Professional Documents
Culture Documents
Sơ Đ Ea
Sơ Đ Ea
Uploaded by
Mạnh Bùi0 ratings0% found this document useful (0 votes)
6 views4 pagesOriginal Title
SƠ ĐỒ EA
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views4 pagesSơ Đ Ea
Sơ Đ Ea
Uploaded by
Mạnh BùiCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 4
United States Patent 1
Willis, Jr. et al.
{54] PROCESS FOR ETHANOLAMINES
175] Inventors: Stephen B, Willis, Jr Joseph D.
Henry, both of Lake Jackson, Tex.
[73] Assignee: The Dow Chemical Company,
Midland, Mich.
[21] Appl. No: 238,894
(22) Filed: Feb. 27, 1981
(51} Int. cls .. COTE 89/02; COTC 85/04
(3) US.a. 564/471; 564/475,
364/476
364/475, 477, 476
[58] Field of Search
[6] References Cited
U.S. PATENT DOCUMENTS
2051486 8/1936 Kautter
2,586,167 2/1982. Wilson ..
3)131)132 4/1964 Moss et al.
3,639,403 2/1972 Mublbaver
3,849,262 11/1974 Cocuzza
- 564/477
304/475 X
564/475 X
564/877 X
564/875 X
ny 4,355,181
[45] Oct, 19, 1982
. S6a/47T
seasart
4,119,670 10/1978. Tsuchiya
4,169,856 10/1979 Cocuzza etal.
FOREIGN PATENT DOCUMENTS
540963. 5/1957 Canad
1543681 7/1969. Fed. Rep. of Germany
7312683 3/1974 Netherlands a
Primary Examiner—John Doll
Attorney, Agent, or Firm—A. Cooper Ancona
(57) ABSTRACT
Ina process of making ethanolamines by reacting ethyl-
ene and ammonia and wherein ammonia is used in ex-
cess of the stoichiometric amount, the improvement
comprising flashing and liquification at least part of the
excess ammonia in the effluent prior to recycling. This
‘moderates the operating conditions and simplifies the
equipment needed to recycle the balance of the ammo-
nia,
saan?
564/475
504/877
4 Claims, 1 Drawing Figure
U.S. Patent Oct. 19, 1982 4,355,181
4,355,
1
PROCESS FOR ETHANOLAMINES
BACKGROUND OF THE INVENTION
This invention relates to an improved process for
preparing ethanolamines by reacting ethylene oxide
with ammonia. From another viewpoint, the invention
relates to a process for effectively recovering the unre-
acted ammonia in the reaction of ethylene oxide (EO)
with ammonia (NHS).
Ethanolamines find a wide range of applicants as
detergents, emulsifiers, gas absorbents, corrosion inhi
tors, gloss-imparting agents, polishes, textile treating
assistants, and raw materials for herbicides, pharmaceu-
ticals, etc.
They can be produced by the reaction of ethylene
halohydrins or ethylene oxide with ammonia, but the
current commercial-scale production of ethanolamines
almost entirely relies on the reaction of ethylene oxide
with ammonia,
The reaction of ethylene oxide addition to ammonia
not only yields monoethanolamine (MEA), but also
diethanolamine (DEA) and triethanolamine (TEA).
“The ratios of the ethanolamines in the reaction product
is determined by the mol ratio of ammonia to ethylene
oxide. For example, to obtain a reaction product con-
taining # major proportion of MEA, a large excess of
ammonia should be reacted with ethylene oxide. This
procedure requires a heating source, for example a large 39
quantity of steam, to recover the excess of ammonia
after the reaction
The addition reaction between ethylene oxide and
ammonia is an exothermic reaction. In known processes
using reactors with shell and tubes, itis the common
practice to cool the outside of a reaction zone with
cooling water, etc. so as to restrict the reaction tempera-
ture to 50° to 150° C. It is also known to react ethylene
‘oxide and ammonia adiabatically, and desorb the ammo-
nia by releasing the elevated pressure. Since the inside
of the reactor attains high temperatures and pressures, a
large-scale apparatus is required, and the resulting etha-
nolamines, especially TEA, tend to be colored. In this
process, the equipment needed becomes larger, the
larger the ratio of NH3/EO. Water is employed both as
a catalyst and an absorbent in the reaction, the latter
facilitating recycle of the excess ammonia.
