Professional Documents
Culture Documents
Impact of - Arginine Metabolism On Immune Response and Anticancer Immunotherapy
Uploaded by
FistiOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Impact of - Arginine Metabolism On Immune Response and Anticancer Immunotherapy
Uploaded by
FistiCopyright:
Available Formats
Mini Review
published: 16 March 2018
doi: 10.3389/fonc.2018.00067
Sun-Hee Kim, Jason Roszik, Elizabeth A. Grimm and Suhendan Ekmekcioglu*
Department of Melanoma Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX,
United States
The progression from neoplastic initiation to malignancy happens in part because of the
failure of immune surveillance. Cancer cells successfully escape immune recognition
and elimination and create an immune-suppressive microenvironment. A suppressive
metabolic microenvironment may also contribute to ineffective T-cell function. Tumor
progression is characterized by a complex network of interactions among different cell
types that cooperatively exploit metabolic reprogramming. As we start to recognize
that cancer cells use different metabolism processes than normal cells do, a better
Edited by:
understanding of the functional mechanisms of the regulation and reprogramming of the
Sherven Sharma,
VA Greater Los Angeles metabolic landscape in cancer cells is crucial to successful immunotherapy strategies.
Healthcare System (VHA), However, the exact role of metabolism in T cells and in the tumor microenvironment is
United States
not known. One pathway that plays an important role in the regulation of immune cell
Reviewed by:
Pin Wu,
reactivity is arginine metabolism, which has complex cellular functions. l-arginine and
Zhejiang University, China its downstream metabolites (e.g., ornithine and citrulline) could be essential to T-cell
William K. Decker, activation and thus modulate innate and adaptive immunity to further promote tumor
Baylor College of Medicine,
United States survival and growth. Identifying metabolic targets that mediate immunosuppression
*Correspondence: and are fundamental to sustaining tumor growth is key to increasing the efficacy of
Suhendan Ekmekcioglu immunotherapies.
sekmekcioglu@mdanderson.org
Keywords: arginine, NO synthase, arginase-1, cyclooxygenase-2, microsomal prostaglandin E synthase-1,
immunotherapy, metabolism, immune response
Specialty section:
This article was submitted to
Cancer Immunity and INTRODUCTION
Immunotherapy,
a section of the journal Metabolic pathways and their intermediates have been shown to contribute to the formation of the
Frontiers in Oncology immunosuppressive tumor features that exclude immune cells from the tumor microenvironment
Received: 18 December 2017 or impair their ability to respond. Manipulating these metabolic changes can strengthen antitumor
Accepted: 01 March 2018 immune responses by recovering the functions of the T cells. Thus, cancer immunotherapy strategies
Published: 16 March 2018
focus on metabolism to promote immune cells’ acquisition of sufficient metabolites to preserve their
Citation: antitumor responses.
Kim S-H, Roszik J, Grimm EA and Tumor progression is characterized by a complex network of communication among different
Ekmekcioglu S (2018) Impact of
cell types and cancer cells that stimulates the metabolic reprogramming of the cancer cells and thus
l-Arginine Metabolism on
Immune Response and
influences their functionality. Our research suggests that deleterious tumor inflammatory processes
Anticancer Immunotherapy. occur in response to microenvironmental communications and are driven by intrinsic inflammatory
Front. Oncol. 8:67. processes (1). Much recent interest and new immune system data suggests that cellular bioenergy
doi: 10.3389/fonc.2018.00067 links metabolism with inflammation and immunity to protect normal cells from immune death
Frontiers in Oncology | www.frontiersin.org 1 March 2018 | Volume 8 | Article 67
Kim et al. Arginine Metabolism on Immune Response
(i.e., checkpoint inhibition) and to restore homeostasis with
minimal tissue damage (2), and provides a complex balance to
make it beneficial for the host. However, cancer cells with these
self-protective mechanisms become resistant to immune attack.
Clinically evident cancers are those that have evaded the immune
response. Inflammation and immune evasion are hallmarks of
cancer progression, as evidenced by the fact that cancer patients
with fewer immune cells in their biopsied tissues have poorer
responses to immunotherapies (3). These cells may include the
direct involvement of immune cells, including macrophages,
T cells, B cells, natural killer cells, dendritic cells, and myeloid-
derived suppressor cells (MDSCs) (4).
