Professional Documents
Culture Documents
The Birth and Death of Proteins: Some Key Concepts
Uploaded by
Luke Shanti0 ratings0% found this document useful (0 votes)
9 views2 pagesOriginal Title
translation
Copyright
© © All Rights Reserved
Available Formats
DOC, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
9 views2 pagesThe Birth and Death of Proteins: Some Key Concepts
Uploaded by
Luke ShantiCopyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
You are on page 1of 2
The birth and death of proteins
Some key concepts
Proteins (polypeptides) are synthesised by translating the sequence encoded in the mRNA in the form of
a non-overlapping, degenerate triplet code.
This triplet code dictates translation of specific RNA triplet codons to amino acids and occurs in the
cytosol.
The AUG start codon for methionine is most common, specifying the NH2-terminus of most proteins
An open reading frame (ORF) is the uninterrupted triplet sequence in mRNA from a specific start codon
to the stop codon, which is translated into a linear sequence of amino acids in a polypeptide chain.
Mutations in the base sequence can alter ORF and subsequent proteins
Types of mutations
Deletions or Insertions: 1bp to several Mbp
Single base substitutions
Missense mutations: replace one amino acid codon with another
Nonsense mutations: replace amino acid codon with stop codon
Splice site mutations: create or remove exon-intron boundaries
Frameshift mutations: alter the ORF due to base substitutions
Dynamic mutations: changes in the length of tandem repeat elements
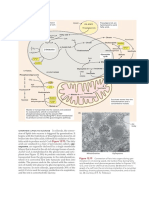
Translation requires 3 main components to come together
1) Messenger RNA:This class of RNAs are the genetic coding templates used by the translational machinery
to determine the order of amino acids incorporated into an elongating polypeptide in the process of
translation.
2) Transfer RNA: A class of small RNAs that form covalent bonds to amino acids and allows correct
insertion of amino acids into the elongating polypeptide chain.
3) Ribosomes: Ribosomal RNA (rRNA) assembled, together with numerous ribosomal proteins, to form the
ribosomes. Ribosomes engage the mRNAs and form a catalytic domain into which the tRNAs enter with
their attached amino acids. The proteins of the ribosomes catalyze all of the functions of polypeptide
synthesis
Translation has 2 important recognition steps
1) Correct aminoacylation (‘charging’): tRNA has to be charged (aminoacylated with the correct amino
acid) to allow the amino acid to participate in peptide bond formation. This is brought about by the
enzyme, aminoacyl-tRNA synthetases (aaRSs) which covalently attach the correct amino acid to
tRNA (specified by anticodon) in an ATP dependent process. The specificity of aaRSs to select the
right tRNA to be acylated (especially since most tRNAs are structurally similar) is done by
recognising specific tRNA identifiers present on the acceptor step & anticodon loop. e.g. AlaRSs
recognise G3.U70 bp.
2) Select the correct charged tRNA as specified by mRNA: Less than 61tRNAs are found in cells (based on
conventional Watson-Crick base paring), which suggests that a single tRNA anticodon would
recognise more than one mRNA codon. This is the ‘Wobble hypothesis’ which explains this non-
standard pairing of the 3 base position of the mRNA (codon) with the 1 st position on the tRNA
(anticodon).
