Professional Documents
Culture Documents
Ed073p1174 (1) 3
Ed073p1174 (1) 3
Uploaded by
joaquinOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ed073p1174 (1) 3
Ed073p1174 (1) 3
Uploaded by
joaquinCopyright:
Available Formats
In the Laboratory
Determination of Phosphate in Cola Beverages Using
Nonsuppressed Ion Chromatography
An Experiment Introducing Ion Chromatography for Quantitative Analysis
M. A. Bello and A. Gustavo González
Departamento de Química Analítica, Universidad de Sevilla, 41012 Sevilla, Spain
The determination of inorganic anions was very dif- The concentrate solution A is prepared by dissolv-
ficult until the development of ion chromatography (IC) ing 16 g of sodium gluconate, 18 g of boric acid, and 25 g
(1). IC is an HPLC version of ion exchange that has be- of sodium tetraborate in a mixture 25:75 v/v of
Downloaded via PONTIFICIA UNIV CATOLICA DE CHILE on November 18, 2021 at 01:35:35 (UTC).
come the method of choice for routine anion analysis (2). glycerin:water to 1 L. The working eluent is prepared
See https://pubs.acs.org/sharingguidelines for options on how to legitimately share published articles.
In IC, ions are usually detected by measuring the con- by mixing 12 mL of concentrate solution A, 20 mL of n-
ductivity of eluate (3). The two techniques for conduc- butanol, 120 mL of acetonitrile, and water up to 1 L (pH
tivity detection are “single column” or “nonsuppressed” 8.4). This eluent is degassed with a flow of helium and
ion chromatography (NSIC) and “suppressed” ion chro- microfiltered (0.45 µm) before use. The background con-
matography (SIC). Although SIC presents the advantage ductivity of the eluent should be about 180 µS.
of a signal-to-noise ratio about one order of magnitude
Reagents
better than NSIC, NSIC is often selected because of its
simple instrumentation. With the addition of a conduc- Potassium dihydrogen phosphate, boric acid, sodium
tivity detector, any standard HPLC system can perform gluconate, sodium tetraborate decahydrate, glycerin, n-
NSIC. Moreover, NSIC presents several analytical ad- butanol, and acetonitrile must be of analytical grade or
vantages: calibration from borate–gluconate is more lin- better. The use of Milli-Q treated water is strongly rec-
ear than with eluents commonly used in SIC (4); and ommended.
anion exchange and conductivity detection of very weak A standard stock solution of 1000 mg/L phosphate
acids (e.g., borate, cyanide, sulfide, silicate) can be per- is prepared for calibration.
formed only by NSIC (5). Procedures
Because of the importance of IC, we felt a need for
From the standard phosphate stock solution, at least
developing a series of IC experiments that could be in-
five standards covering the range 1–10 mg/L of phos-
tegrated into existing laboratory courses in analytical
phate are prepared by dilution.
chemistry. In the present paper we report one of these
About 100 mL of cola is boiled for 20 min in a bea-
experiments: The determination of phosphate in cola
ker covered by a watch glass and then sonicated for 10
beverages by NSIC.
min or treated with a flow of nitrogen to remove carbon
Phosphate, when present at minor or trace levels, is
dioxide. Samples are prepared for analysis by diluting
currently determined spectrophotometrically, preferably
the degassed cola 1:50 with water and passing it through
as molybdenum blue or using the phosphovanadomolyb-
a Waters C18 SEP-Pak Plus cartridge (short body) to pre-
date method (6). These standard procedures are tiresome
vent retention of neutral organics via hydrophobic in-
compared with recent procedures based in IC (7–9).
teractions with the column packing (11). Each solution
Colas are actually relatively rich in phosphoric acid,
is filtered through a 0.45-µm filter unit before injection.
having concentrations of about 5 × 10{3 M (10), and may
Figures 1 and 2 depict chromatograms correspond-
be considered as very suitable laboratory samples for the ing to Coca-Cola and Pepsi-Cola, respectively. Phosphate,
experiments (Coca-Cola® , Coca-Cola Light® , Pepsi- which appears as peak I, elutes in approximately 11.30
Cola® , Pepsi Diet® , Crystal Pepsi®, Schweppes Black min. Peak II corresponds to sulfate, whose presence will
Cola®, Tab®, etc.). not be discussed here.