Some of the mechanisms the immune system uses to fight
infectious diseases also promote immune suppression in the tumor
microenvironment. Tumor resistance to the immune response is Figure 1 | Potential mechanism of arginine metabolism in cancer cells
versus immune-infiltrating cells in human melanoma. After arginine enters
assisted by the immunoediting of the immune signals induced by mammalian cells through the membrane-bound transporters CAT1 and
the tumors and their metabolites (2), possibly including that of CAT2B, it is metabolized by one of the NO synthase (NOS) enzymes to
checkpoint inhibitor upregulation, via inflammatory mechanisms produce nitric oxide (NO), which is associated with immune suppression and
mediated by arginine metabolism (5). This review presents the results in the expression of specific markers and functional changes.
role of arginine metabolism in the major inflammatory processes
that we have found to drive the survival of melanoma and other
cancers, including those processes underlying these tumors’ is constitutively expressed in most tissues, whereas CAT2B is
resistance to therapy, particularly immunotherapy. cytokine inducible (8). Thus, we speculate that the diverse expres-
sion of CAT2B may be associated with the inflammatory factors
expressed in human melanoma cells.
ARGININE METABOLISM AS A MAJOR After arginine enters mammalian cells through the membrane-
REQUIREMENT FOR INFLAMMATORY bound transporters CAT1 and CAT2B, it is metabolized by one of
NETWORKS AND PRODUCTS the NOS enzymes. The enzymatic production of NO is cell-type
specific, with cytokine-driven inducible NOS (iNOS) noted ini-
Human tumors support an immunosuppressive microenviron- tially for the burst of higher levels as part of the pathogen defense
ment that often prevents effective immunotherapy. Because of system. Neuronal cells use neuronal NOS to produce NO for sign-
their effectiveness and the long-term responses they elicit, cancer aling. The third NOS, endothelial NOS, regulates NO production
immunotherapies have changed the landscape of cancer care. in endothelia and is responsible for vascular relaxation (9). In pre-
Despite the major breakthroughs in cancer immunotherapy of vious studies, we found that the selectively increased metabolism
the past decade, the success to date does not meet the promise of l-arginine in melanoma and other human tumors is associated
of the approach. Different physiological mechanisms can prevent with immune suppression and neovascularization and that the
durable responses to immunotherapy. Some of these mechanisms arginine metabolic product of NO results in the expression of
have been characterized molecularly and may be triggered by the specific markers of tumor progression and in functional changes
tumor microenvironment, resulting in impaired immune effector (10, 11). We and others have also found that increased arginine
cell function. metabolism in melanoma tumors and their microenvironment
Arginine metabolism is one of the mechanisms responsible mediates a previously unappreciated system that is fundamental
for tumor progression and it is highly compartmentalized due to to sustaining the growth of many cancers while specifically caus-
expression of enzymes involved in arginine metabolism in vari- ing immunosuppression (11–14).
ous cells. l-arginine is a multipurpose amino acid that also serves The metabolism of arginine by a family of NOSs generates
as a precursor for multiple metabolites, including polyamines and NO, which can affect molecular pathways in melanoma cells and
nitric oxide (NO), which have strong immunomodulatory prop- the melanoma microenvironment by defined posttranslational
erties (Figure 1). l-arginine is the substrate for four enzymes, modifications. NO is a relatively stable intracellular molecule that
several of which exist as multiple isoforms: NO synthases (NOSs), diffuses through lipid bilayers and persists for at least 7 s (15).
arginases (ARGs), glycine aminidotransferase, and l-arginine NO can react with superoxide to form peroxynitrite, which
decarboxylase (6). To encounter the enzymes involved in its rapidly forms two posttranslational modifications on proteins,
metabolism, l-arginine must be transported through the plasma nitrosylation of thiols and nitration of tyrosines (NT) (16, 17).