Polypeptide synthesis (translation) can be divided into 3 main steps
1) Chain Initiation
Initiation usually begins at the first AUG from the 5’ end of the mRNA. Recognition of the codon is
mediated by a series of eukaryotic initiation factors (eIFs). The ribosome assembles along with the
mRNA and charged initiator tRNA (tRNAimet). The small (40S) and large subunit (60S) are usually
kept apart by eIF3 and eIF6 when not engaged in translation. Initiation is a 4 step process as follows
Step 1: Formation of pre-initiation complex. The 40S-eIF3 is bound by eIF1A to a ternary complex of
tRNAimet, eIF2 and GTP
Step 2: Formation of initiation complex (cap binding of mRNA to 40S)
Step 3: Positioning at start codon. The initiation complex slides along mRNA using the helicase activity
of eIF4. eIF4 uses ATP hydrolysis to unwinds the mRNA secondary structure. Initiation complex
stops at the start site AUG. This recognition allows an irreversible GTP hydrolysis of eIF2
preventing any further unwinding
Step 4: Association of large subunit (60S). Irreversible GTP hydrolysis mediates the association of 60S-
eIF6 (large subunit ) to the small subunit by the action of eIF5. This becomes the P site
2) Chain Elongation
This step is mediated by eukaryotic elongation factors (eEFs). The main steps in this process involve
entry of the aatRNA, conformational change in the ribosome structure, formation of the peptide bind
and the translocation of the ribosome to the next codon on the mRNA. These can be summarised as
follows
Step 1. aatRNA binding
aatRNA binds to A site on ribosome by base pairing with codon
Step 2. Conformation change in ribosome: induced by GTP hydrolysis of EF1a
Step 3: Transpeptidation
C terminal of polypeptide uncoupled from P site tRNA and peptide bond transferred to amino acid
on A site tRNA
catalysed by peptidyltransferase
Step 4: Translocation
GTP hydrolysis of EF2 causes 2nd conformational change P site tRNA is transferred to E site
Simultaneous transfer of A site tRNA moved to P site
3) Chain termination
Release factors recognise and bind to stop codons. This induces peptidyl transferase to transfer peptidyl
group to water instead of aatRNA and the nascent polypeptide is released. Uncharged tRNA is released
from the 80S ribosome and the inactive ribosome then releases the mRNA.
Post translational folding and / or modification
The amino acid sequence of a protein determines its folding into a specific 3-D conformation. This folding
is mediated by molecular chaperones (e.g. Hsp70) or chaperonins (Hsp60 complexes). Nearly every
protein is modified after synthesis on the ribosome. These modifications are essential and dictate the
activity, life span or the cellular location of proteins. During modification, various chemical groups (e.g
acetyl, phosphoryl, hydroxyl, glycosyl etc) are added to the N or C terminal or within internal residues
of the polypeptide.
Death of proteins: Proteins that are misfolded, denatured, in excess or extracellular in origin are targeted
for degradation within lysosomes. Another pathway is by the addition of ubiquitin to lysine residues,
which is recognised are destroyed by the proteosome complex. Degradation of proteins can be a part of
normal cell processes (cell cycle) or may be implicated in disease, especially neurodegenerative diseases
(Parkinsons. Alzheimers etc).