Quantitation of phosphate in the cola samples is
Experimental Procedure achieved through a calibration curve. Using the peak
height as analytical signal, a calibration line covering
Apparatus the range of 1–10 mg/L phosphate proves suitable for
A Waters 501 HPLC pump is used with a Waters all the colas studied.
IC-Pak A HR column and a Waters Guard Pak pre-
column. Samples are injected by using a Rheodyne-type Discussion
injector with a 100-µL loop, and detected using a Wa-
ters 341 conductivity detector. Peak evaluation is made Each student should obtain at least 3 different co-
with an Hewlett Packard HP3395 integrator. las. After the session, students calculate the concentra-
The detector sensitivity selected for experiments was tion of phosphate in the cola samples and also its rela-
1 µS and the eluent flow rate was 1 mL/min. tive standard deviation (RSD) from replicate injections
of the same cola brand (repeatability). From the students’
Eluent Preparation reports, the instructor may evaluate the reproducibility
using the results obtained by different students for the
1174 Journal of Chemical Education • Vol. 73 No. 12 December 1996
In the Laboratory
same cola brands.
Table 1. Phosphate Contents of the Analyzed
In our case (10 students, 2 instructors, and one ses-
Cola Beverages
sion of 3 hours), the repeatability RSD ranged from 0.5
to 1.5%. We calculate the reproducibility RSD to lie Phosphatea Reproducibility
Cola brand
within 2.8–5.6%. Table 1 shows the average concentra- (mg/L) (% RSD)
tion and the reproducibility of phosphate found in sev- Coca-Cola 530 2.8
eral cola beverages by the students in one session. Based Coca-Cola Light 500 4.0
on the reproducibilities, the mean values were appro- Pepsi-Cola 540 2.7
priately rounded.
As a first insight, a relative difference among brands Pepsi Diet 480 4.2
is detectable. The application of one-way ANOVA to the Crystal Pepsi 450 5.6
collected results (at least 4 data for each analyzed cola Schweppes Black Cola 570 3.5
brand) confirmed that the brands differ significantly.
Moreover, from the estimate of the within-sample vari- Tab 440 3.4
ance, the least significant difference (12) among means a
Expressed as orthophosphoric acid.
was calculated, giving 27. Comparing this value with the
differences between the means one can see that only the
couples Crystal Pepsi/Tab, Coca-Cola Light/Pepsi Diet, Literature Cited
and Coca-Cola/Pepsi-Cola do not differ significantly in
the content of phosphate. 1. Walton, H. F.; Rockling, R. D. Ion Exchange in Analytical Chemistry; CRC:
Boston, 1990; p 59.
The pH of degassed undiluted colas ranged between 2. Harris, D. C. Quantitative Chemical Analysis; W. H. Freeman: New York,
2.4 and 2.5, in good agreement with the observations of 1995; p 706.
3. Small, H.; Stevens, T. S.; Bauman, W. C. Anal. Chem. 1975, 47, 1801–1809.
Murphy (10). Using the first ionization constant for phos- 4. Schmuckler, G.; Jagoe, A. L.; Girard, J. E.; Buell, P. E. J. Chromatogr. 1986,
phoric acid, Ka = 7.5 × 10{3, the total concentration of 356, 413–419.
5. Okada, T.; Kuwamoto, T. Anal. Chem. 1985, 57, 829–833.
phosphoric acid is calculated. The results range within 6. Williams, W. J. Handbook of Anion Determination; Butterworths: London,
6.09 × 10 {3 and 4.50 × 10{3 M, which correspond to 597– 1979; pp 435, 487.