membrane via cationic amino acid transporters (CATs) and A second arginine metabolism via ARG leads to proline and
metabolized by NOS enzymes (7). Using the Cancer Cell Line polyamine production, which may also contribute to tumor
Encyclopedia database, we found that CAT2B expression var- progression. Therefore, overall arginine metabolism depends on
ies among human melanoma cells and that a subset of human the activity of the NOS and ARG enzyme families. NOS oxidizes
melanoma cells has a very high level of CAT2B expression, but arginine to citrulline and NO, and ARG hydrolyzes arginine into
that CAT1 expression is similar among all the cell lines. CAT1 ornithine and urea. We and others have demonstrated that many
Frontiers in Oncology | www.frontiersin.org 2 March 2018 | Volume 8 | Article 67
Kim et al. Arginine Metabolism on Immune Response
human tumors, including melanoma, express iNOS (18–23). iNOS potent immune suppressant (26). In melanoma, specifically,
expression and NO production have been shown to promote tumor-derived COX activity is the key suppressor of type I
many cancers’ growth, survival, and resistance to therapy. One interferon-mediated tumor elimination and induces an inflam-
recent study of the genomic and transcriptomic features of the matory signature associated with progression (27). The PGE2-
response of metastatic melanoma to anti-programmed cell death mediated stimulation of immunosuppressive cells, including
protein 1 (PD-1) therapy revealed that a transcriptional signature T regulatory cells (Tregs) and MDSCs, may also be an important
is related to innate anti-PD-1 resistance (24). Using the same mechanism of immune suppression. PGE2 has an important role
clinical dataset, we found that iNOS expression in metastatic in redirecting the differentiation of human dendritic cells into
melanoma is associated with response to anti-PD-1 therapy MDSCs, and the inhibition of COX-2 or PGE2 receptors abol-
(unpublished data). Therefore, understanding the regulation of ishes MDSCs’ functions and their CXCR4-CXCL12-mediated
immune cell response and functions of tumor-expressed iNOS attraction to the cancer environment (28). PGE2 stimulates the
will provide significant opportunities for therapeutic interven- de novo conversion of Tregs from naïve CD4+ T cells (29), and
tion. ARG-1 activity is associated with polarized, protumoral Tregs expressing PGE2 receptors are preferentially recruited to
M2 tumor-associated macrophages (25). Suppression of T-cell factors expressed by COX-2-expressing tumor cells (30). Elevated
activation, proliferation, and differentiation by macrophage MDSCs and Tregs suppress T-cell infiltration into the tumor site
ARG-1 dominated by M2 macrophages and interactions between and/or inhibit T-cell activity. In a previous study of stage III
macrophage metabolism and M1/M2 polarization is an urgent melanoma, our tissue microarray analysis demonstrated that
matter to be resolved in cancer. Together, ARG and NOS enzymes high mPGES1 staining intensity was significantly associated with
metabolize arginine and are critical components of immune sup- low CD8 levels, and we found that patients whose tumors had a
pression pathways, and the metabolic products of these enzymes high-mPGES1, low-CD8 expression signature had a significantly
are imperative mediators of T-cell function in cancer (12). increased risk of death (31).
The importance of arginine metabolism as a novel field of Several recent reports suggest that targeting PGE2 metabolism
investigation includes that of arginine depletion retarding the could help reduce programmed death-ligand 1 (PD-L1)-mediated
growth of some cancers (26), whereas others report that arginine immune suppression (27, 32, 33). One study showed that COX-2
supplementation enhances antitumor effects, probably by enhan and PD-L1 were expressed in both primary melanoma lesions
cing immune function (11, 27). Arginine supplementation has and non-matching lymph node metastases and that the inhibition
been discovered to stimulate T cell and natural killer cell activity of COX-2 activity by celecoxib downregulated PD-L1 expression
and promotes the production of pro-inflammatory cytokines in both BRAFV600E A375 and NRASQ61R SK-MEL-2 mela-
(28, 29). The roles of arginine in the context of NOS and ARG noma cell lines (32). In another study, an analysis of murine bone
activity and in tumor-derived NO need to be understood. marrow cells cocultured with murine MBT-2 bladder tumor cells
demonstrated that the COX-2/mPGES1/PGE2 pathway regulates
PD-L1 expression in tumor-associated macrophages and MDSCs
ARGININE METABOLISM AND NOS (33). A third study showed that conventional nonsteroidal anti-
AND CYCLOOXYGENASE inflammatory drugs and COX-2 inhibitors combined with an
anti-PD-1 monoclonal antibody promoted a much more rapid
Preliminary data from our laboratory and others (24, 25) indicate regression of mouse melanoma tumors than the anti-PD-1
that NO activates cyclooxygenase-2 (COX-2) and other inflam- antibody alone did (27). This result suggests that the COX-2/
matory mediators, thereby creating a pro-oxidant microenviron- mPGES1/PGE2 pathway contributes to immune evasion and that
ment that supports cancer cell growth and suppresses antitumor nonsteroidal anti-inflammatory drugs and COX-2 inhibitors are
immunity. iNOS/NO positively regulates the production of useful adjuvants to immune-based anticancer therapies.