You might also like
- The Translation: Translation Is A Process by Which The Genetic Code Contained Within An mRNADocument5 pagesThe Translation: Translation Is A Process by Which The Genetic Code Contained Within An mRNAShahriar ShamimNo ratings yet
- MB Chapter 6 TranslationDocument31 pagesMB Chapter 6 TranslationMustee TeferaNo ratings yet
- Transcription: Protein Biosynthesis Is The Process by Which BiologicalDocument4 pagesTranscription: Protein Biosynthesis Is The Process by Which BiologicalAnand RajNo ratings yet
- Agus Limanto Faculty of Medicine UkridaDocument26 pagesAgus Limanto Faculty of Medicine UkridaMrs GeekNo ratings yet
- TranslationDocument90 pagesTranslationspitzmark2030No ratings yet
- Regulasi Ekspresi GenDocument41 pagesRegulasi Ekspresi GenJohn'sSujonoNo ratings yet
- Lec. 6Document10 pagesLec. 6Dr. Mohamed ShamsNo ratings yet
- Translation: From Messenger RNA To ProteinDocument71 pagesTranslation: From Messenger RNA To Proteinbombertest1No ratings yet
- Protein BiosynthesisDocument21 pagesProtein BiosynthesisMyrrh Tagurigan TrainNo ratings yet
- 13 Miller Chap 4b LectureDocument21 pages13 Miller Chap 4b LectureGerone Tolentino AtienzaNo ratings yet
- TRANSLATIONDocument19 pagesTRANSLATIONUsha SuthersonNo ratings yet
- LECTURE 12 TranslationDocument17 pagesLECTURE 12 TranslationAditi SharmaNo ratings yet
- DNA Translation - Dr. Mohammed OsmanDocument43 pagesDNA Translation - Dr. Mohammed Osmanمعتز محمدNo ratings yet
- Prokaryotic Translation MechanismDocument22 pagesProkaryotic Translation MechanismManoj JoshiNo ratings yet
- Gene ExpressionDocument58 pagesGene ExpressionJunirose PanesNo ratings yet
- TDNº3 Part2Document3 pagesTDNº3 Part2selmi bouzidNo ratings yet
- Translation of mRNA (Protein Synthesis)Document80 pagesTranslation of mRNA (Protein Synthesis)Cok AnggaNo ratings yet
- Protein Synthesis-1Document2 pagesProtein Synthesis-1Stanley OdiraNo ratings yet
- DNA Central Dogma ExplainedDocument51 pagesDNA Central Dogma ExplainedIlsya PertiwiNo ratings yet
- Protein BiosynthesisDocument7 pagesProtein BiosynthesisOlusola OtasanyaNo ratings yet
- Transcription &translation: Mol. Biology Lec-4 TranscriptionDocument12 pagesTranscription &translation: Mol. Biology Lec-4 TranscriptionAhmed Ali AssafNo ratings yet
- Translation and Regulation of Gene ExpressionDocument51 pagesTranslation and Regulation of Gene ExpressionP. Jacksen Sam PaulNo ratings yet
- Chapter 6 GeneticsDocument12 pagesChapter 6 GeneticsAmer ToutonjiNo ratings yet
- Translation Project OverviewDocument12 pagesTranslation Project OverviewNEET STUDIESNo ratings yet
- Translation AnoverviewDocument32 pagesTranslation AnoverviewshitalchandrasitNo ratings yet
- Microm 410 Midterm 2 GlossaryDocument8 pagesMicrom 410 Midterm 2 GlossaryNatalie ChenNo ratings yet
- EXERCISE 7. Molecular Basis of Heredity Gene Action Transcription and TranslationDocument5 pagesEXERCISE 7. Molecular Basis of Heredity Gene Action Transcription and TranslationMohamidin MamalapatNo ratings yet
- ActiveDocs - Biochemistry Review Block IIDocument145 pagesActiveDocs - Biochemistry Review Block IIjavierNo ratings yet
- Cell TranslationDocument15 pagesCell TranslationevilheadNo ratings yet
- BBM Translasi BIOMEDIKDocument79 pagesBBM Translasi BIOMEDIKJessica Buntara SulaimanNo ratings yet