441 mg/L. This suggests that phosphoric acid is the 7. Ryder, D. S. J. Chromatogr. 1986, 354, 438–441.
8. Schmuckler, G.; Brenman, L. LC-GC Int. 1992, 5, 36–38.
strongest acid present in cola, and accordingly the sul- 9. Tanaka, T.; Hiiro, K.; Kawahara, A.; Wakida, S. Bunseki Kagaku 1983, 32,
fate levels found in the analysis could be attributed to 771–773.
10. Murphy, J. J. Chem. Educ. 1983, 60, 420–421.
the water used in the cola formulation. 11. Water Ion Chromatography Cookbook; Manual no. 20195; Millipore Corpo-
ration, Water Chromatography Division: Mildford, 1989.
12. Miller, J. C.; Miller, J. N. Statistics for Analytical Chemistry; Ellis Horwood:
Chichester, 1993; pp 66–69.
Figure 1. Ion chromatogram for 1:50 diluted Coca-Cola ® (I, phos- Figure 2. Ion chromatogram for 1:50 diluted Pepsi-Cola ® (I, phos-
phate; II, sulfate). phate; II, sulfate).
Vol. 73 No. 12 December 1996 • Journal of Chemical Education 1175
You might also like
- SMWW 2882 070Document8 pagesSMWW 2882 070lmb LaboratoriosNo ratings yet
- Application Li Batteries 5110 Icp-Oes 5994-1937en Us AgilentDocument4 pagesApplication Li Batteries 5110 Icp-Oes 5994-1937en Us AgilentxxxNo ratings yet
- 1 Selective Determination of ChlorineDocument7 pages1 Selective Determination of Chlorinevey bieberNo ratings yet
- Zinc CitrateDocument1 pageZinc CitrateKasidit SornchaiNo ratings yet
- 2006 09 06 Experiment 5Document4 pages2006 09 06 Experiment 5Almira Kaye CuadraNo ratings yet
- Iodate and Iodine Speciation by LC-ICPMSDocument6 pagesIodate and Iodine Speciation by LC-ICPMSShubhamNo ratings yet
- Presentation ICDocument32 pagesPresentation ICgitaismala90No ratings yet
- Determination of Potassium in Sea Water: MethodDocument2 pagesDetermination of Potassium in Sea Water: MethodPysadee PysadeeNo ratings yet
- Chem Lab ManualDocument37 pagesChem Lab ManualChris JonathanNo ratings yet
- Cianyde WasteDocument4 pagesCianyde WasteFrancisco BocanegraNo ratings yet
- Bai Bao EC-1998Document5 pagesBai Bao EC-1998Bình GiangNo ratings yet
- Ion Chromatography in Environmental AnalysisDocument23 pagesIon Chromatography in Environmental AnalysisCompras FQ AnaltecNo ratings yet
- Vape Fid AgilentDocument10 pagesVape Fid AgilentBulanbintang klangNo ratings yet
- Ijct 6 (1) 38-42Document5 pagesIjct 6 (1) 38-42Hêny CarlênicNo ratings yet
- Testing Pollutan LevelsDocument4 pagesTesting Pollutan LevelsWanti CantikNo ratings yet
- Determination of Quinine in Tonic Water Using FluorescenceDocument22 pagesDetermination of Quinine in Tonic Water Using FluorescenceAin SufizaNo ratings yet
- Coluna Cromatográfica - Art4Document2 pagesColuna Cromatográfica - Art4thfsctaaNo ratings yet
- Keywords:-Spectrophotometry, Nickel, Triton X-100, 5 - (2'Document9 pagesKeywords:-Spectrophotometry, Nickel, Triton X-100, 5 - (2'International Journal of Innovative Science and Research TechnologyNo ratings yet
- AOAC Official Method 999.15 Vitamin K in MilkDocument2 pagesAOAC Official Method 999.15 Vitamin K in MilkAnju Doraisamy100% (1)