COX-2, microsomal prostaglandin E synthase-1 (mPGES1), and Our data show not only the NO-mediated modulation of
prostaglandin E2 (PGE2). COX-2 and iNOS can be produced tumor-infiltrating leukocyte (TIL) growth but also the COX-
concurrently in several inflammatory conditions (24). In our 2-mediated production of PGE2 in human melanoma. Other
previous study, a tissue microarray analysis of stage III melanoma potential sources of NO from arginine metabolism in cancers
showed that most mPGES1-positive tissues were also positive include MDSCs, which are a heterogeneous population of
for iNOS and had co-localized mPGES1 and iNOS expression. myeloid cells that often infiltrate cancers and sites of inflamma-
n addition, transient iNOS expression and NO donors dramati- tion and infection and have a remarkable ability to suppress T-cell
cally enhanced PGE2 production, suggesting that iNOS and NO responses (30). We have previously reported that iNOS expression
are upstream of PGE2 biosynthesis in melanoma cells. We found in melanoma is significantly associated with apoptosis resistance
that mPGES1 was tyrosine-nitrated by NO. Our data suggest that and shorter patient survival (18, 31). We recently observed global
iNOS regulates mPGES1 activity through a posttranslational NT NT expression in stage IIIc and IV melanomas in humans. NT is
modification, which ultimately enhances PGE2 production; that a marker of NO’s reaction with reactive oxygen species to form
iNOS activates the mPGES1/PGE2 pathway; and that the cross peroxynitrite, which irreversibly nitrates tyrosines. NT expression
talk between iNOS and mPGES1 promotes inflammation, which in tissues is associated with poor TIL growth and poorer response
favors melanoma progression (24). to TIL-based therapy. Protein-associated NT has been identified as
In melanoma and other cancers, PGE2 plays a role in immune a surrogate marker of in situ inflammation, but its role in cellular
responses, acting as both a pro-inflammatory mediator and a immune response has not been studied broadly. One intriguing
Frontiers in Oncology | www.frontiersin.org 3 March 2018 | Volume 8 | Article 67
Kim et al. Arginine Metabolism on Immune Response
study showed that the oxidation of tyrosine to NT on either T-cell our knowledge regarding their role in current immunotherapy
receptor or major histocompatibility complex (MHC) molecules approaches. The extent to which immune cells and tumor cells
can disturb CD8 T cells’ recognition of the MHC class I-restricted in the tumor microenvironment respond to arginine metabolism
epitope of lymphocytic choriomeningitis virus glycoprotein (32). could predict better immunotherapy outcomes.
This study also demonstrated that T cells’ specific recognition of
nitrated epitopes is not limited to MHC class II epitopes but that AUTHOR CONTRIBUTIONS
the conversion of tyrosine to nitrotyrosine can also intensely dis-
turb these cells’ recognition of MHC class I-restricted epitopes. If, All authors (S-HK, JR, EG, and SE) were involved in building the
like CD4 T cells, CD8 T cells can recognize epitopes containing concept and participated in the writing based on their expertise
an NT posttranslational modification as antigenic, this would area. S-HK was involved mainly in writing; JR was involved in
certainly affect TIL activation in melanoma patients. concept and analyses, EG was involved in sharing her expertise,
reviewing, and editing; and SE was mainly involved in writing,
CONCLUSION concept design, reviewing, and editing.
Following the success of checkpoint inhibitors in many tumor ACKNOWLEDGMENTS
types, immunotherapy has become a major focus of cancer
researchers and clinicians. Our mission is to guide oncology This work was supported by MD Anderson’s Melanoma SPORE
research by improving our understanding of the role of tumors’ (P50 CA093459; S-HK, EG, and SE); the National Institutes of
interactions with their microenvironment and with immune Health through MD Anderson’s Cancer Center Support Grant
cells functions in particular. Arginine metabolism is one of the (P30-CA016672; EG); the Dr. Miriam and Sheldon G. Adelson
mechanisms responsible for immune response to tumor progres- Medical Research Foundation (EAG); MD Anderson’s Melanoma
sion. Because different cells express enzymes involved in the Moon Shot Program (JR and EG); and the Jim Mulva Foundation;
process, arginine metabolism is highly dysregulated in cancer. and AIM Foundations. We also thank Joseph A. Munch of the
The concept of antitumor immune response, based on usage of MD Anderson Department of Scientific Publications for carefully
arginine via NOS and ARG, is timely topic to focus on to set editing the manuscript and providing valuable comments.