The present improved process, by employing a high
pressure flash distillation and liquification of part of the
NH avoids the need for ever larger absorbers or com- 50
pressors as the ratio of NH3/EO increases. This also
reduces the severity of the process conditions necessary
torrecycle the ammonia when the ratio of H20/NHS fed
to the reactor is limited (reduced) for economic reasons.
‘The direct liquefaction of NH3is also a convenient and
economical method to prepare NH for recycle in the
anhydrous reaction of NH3 end EO.
SUMMARY OF THE INVENTION
‘An improved process for making ethanolamines by
the reaction of ethylene oxide and ammonia wherein at
least a part of the excess ammonia from the reaction is
flashed and condensed, while the remaining excess am-
‘mona is absorbed in water or liquified by compression
and/or refrigeration prior to being recycled to the reac-
tor. This improvement allows the size of the ammonia
absorber and stripper to become essentially independent
of the NH3/EO ratio as well as reduces the size of com-
5
2
35
0
“6
35
©
6
181
2
ppressors or reftigeration units needed or eliminates
them entirely.
DETAILED DESCRIPTION OF THE
INVENTION
‘The process of the present invention is adaptable to
any process in which a relatively volatile reactant must
bbe used in significant excess in order to control the
product distribution from the reaction. The use of the
invention is especially indicated if the reactant is cus-
tomarily prepared for recycle by absorption into a
uid such as water since the amount of the absorbing.
liguid should be minimized for economic reasons. At-
tempts to achieve economically desirable levels of ab-
sorbing liquid often lead to excess pressures and result-
ing high temperatures in the bottom of the stripping.
‘column orto the addition of compressors and/or refrig-
eration units. Such high temperatures are undesirable
because they often result in products of inferior quality,
while the addition of more equipment has obvious dis-
advantages.
The invention is illustrated in the process of making
ethanolamines by reacting ammonia and ethylene oxide.
‘The product isa mixture of monoethanolamine together
with lesser amounts of di- and triethanolamines. An
excess of ammonia favors the formation of the monoeth-
anolamine. The excess ammonia which does not react is
customarily absorbed into water and recycled to the
process. The concentration of the ammonia/water solu-
tion isa function of the temperature of the water and the
ammonia pressure in the absorber. It is economically
desirable to limit the amount of water fed to the reactor
since this water must be later separated from the prod-
ucts of the reaction. This separation requires either
considerable energy or the addition of expensive equip-
ment such as multiple effect evaporators to minimize
this energy use. The present invention involves an im-
provement which limits the amount of energy and
equipment associated with the recycling of the excess
ammonia. A high pressure flash is employed immedi-
ately following the reactor where a portion of the am-
‘monia that is not reacted is flashed overhead leaving the
reaction product amines as the bottoms. The remaining
ammonia left dissolved in the ethanolamines product is
distilled off at a lower pressure in a first distillation
column and water is removed from the product in a
second distillation column. The ammonia from the high
pressure flash is directly condensed and sent to a
makeup or holding tank while that removed as the over-
head in the first distillation is sent to a water absorber
tunit. The water removed from the amines product is
sent to the water absorber unit. The aqueous ammonia
from the absorber unit is sent to the makeup or holding
tank and from there recycled to the reactor as 85-90%
ammonia, there being enough water present to act as
catalyst for the reaction
‘A representative process is described in connection
with the drawing.