- 2022-05-15 L8 - Protein SynthesisDocument64 pages2022-05-15 L8 - Protein SynthesisTamara ElyasNo ratings yet
- Genetic Codon: 3 NucleotidesDocument25 pagesGenetic Codon: 3 Nucleotidesmus zaharaNo ratings yet
- AMPDocument3 pagesAMPRaj KumarNo ratings yet
- Protein SynthesisDocument3 pagesProtein SynthesisabdullaNo ratings yet
- Protein Synthesis (Translation) - Mechanism and Control in Pro - and Eukaryotes.-3Document9 pagesProtein Synthesis (Translation) - Mechanism and Control in Pro - and Eukaryotes.-3carlottabovi28No ratings yet
- DNA-Protein Synthesis: Transcription, Translation & RegulationDocument33 pagesDNA-Protein Synthesis: Transcription, Translation & RegulationSharlene OngNo ratings yet
- Protein SynthesisDocument135 pagesProtein SynthesisCarlaNo ratings yet
- Transcriptiontranslation 170210171328Document34 pagesTranscriptiontranslation 170210171328Farah B. BtoushNo ratings yet
- Otot 2Document106 pagesOtot 2anita parwatiNo ratings yet
- Bio Short Notes FinalDocument5 pagesBio Short Notes FinalMUHAMMAD HAFIZ FAZRISHAH BIN JEFFRY KIMNo ratings yet
- TranslationDocument3 pagesTranslationmeryjuvelhNo ratings yet
- Advanced Chemistryprize2009 PDFDocument24 pagesAdvanced Chemistryprize2009 PDFJeremy GordonNo ratings yet
- TranslasiDocument73 pagesTranslasitamara hannestoNo ratings yet
- Protein Synthesis: Transcription & TranslationDocument4 pagesProtein Synthesis: Transcription & TranslationtshahriyarNo ratings yet
- Translation 30th March 2009Document16 pagesTranslation 30th March 2009api-26794308No ratings yet
- L5-6 Translation in ProkaryotesDocument17 pagesL5-6 Translation in ProkaryotesArchana BhartiNo ratings yet
- Lects 2016 17 20translationDocument71 pagesLects 2016 17 20translationpresentmed100% (3)
- Translation: Protein Synthesis in 3 PhasesDocument28 pagesTranslation: Protein Synthesis in 3 PhasesPaolo NaguitNo ratings yet
- Translation - IDocument35 pagesTranslation - ISahil RanaNo ratings yet
- 24 LN Protein Synthesis CNRAADocument48 pages24 LN Protein Synthesis CNRAADakshitha DharmakeerthiNo ratings yet
- Lecture 10 Protein Synthesis, Post-Translational Modificationsand, Regulation of Protein Synthesis MD2 Part II 2023 by DR Mohamed AbdelbakyDocument49 pagesLecture 10 Protein Synthesis, Post-Translational Modificationsand, Regulation of Protein Synthesis MD2 Part II 2023 by DR Mohamed Abdelbakysoushinelall2007No ratings yet
- The Genetic Code: Translation ProcessDocument93 pagesThe Genetic Code: Translation ProcessErics EfranyNo ratings yet
- Basic Molecular Biology: Translation Process and Genetic Code DecodingDocument51 pagesBasic Molecular Biology: Translation Process and Genetic Code DecodingAleena MustafaNo ratings yet
- Protein SynthesisDocument14 pagesProtein SynthesisOginda MokoroNo ratings yet
- MBII - L23 - Translation 2Document9 pagesMBII - L23 - Translation 2Miles NsgNo ratings yet
- TUTORIAL: DNA BIOLOGY and TECHNOLOGY 1. Describe The Biochemical CompositionDocument6 pagesTUTORIAL: DNA BIOLOGY and TECHNOLOGY 1. Describe The Biochemical Compositionaesha89No ratings yet
- 05b. Part 2 of Nucleic Acids For BSRadTechDocument26 pages05b. Part 2 of Nucleic Acids For BSRadTechBea Abigail BrocalNo ratings yet
- From Dna To ProteinDocument6 pagesFrom Dna To ProteinMadona BadoevNo ratings yet
- S13 Review Sheet Exam 2 PDFDocument1 pageS13 Review Sheet Exam 2 PDFbobbyNo ratings yet