REFERENCES 12. Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, et al. l-arginine
modulates T cell metabolism and enhances survival and anti-tumor activity.
1. Ekmekcioglu S, Davies MA, Tanese K, Roszik J, Shin-Sim M, Bassett RL Jr, Cell (2016) 167:829–842e813. doi:10.1016/j.cell.2016.09.031
et al. Inflammatory marker testing identifies CD74 expression in melanoma 13. Fletcher M, Ramirez ME, Sierra RA, Raber P, Thevenot P, Al-Khami AA,
tumor cells, and its expression associates with favorable survival for stage et al. l-Arginine depletion blunts antitumor T-cell responses by inducing
III melanoma. Clin Cancer Res (2016) 22:3016–24. doi:10.1158/1078-0432. myeloid-derived suppressor cells. Cancer Res (2015) 75:275–83. doi:10.1158/
CCR-15-2226 0008-5472.CAN-14-1491
2. Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell (2010) 14. Norian LA, Rodriguez PC, O’Mara LA, Zabaleta J, Ochoa AC, Cella M, et al.
140:771–6. doi:10.1016/j.cell.2010.03.006 Tumor-infiltrating regulatory dendritic cells inhibit CD8+ T cell function via
3. Reuben A, Spencer CN, Prieto PA, Gopalakrishnan V, Reddy SM, Miller JP, l-arginine metabolism. Cancer Res (2009) 69:3086–94. doi:10.1158/0008-5472.
et al. Genomic and immune heterogeneity are associated with differential CAN-08-2826
responses to therapy in melanoma. NPJ Genom Med (2017) 2:10. doi:10.1038/ 15. Habib S, Ali A. Biochemistry of nitric oxide. Indian J Clin Biochem (2011)
s41525-017-0013-8 26:3–17. doi:10.1007/s12291-011-0108-4
4. Veglia F, Gabrilovich DI. Dendritic cells in cancer: the role revisited. Curr Opin 16. Kovacs I, Lindermayr C. Nitric oxide-based protein modification: formation
Immunol (2017) 45:43–51. doi:10.1016/j.coi.2017.01.002 and site-specificity of protein S-nitrosylation. Front Plant Sci (2013) 4:137.
5. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. doi:10.3389/fpls.2013.00137
Nature (2008) 454:436–44. doi:10.1038/nature07205 17. Yakovlev VA, Mikkelsen RB. Protein tyrosine nitration in cellular signal trans-
6. Popolo A, Adesso S, Pinto A, Autore G, Marzocco S. l-arginine and its metab- duction pathways. J Recept Signal Transduct Res (2010) 30:420–9. doi:10.3109/
olites in kidney and cardiovascular disease. Amino Acids (2014) 46:2271–86. 10799893.2010.513991
doi:10.1007/s00726-014-1825-9 18. Ekmekcioglu S, Ellerhorst JA, Prieto VG, Johnson MM, Broemeling LD,
7. Hatzoglou M, Fernandez J, Yaman I, Closs E, Regulation of cationic amino Grimm EA. Tumor iNOS predicts poor survival for stage III melanoma
acid transport: the story of the CAT-1 transporter. Annu Rev Nutr (2004) patients. Int J Cancer (2006) 119:861–6. doi:10.1002/ijc.21767
24:377–99. doi:10.1146/annurev.nutr.23.011702.073120 19. Johansson CC, Egyhazi S, Masucci G, Harlin H, Mougiakakos D, Poschke I,
8. Simmons WW, Closs EI, Cunningham JM, Smith TW, Kelly RA. Cytokines and et al. Prognostic significance of tumor iNOS and COX-2 in stage III malignant
insulin induce cationic amino acid transporter (CAT) expression in cardiac cutaneous melanoma. Cancer Immunol Immunother (2009) 58:1085–94.
myocytes. Regulation of l-arginine transport and no production by CAT-1, doi:10.1007/s00262-008-0631-1
CAT-2A, and CAT-2B. J Biol Chem (1996) 271:11694–702. doi:10.1074/ 20. Grimm EA, Sikora AG, Ekmekcioglu S. Molecular pathways: inflammation-
jbc.271.20.11694 associated nitric-oxide production as a cancer-supporting redox mechanism
9. Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. and a potential therapeutic target. Clin Cancer Res (2013) 19:5557–63.
