings0% found this document useful (0 votes)
6 views1 pageOriginal Title
Melting Point Procedure (2)
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views1 pageMelting Point Procedure
Melting Point Procedure
Uploaded by
oyamaCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
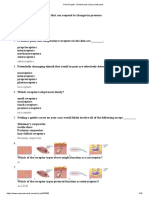
Melting Point Summary
The table below has been edited by Dr Goosen to be commensurate with your practical.
Table 6.2: Procedural summary for obtaining a melting point.
Load the two samples into
separate capillary tubes by Place BOTH samples
jabbing the open end of the into the apparatus.
tube into the solid in the vial Start heating. Label the brown envelope on
provided. Heat at a medium rate your bench as in the example
Note the sample numbers. to 130oC. Then above.
Close the vials. heat very slowly Put both melting point tubes in
With closed end down, drop (2 C every minute).
o
the brown envelope together
the tubes down a long Write down the with your tlc plate.
hollow tube so that it hits temperature ranges of Leave the envelope on your
the benchtop and packs the each compound. bench at the end of the
sample into the closed end Let the instrument cool practical.
of the tube. to 130oC to be ready
Load the sample to a height for the next student.
of 2-3mm.
Melting Points of unknown compounds:
Acetyl Salicylic acid = 135o, Paracetamol = 169o, Salicylic acid 159oC
Contributor: Lisa Nichols (Butte Community College). Organic Chemistry Laboratory Techniques is licensed
under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. Complete
text is available online.
You might also like
- 71+10 New Science Project Junior (with CD): learning science - the fun wayFrom Everand71+10 New Science Project Junior (with CD): learning science - the fun wayNo ratings yet
- Solubility of Salt v.12.15Document5 pagesSolubility of Salt v.12.15veronicaNo ratings yet
- Solubility of Salt v.8.13Document5 pagesSolubility of Salt v.8.13thepizza1949No ratings yet
- 51 Transfer of Heat by RadiationDocument5 pages51 Transfer of Heat by RadiationS'chneider AgudeloNo ratings yet
- Chemistry - Ex - No.1 & 2 - Core ExperimentsDocument5 pagesChemistry - Ex - No.1 & 2 - Core ExperimentsRagul VaidyaNo ratings yet
- Greenhouse LabDocument2 pagesGreenhouse LabBob SteinNo ratings yet
- B.Sc. SEM IDocument4 pagesB.Sc. SEM IsameerNo ratings yet
- Melting PointsDocument5 pagesMelting PointsDrAmit VermaNo ratings yet
- Enthalpy of Solution and ReactionDocument5 pagesEnthalpy of Solution and ReactionCarmen GoguNo ratings yet
- Dav School - Adambakkam Xi STD Chemistry Practical Manual Concept Based Experiments Cutting of Glass Tube / Glass RodDocument36 pagesDav School - Adambakkam Xi STD Chemistry Practical Manual Concept Based Experiments Cutting of Glass Tube / Glass RodRaje SwariNo ratings yet
- Simple Equilibrium Distillation Lab ReportDocument6 pagesSimple Equilibrium Distillation Lab ReportFarahSyazwaniNo ratings yet
- Class XI Determination of Melting and Boiling PointsDocument4 pagesClass XI Determination of Melting and Boiling PointsPratyush KumarNo ratings yet
- 11 - Practical Term 1Document6 pages11 - Practical Term 1OJASisLiveNo ratings yet
- Flow Chart Exp 2Document4 pagesFlow Chart Exp 2sf444wgt8rNo ratings yet
- Diels Alder Reaction of Anthracene IIDocument7 pagesDiels Alder Reaction of Anthracene IIPrayag Ranjan SahuNo ratings yet
- 2.01 Combustion Enthalpies by Bomb Calorimetry: 2 Background InformationDocument6 pages2.01 Combustion Enthalpies by Bomb Calorimetry: 2 Background InformationGonzalo Jose SequeiraNo ratings yet
- Solubility of NaClDocument5 pagesSolubility of NaClGustavo ZagoNo ratings yet
- 42 Heat TransferDocument6 pages42 Heat TransferChess ManNo ratings yet
- Newmelting Point LabDocument9 pagesNewmelting Point LabsoulsodaNo ratings yet
- Experiment 5 in Chemistry For Engineers - ThermochemistryDocument2 pagesExperiment 5 in Chemistry For Engineers - Thermochemistrykristine lorenteNo ratings yet
- Specific Heat Set: Instructions and Experiments For TheDocument6 pagesSpecific Heat Set: Instructions and Experiments For The220110No ratings yet
- Experiment DistillationDocument3 pagesExperiment DistillationHanna AnneNo ratings yet
- WoodliceDocument13 pagesWoodliceStacy100% (4)
- Vapro Pressure and Heat Heat of VaporazationDocument5 pagesVapro Pressure and Heat Heat of VaporazationStephen Rey CaldeaNo ratings yet