- Bsis25 Tibc 2018Document4 pagesBsis25 Tibc 2018Houssam DjeradNo ratings yet
- Isolation of RNADocument2 pagesIsolation of RNAALLEN SERGIO DIZONNo ratings yet
- (Shimadzu) Appl News No A636 Analysis of Fe and MN in Tap Water by Flameless AA MethodDocument2 pages(Shimadzu) Appl News No A636 Analysis of Fe and MN in Tap Water by Flameless AA MethodfaridsidikNo ratings yet
- USP LidocaineDocument2 pagesUSP LidocaineRogxr JencasearNo ratings yet
- 5 - Determinação SDocument12 pages5 - Determinação SEudson VictorNo ratings yet
- s00604 007 0733 ZDocument6 pagess00604 007 0733 Zprakashjyoti0901No ratings yet
- Acids Org Sb-Aq 5989-1265Document8 pagesAcids Org Sb-Aq 5989-1265pacocurroNo ratings yet
- Aoac 979.08Document1 pageAoac 979.08blink scientificNo ratings yet
- 8271-Article Text-29026-1-10-20130629 PDFDocument4 pages8271-Article Text-29026-1-10-20130629 PDFPankaj DahalNo ratings yet
- Analysis of Organic Acids On An Agilent Infinitylab Poroshell 120 Hilic-Z ColumnDocument4 pagesAnalysis of Organic Acids On An Agilent Infinitylab Poroshell 120 Hilic-Z ColumnDANIELNo ratings yet
- PH Sensor Final SC24V GSDocument4 pagesPH Sensor Final SC24V GShenryhariyadiNo ratings yet
- Analysis Methods For Zinc Plating BrightenersDocument5 pagesAnalysis Methods For Zinc Plating BrightenersYasir ZohaNo ratings yet
- Total Cyanide Using UV Digestion and Amperometric Detection (ASTM D7511 09e Equivalent Method)Document6 pagesTotal Cyanide Using UV Digestion and Amperometric Detection (ASTM D7511 09e Equivalent Method)maruf amaludinNo ratings yet
- The Determination of Mercury in Plastics by CVG-ICP-OESDocument1 pageThe Determination of Mercury in Plastics by CVG-ICP-OESAri VäisänenNo ratings yet
- E38IA049EN A ApplReport QuickRoutineSVTDigestion of Fe andCU OreDocument4 pagesE38IA049EN A ApplReport QuickRoutineSVTDigestion of Fe andCU OreAl KayprofNo ratings yet
- Á641Ñ Completeness of Solution: 310 Á631ñ Color and Achromicity / Physical Tests USP 37Document2 pagesÁ641Ñ Completeness of Solution: 310 Á631ñ Color and Achromicity / Physical Tests USP 37David SRNo ratings yet
- Decker. R. Oldenburg, S. Covalent Bioconjugation of Antibodies To Carboxyl Terminated NanoparticlesDocument1 pageDecker. R. Oldenburg, S. Covalent Bioconjugation of Antibodies To Carboxyl Terminated NanoparticlesStella AguirreNo ratings yet
- EuSalt AS016-2005 Chloride - Potentiometric MethodDocument4 pagesEuSalt AS016-2005 Chloride - Potentiometric MethodBadini ChanalNo ratings yet
- CPB 35 5010Document5 pagesCPB 35 5010Larisa CatautaNo ratings yet
- Synthesis and Properties of Cardanol BasDocument12 pagesSynthesis and Properties of Cardanol Bas210353 Trần Thị Ngọc HiềnNo ratings yet
- Analysis Methods: S. N. Parameters Brief Description Test MethodsDocument1 pageAnalysis Methods: S. N. Parameters Brief Description Test Methodsसुनिल बाबु खत्रीNo ratings yet