Eur Heart J (2012) 33:829–37. doi:10.1093/eurheartj/ehr304 doi:10.1158/1078-0432.CCR-12-1554
10. Wang Y, Hu S, Gabisi AM Jr, Er JA, Pope A, Burstein G, et al. Developing an 21. Heinecke JL, Ridnour LA, Cheng RY, Switzer CH, Lizardo MM, Khanna C, et al.
irreversible inhibitor of human DDAH-1, an enzyme upregulated in mela- Tumor microenvironment-based feed-forward regulation of NOS2 in breast
noma. ChemMedChem (2014) 9:792–7. doi:10.1002/cmdc.201300557 cancer progression. Proc Natl Acad Sci U S A (2014) 111:6323–8. doi:10.1073/
11. Yoon JK, Frankel AE, Feun LG, Ekmekcioglu S, Kim KB. Arginine deprivation pnas.1401799111
therapy for malignant melanoma. Clin Pharmacol (2013) 5:11–9. doi:10.2147/ 22. Tanese K, Grimm EA, Ekmekcioglu S. The role of melanoma tumor-derived
CPAA.S37350 nitric oxide in the tumor inflammatory microenvironment: its impact on the
Frontiers in Oncology | www.frontiersin.org 4 March 2018 | Volume 8 | Article 67
Kim et al. Arginine Metabolism on Immune Response
chemokine expression profile, including suppression of CXCL10. Int J Cancer 30. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of
(2012) 131:891–901. doi:10.1002/ijc.26451 the immune system. Nat Rev Immunol (2009) 9:162–74. doi:10.1038/nri2506
23. Ekmekcioglu S, Grimm EA, Roszik J. Targeting iNOS to increase efficacy of 31. Tang CH, Grimm EA. Depletion of endogenous nitric oxide enhances
immunotherapies. Hum Vaccin Immunother (2017) 13:1105–8. doi:10.1080/ cisplatin-induced apoptosis in a p53-dependent manner in melanoma cell
21645515.2016.1276682 lines. J Biol Chem (2004) 279:288–98. doi:10.1074/jbc.M310821200
24. Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. 32. Visigalli R, Bussolati O, Sala R, Barilli A, Rotoli BM, Parolari A, et al. The
Genomic and transcriptomic features of response to anti-PD-1 therapy in stimulation of arginine transport by TNFalpha in human endothelial cells
metastatic melanoma. Cell (2016) 165:35–44. doi:10.1016/j.cell.2016.02.065 depends on NF-kappaB activation. Biochim Biophys Acta (2004) 1664:45–52.
25. Tham M, Tan KW, Keeble J, Wang X, Hubert S, Barron L, et al. Melanoma- doi:10.1016/j.bbamem.2004.04.001
initiating cells exploit M2 macrophage TGFbeta and arginase pathway for 33. Prima V, Kaliberova LN, Kaliberov S, Curiel DT, Kusmartsev S. COX2/
survival and proliferation. Oncotarget (2014) 5:12027–42. doi:10.18632/ mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated
oncotarget.2482 macrophages and myeloid-derived suppressor cells. Proc Natl Acad Sci U S A
26. Lind DS. Arginine and cancer. J Nutr (2004) 134:2837S–41S. doi:10.1093/ (2017) 114:1117–22. doi:10.1073/pnas.1612920114
jn/134.10.2837S
27. Feun L, You M, Wu CJ, Kuo MT, Wangpaichitr M, Spector S, et al. Arginine Conflict of Interest Statement: The authors declare that the research was con-
deprivation as a targeted therapy for cancer. Curr Pharm Des (2008) ducted in the absence of any commercial or financial relationships that could be
14:1049–57. doi:10.2174/138161208784246199 construed as a potential conflict of interest.
28. Rodriguez PC, Quiceno DG, Ochoa AC. l-arginine availability regulates
T-lymphocyte cell-cycle progression. Blood (2007) 109:1568–73. doi:10.1182/ Copyright © 2018 Kim, Roszik, Grimm and Ekmekcioglu. This is an open-access
blood-2006-06-031856 article distributed under the terms of the Creative Commons Attribution License (CC
29. Lamas B, Vergnaud-Gauduchon J, Goncalves-Mendes N, Perche O, Rossary A, BY). The use, distribution or reproduction in other forums is permitted, provided
Vasson MP, et al. Altered functions of natural killer cells in response to the original author(s) and the copyright owner are credited and that the original
l-arginine availability. Cell Immunol (2012) 280:182–90. doi:10.1016/j.cellimm. publication in this journal is cited, in accordance with accepted academic practice. No
2012.11.018 use, distribution or reproduction is permitted which does not comply with these terms.