- Nursing Education EssentialsDocument11 pagesNursing Education EssentialsLuke ShantiNo ratings yet
- Photosynthesis QuizDocument1 pagePhotosynthesis QuizLuke ShantiNo ratings yet
- Photosynthesis QuizDocument1 pagePhotosynthesis QuizLuke ShantiNo ratings yet
- Chm333 Spring 2003 Professor Christine HrycynaDocument1 pageChm333 Spring 2003 Professor Christine HrycynaLuke ShantiNo ratings yet
- OX Phos Handout ThinkwellDocument1 pageOX Phos Handout ThinkwellSiddarth PalletiNo ratings yet
- Amino Acids NotesDocument13 pagesAmino Acids NotesCassy WalkerNo ratings yet
- Nucleotides and Nucleic AcidsDocument54 pagesNucleotides and Nucleic AcidsLuke ShantiNo ratings yet
- TCA Handout 2Document1 pageTCA Handout 2Luke ShantiNo ratings yet
- Proteins Protocol LowryDocument5 pagesProteins Protocol LowryAnonymous 5NXc6NuNo ratings yet
- Asfldjsohspring 2013 Lecture 1hkjsgdiuaDocument9 pagesAsfldjsohspring 2013 Lecture 1hkjsgdiuavicbart11No ratings yet
- Glycolysis and TCA ThinkwellDocument1 pageGlycolysis and TCA ThinkwellRijvi MauryaNo ratings yet
- Amplified Fragment Length Polymorphism Fingerprinting of 16 Banana Cultivars (Musa CVS.)Document7 pagesAmplified Fragment Length Polymorphism Fingerprinting of 16 Banana Cultivars (Musa CVS.)Luke ShantiNo ratings yet
- Amino Acid 2Document7 pagesAmino Acid 2Luke ShantiNo ratings yet
- Musa ArunachalDocument8 pagesMusa ArunachalLuke ShantiNo ratings yet
- Banana DiversityDocument2 pagesBanana DiversityLuke ShantiNo ratings yet
- Gender Sensitive Issues and Women EmpowermentDocument9 pagesGender Sensitive Issues and Women EmpowermentLuke ShantiNo ratings yet
- PDFDocument4 pagesPDFAngelica ErguizaNo ratings yet
- N Acetyl-Coa: Overview: Lipids To SucroseDocument1 pageN Acetyl-Coa: Overview: Lipids To SucroseLuke ShantiNo ratings yet
- Nursing Education EssentialsDocument11 pagesNursing Education EssentialsLuke ShantiNo ratings yet
- Wild Edible Plants and Their UtilizationDocument9 pagesWild Edible Plants and Their UtilizationLuke ShantiNo ratings yet
- Nursing Education EssentialsDocument11 pagesNursing Education EssentialsLuke ShantiNo ratings yet
- Improving the Visibility of Nurses in HealthcareDocument17 pagesImproving the Visibility of Nurses in HealthcareLuke ShantiNo ratings yet
- Improving the Visibility of Nurses in HealthcareDocument17 pagesImproving the Visibility of Nurses in HealthcareLuke ShantiNo ratings yet
- Abiotic Stress Management QuizDocument2 pagesAbiotic Stress Management QuizLuke ShantiNo ratings yet
- Code of Ethics For Nurses in India: 1.the Nurse Respects The Uniqueness of Individual in Provision of Care - NurseDocument5 pagesCode of Ethics For Nurses in India: 1.the Nurse Respects The Uniqueness of Individual in Provision of Care - NurseShivani MalviyaNo ratings yet
- Improving the Visibility of Nurses in HealthcareDocument17 pagesImproving the Visibility of Nurses in HealthcareLuke ShantiNo ratings yet
- Crop Growth Regulators BA 4370Document9 pagesCrop Growth Regulators BA 4370Chongtham Allaylay DeviNo ratings yet
- Abiotic Stress Management in Vegetable Crops VSC606Document1 pageAbiotic Stress Management in Vegetable Crops VSC606Luke ShantiNo ratings yet
- Lesson 5 Protein SynthesisDocument8 pagesLesson 5 Protein SynthesisMarc Laurence LadoresNo ratings yet
- PCR, RT-PCR, Nested-Pcr, Multiplex PCR, Quantitative PCRDocument116 pagesPCR, RT-PCR, Nested-Pcr, Multiplex PCR, Quantitative PCRYunizardiNo ratings yet