- Analysis Methods: S. N. Parameters Brief Description Test MethodsDocument1 pageAnalysis Methods: S. N. Parameters Brief Description Test Methodsसुनिल बाबु खत्रीNo ratings yet
- AN237 LC Benzalkonium Chloride LPN2313 - EDocument6 pagesAN237 LC Benzalkonium Chloride LPN2313 - EDaniel AgungNo ratings yet
- 42118-LPN 1876-01 - CyanideDocument7 pages42118-LPN 1876-01 - CyanidehuongktbNo ratings yet
- PH BceDocument5 pagesPH BceKrish KNo ratings yet
- Fluitest U/Csf: Ultrasensitive ProteinDocument4 pagesFluitest U/Csf: Ultrasensitive ProteinDarko MaksimovicNo ratings yet
- Zinc Sulfate Ophthalmic SolutionDocument1 pageZinc Sulfate Ophthalmic SolutionKasidit SornchaiNo ratings yet
- (Aparece-Reyes) Recorded DefenseDocument27 pages(Aparece-Reyes) Recorded DefenseellaNo ratings yet
- Analysis of Sugars Using An Agilent Infinitylab Poroshell 120 Hilic-Z ColumnDocument6 pagesAnalysis of Sugars Using An Agilent Infinitylab Poroshell 120 Hilic-Z ColumnDANIELNo ratings yet
- Ijca 30a (6) 531-535Document5 pagesIjca 30a (6) 531-535Zia KhanNo ratings yet
- Ion Pair ChromatographyDocument9 pagesIon Pair ChromatographyLeo EspositoNo ratings yet
- 8 - Sodium Benzoate USP CurrentDocument1 page8 - Sodium Benzoate USP Currentasmae.labindusNo ratings yet
- Separasi Anion BromidaDocument4 pagesSeparasi Anion BromidaAdamNo ratings yet
- ALP Single ReagentDocument2 pagesALP Single ReagentJames 'jps' SimanjuntakNo ratings yet
- Ion Pairing Chromatogr DionexDocument8 pagesIon Pairing Chromatogr DionexNguyen DungNo ratings yet
- Synthesis, Characterization, and Use of A Cobalt (l1) Complex As An NMR Shift ReagentDocument2 pagesSynthesis, Characterization, and Use of A Cobalt (l1) Complex As An NMR Shift ReagentAliceNo ratings yet
- Zinc CarbonateDocument2 pagesZinc CarbonateKasidit SornchaiNo ratings yet
- Removal of Nitrates From Water in The Presence of Competitors Anions Using Purolite ResinsDocument13 pagesRemoval of Nitrates From Water in The Presence of Competitors Anions Using Purolite ResinsClau GrsNo ratings yet
- A Simple Spectrophotometric Assay For The Measurement of Soluble Silica in WaterDocument3 pagesA Simple Spectrophotometric Assay For The Measurement of Soluble Silica in WaterendorengasNo ratings yet
- Articulo Importante ElectroquimicaDocument4 pagesArticulo Importante ElectroquimicaMarcial Fuentes EstradaNo ratings yet
- Applications of Zeeman Graphite Furnace Atomic Absorption Spectrometry in the Chemical Laboratory and in ToxicologyFrom EverandApplications of Zeeman Graphite Furnace Atomic Absorption Spectrometry in the Chemical Laboratory and in ToxicologyC. MinoiaNo ratings yet
- Alloy Steel ChartDocument4 pagesAlloy Steel Chartmodi_mihirNo ratings yet
- Jurnal Sabun Mandi Yg Ini Juga BisaDocument9 pagesJurnal Sabun Mandi Yg Ini Juga BisaTiara Salsabillah AzwaNo ratings yet
- 9701 s04 QP 4Document12 pages9701 s04 QP 4Hubbak KhanNo ratings yet
- 453 - Pdfsam - Timmehaus - Plant Design and Economics For Chemical EngineersDocument4 pages453 - Pdfsam - Timmehaus - Plant Design and Economics For Chemical EngineersYazan SalehNo ratings yet