Frontiers in Oncology | www.frontiersin.org 5 March 2018 | Volume 8 | Article 67
You might also like
- Bronchial Thermoplasty and Guided Bronchoscopy Part OneDocument25 pagesBronchial Thermoplasty and Guided Bronchoscopy Part OneFistiNo ratings yet
- Jurnal Fungi InhaledDocument13 pagesJurnal Fungi InhaledFistiNo ratings yet
- 10.1016@S1470 20451930318 3Document10 pages10.1016@S1470 20451930318 3FistiNo ratings yet
- Pharmacy Professionals ' Experiences and Perceptions of Providing NHS Patient Medicines Helpline Services: A Qualitative StudyDocument11 pagesPharmacy Professionals ' Experiences and Perceptions of Providing NHS Patient Medicines Helpline Services: A Qualitative StudyFistiNo ratings yet
- Alsaraf 2019 IOP Conf. Ser. Mater. Sci. Eng. 571 012082Document11 pagesAlsaraf 2019 IOP Conf. Ser. Mater. Sci. Eng. 571 012082FistiNo ratings yet
- Characteristics and Outcomes of Acute Kidney Injury in Hospitalized COVID-19 Patients: A Multicenter Study by The Turkish Society of NephrologyDocument25 pagesCharacteristics and Outcomes of Acute Kidney Injury in Hospitalized COVID-19 Patients: A Multicenter Study by The Turkish Society of NephrologyFistiNo ratings yet
- Biopharmaceutics Aspect of Drug Development: Prof. HENNY LUCIDA, PH.D, Apt Andalas UniversityDocument19 pagesBiopharmaceutics Aspect of Drug Development: Prof. HENNY LUCIDA, PH.D, Apt Andalas UniversityFistiNo ratings yet
- Pediatric Parenteral Nutrition Kelly Kopec PharmDocument8 pagesPediatric Parenteral Nutrition Kelly Kopec PharmFistiNo ratings yet
- Patofisiologi: Apt. Najmiatul Fitria, M.Farm., PH.DDocument4 pagesPatofisiologi: Apt. Najmiatul Fitria, M.Farm., PH.DFistiNo ratings yet
- Nilai-Nilai Edukatif Dalam Lirik Nyanyian Onang-Onang Pada Acara Pernikahan Suku Batak Angkola Kabupaten Tapanuli Selatan Provinsi Sumatera UtaraDocument15 pagesNilai-Nilai Edukatif Dalam Lirik Nyanyian Onang-Onang Pada Acara Pernikahan Suku Batak Angkola Kabupaten Tapanuli Selatan Provinsi Sumatera UtaraFistiNo ratings yet
- Ergot Alkaloid BiosynthesisDocument15 pagesErgot Alkaloid BiosynthesisFistiNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- PATOLOGIDocument361 pagesPATOLOGIfe100% (1)
- Topnotch Surgery Supplement HandoutDocument85 pagesTopnotch Surgery Supplement HandoutSarah Michelle NiduaNo ratings yet
- Risk of Opportunistic Infections Associated With Long Term GlucocorticoidDocument20 pagesRisk of Opportunistic Infections Associated With Long Term GlucocorticoidRafael CamachoNo ratings yet
- Impact of Gut Microbiome On Skin Health Gut Skin Axis Observed Through The Lenses of Therapeutics and Skin DiseasesDocument30 pagesImpact of Gut Microbiome On Skin Health Gut Skin Axis Observed Through The Lenses of Therapeutics and Skin DiseasesLudmila Lopes Silva FigueredoNo ratings yet
- Unit Exam #1: Blood Vessels: B.) Advanced Glycation End ProductsDocument85 pagesUnit Exam #1: Blood Vessels: B.) Advanced Glycation End ProductsCherry RahimaNo ratings yet
- PhonophoresisDocument22 pagesPhonophoresisnithin nair88% (8)
- Igg4-Related Disease: A Reminder For Practicing PathologistsDocument8 pagesIgg4-Related Disease: A Reminder For Practicing PathologistsMariela Judith UCNo ratings yet
- ODY Efenses: Ap Biology Name Animals Form & Function Activity #4 Date HourDocument5 pagesODY Efenses: Ap Biology Name Animals Form & Function Activity #4 Date Hourancientblackdragon0% (1)