Thus, ethylene oxide from tank 17 and ammonia from
tank 16 are introduced into a reactor 10, together with
sufficient water to catalyze the reaction, at a mol ratio
of about 5 mols NH3 per mol of EO. The effluent prod-
‘ct is sent fo flash column 11, operated at a pressure of
~300 psig. Ammonia is taken overhead and sent to a
condenser 14 and thence to makeup tank 16. The re-
maining ammonia, water and mixture of ethanolamines
is taken off the bottom of column 11 and introduced into
distillation column 12 operated at about 30 psig and at a
4,355,181
3
temperature of about 140° C. Ammonia is taken over-
hhead and sent to absorber 15 where it is absorbed in
water. The water and amines are taken off the bottom of
column 12 and sent to a second distillation column 13
operated under reduced pressure. Water is taken over-
head and sent to absorber 15. Amines are taken out of
the bottom of column 13 and sent to separation columns
(not shown) where the mono-, di- and trithanolamines
are recovered. The NH; from condenser 14 and aque-
ous NHS from absorber 15 are conveniently sent to tank
16 and thence to reactor 10.
‘The mol ratio of NH3 to EO can be from about 3/1 to
about 40/1 depending upon the desired product mix.
‘The reactor 10 is operated at a temperature of from
about 50° to about 175° C. and preferably from about
100° to about 130° C. Water employed as the catalyst is,
generally present at about 0.1 mol per mol of NH3 but
‘may be as high as 1 or as low as 0.05. The pressure in the
ammonia flash column 11 is generally from about 220 to
about 350 psig, but preferably 280 to 310 psig.
Column 12 for removing the remaining ammonia
from the flash column 11 bottoms is generally operated
at about 30 psig, but may be operated at from 15 to
about 70 psig. It is preferably operated at a pressure
such that the temperature of the amines and water in the
bottom of column 12 does not exceed about 150° C.
Generally 70-85% of the total excess ammonia is
removed in the high pressure flash. It is desirable to
remove at least 50% but probably no more than about
90-95% of the excess NH. The % of the excess ammo-
nia removed in the high pressure flash depends on the
amount of water desired to promote the reaction, In the
case in which 0.1 mol of H2O per mol of NH) is used,
approximately 90% of the NH3 would be conveniently
removed in the high pressure flash. This would allow
the absorber to run at relatively mild conditions while
the NH3/H30 solution fed to the reactor would be
about 90% NHs. Operating at about 90% NH) in the
feed under the conditions of the prior art without the
high pressure flash would require excessively high tem-
peratures during distillation, e.g. in column 12, which
are deleterious to the products, producing color and
by-products.
‘The advantages of this improved process are that less
energy is required since a portion of the ammonia is
removed in the high pressure flash without being al
sorbed in water. At the same time, since less ammonia
25
30
3s
0
45
50
55
6
4
removed at low pressures, as in the known process, a
lower temperature is employed which permits a purer
product to be obtained and reduces the corrosion
problems in the aqueous ammonia recycle system.
“Another advantage is that less capital is required for
equipment since smaller volumes of water must be re
led. The plant would be easier to operate as the liq
ammonia recycle system would absorb swings in
NHG/EO mol ratio, leaving the aqueous ammonia recy-
cle section of the plant with a constant feed of water and
‘We claim:
1. Ina process for making alkanolamines by the reac-
tion of an alkylene oxide and ammonia in the liqu
phase, said ammonia being employed in stoichiometric
excess and in which said excess is recovered from the
reaction product and recycled to said reaction, the im-
provement which comprises (1) reacting said ammoni
and said alkylene oxide in the presence of from about
0.05 mol to about 1.0 mol of water per mol of ammonia,
@) removing the reaction product from the reactor and
flashing a portion of the ammonia in a distillation col-
umn under a pressure of from about 220 to about 350,
psig, (3) condensing said portion of ammonia, (4) send
ing the remaining reaction product and remaining am-
‘monia to a low pressure distillation column to remove
said remaining ammonia overhead, (5) sending the bot-
toms of step 4 comprising water and alkanolamines to a
reduced pressure distillation to remove said water, (6)
sending said water to an ammonia absorber unit, (7)
sending said ammonia removed overhead in step 4 to
said absorber unit, (8) recycling said condensed ammo-
nia from step 3, said absorbed ammonia from step 6 and
said water removed in step 5 to said reaction,
2. The process of claim 1 wherein the pressure in step
(@) is maintained at a pressure such that the bottoms
temperature of said distillation does not exceed about
150° C.
3. The process of claim 1 wherein at least 30% of the
excess ammonia is removed in step (2).