- Predict The Product For The Following Reaction.: I II IIIDocument11 pagesPredict The Product For The Following Reaction.: I II IIIzelalem getachewNo ratings yet
- Lime Provides An Economical Way of Soil StabilizationDocument1 pageLime Provides An Economical Way of Soil StabilizationCalynn Tiffany YapNo ratings yet
- Lewis Concept of Acids and BasesDocument6 pagesLewis Concept of Acids and Basescayla mae carlosNo ratings yet
- Dna ExtractionDocument21 pagesDna ExtractionXian LeeNo ratings yet
- ZSUS TDS Metalworking Phosphetal 2-22-21Document2 pagesZSUS TDS Metalworking Phosphetal 2-22-21İsmail YakinNo ratings yet
- August 2022 Report For Dennis T MuzilaDocument11 pagesAugust 2022 Report For Dennis T MuzilaDennis MuzilaNo ratings yet
- Kolation of Oil of Clove and Separation of Eugenol and Acetyl EugenolDocument1 pageKolation of Oil of Clove and Separation of Eugenol and Acetyl EugenolFaFi SaLaNo ratings yet
- SP Carey - Parte BDocument260 pagesSP Carey - Parte BLeanne SilvaNo ratings yet
- Acid Base TitrationDocument6 pagesAcid Base Titrationgunapranes15No ratings yet
- Protein SurfatanDocument19 pagesProtein Surfatanmeilana23No ratings yet
- Lab ChemistryDocument3 pagesLab ChemistryGladies MacancelaNo ratings yet
- CBSE Class 12 Question Paper Solution 2018 Chemistry Set 1Document8 pagesCBSE Class 12 Question Paper Solution 2018 Chemistry Set 1Saran.kNo ratings yet
- Nudelman 2004Document4 pagesNudelman 2004kongaradamuNo ratings yet
- Astm D513Document8 pagesAstm D513CeciliagorraNo ratings yet
- Ion-Exchange Methods and IntercalationDocument4 pagesIon-Exchange Methods and IntercalationHimanshu GuptaNo ratings yet
- Non-Stoichiometric Defects: Hari Prakash Sahu Id-Mu20Mch042 MSC 2Nd SemDocument8 pagesNon-Stoichiometric Defects: Hari Prakash Sahu Id-Mu20Mch042 MSC 2Nd SemomiiNo ratings yet
- Study of Common Food AdulterantsDocument20 pagesStudy of Common Food AdulterantsChaitanya PantulaNo ratings yet
- Experiment: Water, Its Purification and PropertiesDocument6 pagesExperiment: Water, Its Purification and PropertiesLeanna Bautista0% (2)
- Jurnal LamunDocument12 pagesJurnal LamunGita DjafarNo ratings yet
- Jenis, Dan, Sifat, PolimerDocument15 pagesJenis, Dan, Sifat, PolimerpandulewandownskiNo ratings yet
- Analysis Presentation 1Document12 pagesAnalysis Presentation 1Muhammad Hussain KhalilNo ratings yet
- BOB - IdentifyingNutrientsSEDocument6 pagesBOB - IdentifyingNutrientsSEKamil JabiriNo ratings yet
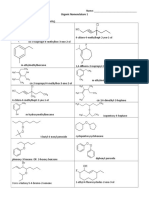
- Organic Nomenclature 1 AnswersDocument2 pagesOrganic Nomenclature 1 AnswersJethro LemosneroNo ratings yet
- Jpa Mineral Trader Pipeline ProjectsDocument2 pagesJpa Mineral Trader Pipeline ProjectsCondebuild StaffNo ratings yet
- Asymptotically Approaching Zero DefectsDocument33 pagesAsymptotically Approaching Zero DefectsAverage JoeNo ratings yet
- CipwnormexcelDocument2 pagesCipwnormexcelHowel TurnerNo ratings yet