- BIO 105L - CHAPTER 6 - Melting Point DeteminationDocument4 pagesBIO 105L - CHAPTER 6 - Melting Point DeteminationFranchiezca AoananNo ratings yet
- 1.1 Introduction of SmallLab KitDocument11 pages1.1 Introduction of SmallLab KitHoongNo ratings yet
- Expt5 SolemneDocument11 pagesExpt5 SolemneArthur Christian SolemneNo ratings yet
- Hayden Cassinelli - Photosynthesis Lab and Report Track C - 5258580Document6 pagesHayden Cassinelli - Photosynthesis Lab and Report Track C - 5258580api-548259020No ratings yet
- ORG LAB Melting Point Determination2009Document8 pagesORG LAB Melting Point Determination2009Yunkai DayNo ratings yet
- ORG LAB Melting Point Determination2009Document8 pagesORG LAB Melting Point Determination2009Nguyen Huu Duy TaiNo ratings yet
- Lab 16Document3 pagesLab 16Alaa Hawary MohamedNo ratings yet
- Specific Heat of Solids: Water 4186 Aluminum 900 Steel 448 Brass 386 Copper 380Document3 pagesSpecific Heat of Solids: Water 4186 Aluminum 900 Steel 448 Brass 386 Copper 380anon_1766400No ratings yet
- Lbych31 Manual 010313Document35 pagesLbych31 Manual 010313Kella OrtegaNo ratings yet
- Lab - Pressure and TemperatureDocument4 pagesLab - Pressure and Temperatureapi-383619824No ratings yet
- Chemistry 11 Lab ManualDocument38 pagesChemistry 11 Lab Manualjkhgvdj mnhsnjkhgNo ratings yet
- ThermalinsulatingmaterialsDocument5 pagesThermalinsulatingmaterialsapi-255617605No ratings yet
- Stefan Boltzmann ApparatusDocument6 pagesStefan Boltzmann ApparatusNik SainiNo ratings yet
- Exp 1 - Melting Points - F17Document5 pagesExp 1 - Melting Points - F17Aditya KumarNo ratings yet
- Melting PointDocument10 pagesMelting Pointapi-246263792No ratings yet
- CWV 03 COMP Another - Look - Freezing PDFDocument4 pagesCWV 03 COMP Another - Look - Freezing PDFTha KantanaNo ratings yet
- EmulsionDocument6 pagesEmulsionأ. علي محمدNo ratings yet
- Handouts14 BPEDocument7 pagesHandouts14 BPEAnna MartinNo ratings yet
- Experiment 1 MgO & SuSO4Document5 pagesExperiment 1 MgO & SuSO4yosi.adriyantosssmrNo ratings yet
- Another Look at Freezing Temperature: ComputerDocument4 pagesAnother Look at Freezing Temperature: ComputerToem ReaseyNo ratings yet
- 11 Experiment 3Document4 pages11 Experiment 3akshat.sh2021No ratings yet
- Lab-Thermal Energy Transfer-Student GuideDocument8 pagesLab-Thermal Energy Transfer-Student GuideCyrusquinonesNo ratings yet
- Cbse Practical Class Xi Expt 1-3Document10 pagesCbse Practical Class Xi Expt 1-3Ramnihash MaddireddyNo ratings yet
- CHM 138 Experiment 2Document3 pagesCHM 138 Experiment 2Ayish MataNo ratings yet
- Boiling Point-Melting Point 2Document1 pageBoiling Point-Melting Point 2HyukLadezaNo ratings yet
- Chem301 Lab ManualDocument42 pagesChem301 Lab ManualIreneVeladoNo ratings yet
- Experiment 1 chem english (氮 鋁 熱)Document14 pagesExperiment 1 chem english (氮 鋁 熱)b0409119cguNo ratings yet
- Experiment 9: Freezing Point Depression Safety HazardsDocument9 pagesExperiment 9: Freezing Point Depression Safety HazardsOscar Martua SinagaNo ratings yet
- CHEM-EXP 7-9 FinalDocument48 pagesCHEM-EXP 7-9 FinalAngel Pico67% (3)
- Chem PlanningDocument4 pagesChem PlanningJiadong YeNo ratings yet
- 9-Simple Distillation (P)Document3 pages9-Simple Distillation (P)Gezem GigantoNo ratings yet
- OMB Alorimetry: ReparationDocument5 pagesOMB Alorimetry: ReparationrajmehaNo ratings yet
- Exothermic Endothermic LabDocument6 pagesExothermic Endothermic LabIwan BfasterNo ratings yet
- Photosynthesis: ExperimentDocument12 pagesPhotosynthesis: ExperimentMonica BingNo ratings yet
- Module 2 - The Human Nervous SystemDocument23 pagesModule 2 - The Human Nervous SystemoyamaNo ratings yet
- Print Chapter 13 Flashcards - Easy NotecardsDocument18 pagesPrint Chapter 13 Flashcards - Easy NotecardsoyamaNo ratings yet
- REdox Titration Intro and Non-Aqueous TitrationsDocument17 pagesREdox Titration Intro and Non-Aqueous TitrationsoyamaNo ratings yet
- Pharmaceutical Chemistry 3 Quality Control I Analytical ChemistryDocument55 pagesPharmaceutical Chemistry 3 Quality Control I Analytical ChemistryoyamaNo ratings yet
- Complexometric TitrationsDocument10 pagesComplexometric TitrationsoyamaNo ratings yet
- Quality of Drugs and Drug Products UpdatedDocument5 pagesQuality of Drugs and Drug Products UpdatedoyamaNo ratings yet
- Am Axol C 62 Pellets MBDocument7 pagesAm Axol C 62 Pellets MBoyamaNo ratings yet
- We Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistsDocument19 pagesWe Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistsoyamaNo ratings yet