- Super Short Notes of Anatomy and Physiology by Pooja????????????Document174 pagesSuper Short Notes of Anatomy and Physiology by Pooja????????????shankarmeti2004No ratings yet
- Bio in For MaticsDocument232 pagesBio in For MaticskrishsreekarNo ratings yet
- (Bruce Alberts, Dennis Bray, Julian Lewis) Biologà PDFDocument1,437 pages(Bruce Alberts, Dennis Bray, Julian Lewis) Biologà PDFdanitza0% (1)
- Introduction to Viruses: Learn About Their Structure, Genes and MoreDocument80 pagesIntroduction to Viruses: Learn About Their Structure, Genes and MoreSaad-ullah SultanNo ratings yet
- BiologyDocument244 pagesBiologyİsmail AtamNo ratings yet
- What Are Introns and Exons - PDFDocument14 pagesWhat Are Introns and Exons - PDFSterlingNo ratings yet
- Class XII: Biology Chapter 6: Molecular Basis of InheritanceDocument9 pagesClass XII: Biology Chapter 6: Molecular Basis of Inheritancevishlesh parmarNo ratings yet
- Review PathologyDocument1,175 pagesReview Pathologykandeepan100% (6)
- 1 Cell As A Unit of Health and DiseaseDocument29 pages1 Cell As A Unit of Health and DiseaseRholter Dave LeeNo ratings yet
- LIFE SCIENCES WINTER CLASSES LEARNERS BOOKLET (Final)Document43 pagesLIFE SCIENCES WINTER CLASSES LEARNERS BOOKLET (Final)Magadani FhatuNo ratings yet
- 4th QUARTER-Module-5-BIOMOLECULESDocument13 pages4th QUARTER-Module-5-BIOMOLECULESStray DogsNo ratings yet
- DNA Translation Written ReportDocument5 pagesDNA Translation Written ReportHerlene SeeNo ratings yet
- Crop LifeDocument7 pagesCrop Life유가연[학생](생명과학대학 유전생명공학과)No ratings yet
- From Gene To Protein - Transcription and TranslationDocument11 pagesFrom Gene To Protein - Transcription and TranslationELOISA N. CASANENo ratings yet
- Eukaryotic TranslationDocument24 pagesEukaryotic TranslationCj ScarletNo ratings yet
- Biochem Lab FinalsDocument35 pagesBiochem Lab FinalsCHARLES RONALD GENATONo ratings yet
- Nucleic Acid MetabolismDocument41 pagesNucleic Acid MetabolismPavani AkkalaNo ratings yet
- Biology Chapter 5Document3 pagesBiology Chapter 5JUDYNo ratings yet
- Virus Replication StrategyDocument6 pagesVirus Replication StrategyShreyash Raj100% (1)
- Regulation of Gene Expression-1Document20 pagesRegulation of Gene Expression-1Iram MalikNo ratings yet
- 3:5 BioDocument4 pages3:5 BioMaya FinkleNo ratings yet
- Polyvinylsulfonic Acid A Low Cost RNase Inhibitor For Enhanced RNA Preservation and Cell Free Protein TranslationDocument9 pagesPolyvinylsulfonic Acid A Low Cost RNase Inhibitor For Enhanced RNA Preservation and Cell Free Protein Translationalessandro8265No ratings yet
- From Gene To Protein: For Campbell Biology, Ninth EditionDocument75 pagesFrom Gene To Protein: For Campbell Biology, Ninth EditionFENo ratings yet
- Biotechnology in Healthcare - An Introduction To Biopharmaceuticals (PDFDrive)Document249 pagesBiotechnology in Healthcare - An Introduction To Biopharmaceuticals (PDFDrive)AddicoNo ratings yet
- 1603LEADERACHIEVERMLABCDEMAZA MAJOR TEST 65760 TEST PDF Fv16Si8njhDocument64 pages1603LEADERACHIEVERMLABCDEMAZA MAJOR TEST 65760 TEST PDF Fv16Si8njhGjgcdNo ratings yet
- Đề Đề Xuất DH NTT YB Lớp 10Document14 pagesĐề Đề Xuất DH NTT YB Lớp 10huytranthaivinhNo ratings yet
- Gene CloningDocument54 pagesGene Cloningshivasharan100% (1)
- Lets SeeDocument986 pagesLets SeeShehnaaz KhanNo ratings yet