4. The process of claim 1 wherein the ratio of NH3 to
EO is from about 3/1 to about 40/1, the pressure em-
ployed in steps 2 and 3 is about 280-310 psig and the
temperature in step 2 is maintained so as to give a mol
ratio of NHs to HzO of from about 1.2/1 to 0.12/1 in the
feed to step 4.
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5814)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1092)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (844)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (348)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Pmce MergedDocument506 pagesPmce MergedDeep SinojiyaNo ratings yet
- Water Technology Lecture 11 Sludge TreatmentDocument46 pagesWater Technology Lecture 11 Sludge TreatmentDeep SinojiyaNo ratings yet
- Module 5 Condensation and BoilingDocument80 pagesModule 5 Condensation and BoilingDeep SinojiyaNo ratings yet
- SYBTech PO221 PC 01b Polymer Classification PPT 2020-07-28Document29 pagesSYBTech PO221 PC 01b Polymer Classification PPT 2020-07-28Deep SinojiyaNo ratings yet
- An Analysis of Fuel Cell Technology For Sustainable Transport in AsiaDocument13 pagesAn Analysis of Fuel Cell Technology For Sustainable Transport in AsiaDeep SinojiyaNo ratings yet
- US4355181Document4 pagesUS4355181Deep SinojiyaNo ratings yet
- Polymer Waste Management: Prof. Amar ArakhDocument42 pagesPolymer Waste Management: Prof. Amar ArakhDeep SinojiyaNo ratings yet
- The Role of Biosurfactants in The Continued Drive For Environmental SustainabilityDocument12 pagesThe Role of Biosurfactants in The Continued Drive For Environmental SustainabilityDeep SinojiyaNo ratings yet
- Movie ReviewDocument3 pagesMovie ReviewDeep SinojiyaNo ratings yet
- T-X-Y Diagram: Antoine's Equation Antoine's Constants Heptane Octane Log P A - B / C + T (C)Document2 pagesT-X-Y Diagram: Antoine's Equation Antoine's Constants Heptane Octane Log P A - B / C + T (C)Deep SinojiyaNo ratings yet
- Chart Title: Antoine Equation: Log P (Bar) A - B / (C + T (C) )Document2 pagesChart Title: Antoine Equation: Log P (Bar) A - B / (C + T (C) )Deep SinojiyaNo ratings yet
- Biodiesel Turmeric Leaves Are The Waste Product TurmericDocument2 pagesBiodiesel Turmeric Leaves Are The Waste Product TurmericDeep Sinojiya0% (1)
- Unit 4 Bitumen - Blowing PDFDocument8 pagesUnit 4 Bitumen - Blowing PDFDeep SinojiyaNo ratings yet
- Simulation Report: Object: MibkDocument2 pagesSimulation Report: Object: MibkDeep SinojiyaNo ratings yet
- $r117hue PDFDocument51 pages$r117hue PDFDeep SinojiyaNo ratings yet
- Unit 1 Refinery Products PDFDocument23 pagesUnit 1 Refinery Products PDFDeep SinojiyaNo ratings yet
- Ist and 2nd Order ResponseDocument22 pagesIst and 2nd Order ResponseDeep SinojiyaNo ratings yet
- Conversion Reactor PDFDocument3 pagesConversion Reactor PDFDeep SinojiyaNo ratings yet
- H.E Report PDFDocument3 pagesH.E Report PDFDeep SinojiyaNo ratings yet
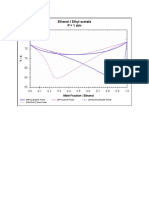
- Ethanol / Ethyl Acetate P 1 Atm: (NRTL) Bubble Points (NRTL) Dew Points (UNIQUAC) Bubble Points (UNIQUAC) Dew PointsDocument1 pageEthanol / Ethyl Acetate P 1 Atm: (NRTL) Bubble Points (NRTL) Dew Points (UNIQUAC) Bubble Points (UNIQUAC) Dew PointsDeep SinojiyaNo ratings yet
- Simulation of Flash Column: S.P.KodolikarDocument8 pagesSimulation of Flash Column: S.P.KodolikarDeep SinojiyaNo ratings yet