- TOPNOTCH Patho Supplement Handout For Sept 2018 UPDATED May 2018Document25 pagesTOPNOTCH Patho Supplement Handout For Sept 2018 UPDATED May 2018Waiwit KritayakiranaNo ratings yet
- (03241750 - Acta Medica Bulgarica) T Helper Cells in The Immunopathogenesis of Systemic Sclerosis - Current TrendsDocument7 pages(03241750 - Acta Medica Bulgarica) T Helper Cells in The Immunopathogenesis of Systemic Sclerosis - Current TrendsTeodorNo ratings yet
- Cellulite: Etiology, Classification, Pathology, and TreatmentDocument8 pagesCellulite: Etiology, Classification, Pathology, and TreatmentDianaNo ratings yet
- Medical Surgical Nursing - RespiratoryDocument15 pagesMedical Surgical Nursing - RespiratoryChristian Esteves75% (4)
- Gingival EnlargementsDocument34 pagesGingival EnlargementsRohan ShahNo ratings yet
- Taco TraliDocument10 pagesTaco TralimanuelNo ratings yet
- Dry EyeDocument126 pagesDry EyeAnonymous PYgnwOWK100% (4)
- Review Article Andrographis Paniculata (Burm. F.) Wall. Ex NeesDocument28 pagesReview Article Andrographis Paniculata (Burm. F.) Wall. Ex NeesKhalijahNo ratings yet
- Stenotrophomona Maltophilia y Bulkorelia Cepacia. Mnadell 2009Document8 pagesStenotrophomona Maltophilia y Bulkorelia Cepacia. Mnadell 2009Laura López Del Castillo LalydelcaNo ratings yet
- Evolution of Nonsteroidal Anti-Inflammatory Drugs (Nsaids) : Cyclooxygenase (Cox) Inhibition and BeyondDocument30 pagesEvolution of Nonsteroidal Anti-Inflammatory Drugs (Nsaids) : Cyclooxygenase (Cox) Inhibition and BeyondnesakusumaNo ratings yet
- Tsifinalpaper Comment CorrectedDocument43 pagesTsifinalpaper Comment CorrectedTsigereda AberaNo ratings yet
- Tao of Forgotten Food Diet - Taoist Herbology 2Document29 pagesTao of Forgotten Food Diet - Taoist Herbology 2ZioAngelNo ratings yet
- Schuessler's Cell SaltsDocument8 pagesSchuessler's Cell SaltsRoshan Jain MNo ratings yet
- Vitamin C-Lipid Metabolites: Uptake and Retention and Effect On Plasma C-Reactive Protein and Oxidized LDL Levels in Healthy VolunteersDocument5 pagesVitamin C-Lipid Metabolites: Uptake and Retention and Effect On Plasma C-Reactive Protein and Oxidized LDL Levels in Healthy VolunteersDhany NurNo ratings yet
- Fphar 11 596099Document17 pagesFphar 11 596099germanNo ratings yet
- Novak Et Al 2011 AllergyDocument10 pagesNovak Et Al 2011 AllergyArturo VeraNo ratings yet
- Maternal Respiratory Sars-Cov-2 Infection in Pregnancy Is Associated With A Robust Inflammatory Response at The Maternal-Fetal InterfaceDocument31 pagesMaternal Respiratory Sars-Cov-2 Infection in Pregnancy Is Associated With A Robust Inflammatory Response at The Maternal-Fetal InterfaceAllan Depieri CataneoNo ratings yet
- Hypersensitivity Word Document-1Document23 pagesHypersensitivity Word Document-1Dan 04No ratings yet
- Psychedelic Drugs in Biomedicine. Review 2017Document14 pagesPsychedelic Drugs in Biomedicine. Review 2017Luis Henrique SalesNo ratings yet
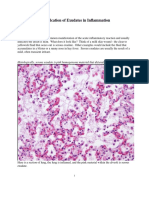
- Identification of Exudates in Inflammation PDFDocument5 pagesIdentification of Exudates in Inflammation PDFlourdes kusumadiNo ratings yet
- PEDIADRUGDocument6 pagesPEDIADRUGPatrice LimNo ratings yet
- Eva-Detko 4-Week ProgramDocument25 pagesEva-Detko 4-Week ProgramVeronika Solntseva100% (3)