Professional Documents
Culture Documents
CELL-DYN Ruby SYSTEM. Field Service Training Workbook
Uploaded by
АлексПрынзинCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CELL-DYN Ruby SYSTEM. Field Service Training Workbook
Uploaded by
АлексПрынзинCopyright:
Available Formats
CELL-DYN Ruby® SYSTEM
Field Service Training Workbook
CELL-DYN Ruby® Field Service Training Workbook 1
204343-101 Dec 2008
The CELL-DYN Ruby Hematology Systems are manufactured and/or distributed by Abbott
Hematology, 5440 Patrick Henry Drive, Santa Clara, CA. 95054, U.S.A. Please direct all
inquiries concerning information in this training guide to the foregoing address.
REVISION STATUS
Part Number Revision Date Pages Revised and Added
204343-101 Dec. 2008 New
All samples (printouts, graphics, displays or screens, etc.) are for information and illustration
purposes only and shall not be used for clinical or maintenance evaluations.
Any product information in this document should be used in conjunction with the latest version of
the Operator’s and Service Manuals. If any discrepancies in information exist within this
document or any other, the latest version of the Operator’s and/or Service Manual takes
precedence.
All Abbott Laboratories product names and trademarks are owned by or licensed to Abbott
Laboratories, its subsidiaries or affiliates. No use of any Abbott trademark, trade name, trade
dress, or product name may be made without the prior written authorization of Abbott
Laboratories, except to identify the product or services of Abbott Laboratories. All other
trademarks brands, product names, and trade names are the property of their respective
companies. All rights reserved.
Except as permitted above, no license or right, expressed or implied, is granted to any person
under any patent, trademark, or other proprietary right of Abbott Laboratories.
Each person assumes full responsibility and all risks arising from use of the information. The
information is presented “AS IS” and may include technical inaccuracies or typographical errors.
Abbott Laboratories reserves the right to make additions, deletions, or modifications to the
information at any time without any prior notification.
Trademark Trademark Statement
CELL-DYN® CELL-DYN is a registered trademark of Abbott Laboratories.
This guide was developed and produced by U.S. Commercial Operations in Irving, TX. it is
intended for training of Abbott Field Service personnel.
Copyright 2008 Abbott Laboratories
2 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
CONTENTS
Training Overview ............................................ .................................. 5
Course Objectives................................................................ ...................................................... 7
Course Agenda .................................................................... ...................................................... 8
Hazards................................................................................ ...................................................... 9
Service Tools ................................................... ............................... 1-1
Activity 1............................................................................... ................................................... 1-5
Activity 2 .............................................................................. ................................................... 1-6
Components and Software .............................. ............................... 2-1
Activity.................................................................................. ................................................... 2-3
Activity Software 1................................................................ ................................................. 2-11
Activity Software 2................................................................ ................................................. 2-19
Basic Operation ............................................... ............................... 3-1
Activity.................................................................................. ................................................... 3-9
Vacuum/Pressure and Fluidics......................... ............................... 4-1
Activity.................................................................................. ................................................... 4-6
Activity.................................................................................. ................................................... 4-9
Optics System.................................................. ............................... 5-1
Activity.................................................................................. ................................................. 5-10
Hemoglobin ..................................................... ............................... 6-1
Activity.................................................................................. ................................................... 6-6
Electronics and Power Systems....................... ............................... 7-1
Activity.................................................................................. ................................................... 7-3
Activity.................................................................................. ................................................... 7-9
Sample Loader ................................................ ............................... 8-1
Activity.................................................................................. ................................................... 8-8
CELL-DYN RUBY Troubleshooting Activities... ............................... 9-1
Miscellaneous .................................................. .............................10-1
Activity.................................................................................. ................................................. 10-3
Activity (AbbottLink) ............................................................. ................................................. 10-6
Activity (Reticulocyte)........................................................... ............................................... 10-13
CELL-DYN Ruby® Field Service Training Workbook 3
204343-101 Dec 2008
CELL-DYN 3200 System Comparison.............. .............................11-1
Basic Operations Activity...................................................... .................................................. 11-4
Power Activity...................................................................... ................................................ 11-11
Fluidics Activity.................................................................... ................................................ 11-17
Sample Loader Activity......................................................... ................................................ 11-25
Optics Activity...................................................................... ................................................ 11-29
PM & Install Activity............................................................. ................................................ 11-33
4 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Training Overview Notes
The CELL-DYN Ruby Field Service Training Program is an
intensive course of presentations and activities designed to provide
the student with the skills and knowledge required to service the
CELL-DYN Ruby System and the CELL-DYN 3200 System. This
course provides the student with the opportunity to orient to these
systems including:
• System components, hardware, and software
• Key normal and abnormal indicators
• Troubleshooting, installation and preventive mainte-
nance procedures
• Concrete actions that they can take to ensure customer
service and business objectives are achieved
The overarching purpose of the CELL-DYN Ruby Field Service
Training Program is to prepare Field Service Personnel for their
role as crucial drivers of customer service excellence.
The benefits of CELL-DYN Ruby Field Service Training Program
are twofold: for Abbott Diagnostics Division, it establishes a set of
consistent processes and procedures for improved customer
service; for Field Service, it provides an essential map through the
often competing demands of day-to-day decision-making.
The information for the Service Training Class will be presented
using the following materials:
• CELL-DYN Ruby and CELL-DYN 3200 System Service
and Support Manual
• CELL-DYN Ruby and CELL-DYN 3200 System Opera-
tor’s Manual
• Technical Service Bulletins and Instrument Service
Advisories
NOTE: All example printouts, graphics, displays, screens, etc. are
for information and illustration purposes only. Actual
printouts, graphics, displays, screens, etc. may vary
depending on software revision, hardware revision, and
instrument.
Service Personnel should keep their laptop updated to contain
current revision levels of materials through timely installation of
DVD updates and/or replication.
CELL-DYN Ruby® Field Service Training Workbook 5
204343-101 Dec 2008
Notes Graphic Conventions
Throughout the text, icons and signal words appear where the
nature of the information warrants special attention.
Note
The note signal word appears adjacent to an important point of
information that is relevant to the current subject matter. The note
is preceded by an envelope icon.
Reference Materials
The laptop icon signals a location recommending the use of
Reference Materials (i.e. Service and Support Manual, TSB, ISA,
Operations Manual, etc.) during training. Alternate media can be
substituted at the instructor’s discretion.
Diagnostic Information
The tool icon signals an important point of diagnostic information
that is relevant to the current subject matter.
Training Objectives
The magnifying glass icon appears next to a module header to
identify the training objectives for the subject matter that follows.
Module Content and/or Instruction
The document icon appears along with a header to identify
training content and/or activity instructions.
Activity
The clock icon appears along with an activity header to identify a
student activity.
6 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Course Objectives
Notes
Course Objectives
After completing the CELL-DYN Ruby Field Service Training
Workbook Program, students should able to:
• Identify the major systems, subsystems and compo-
nents
• Perform basic operation, setup, and maintenance pro-
cedures
• Perform and interpret diagnostic procedures
• Perform removal and replacement procedures
• Troubleshoot to identify root cause of failure
• Repair the instrument
• Perform installation and preventative maintenance pro-
cedures
CELL-DYN Ruby® Field Service Training Workbook 7
204343-101 Dec 2008
Course Objectives
Course Agenda
NOTE:The information below provides a suggested training agenda.
Days and topics may vary.
The class is designed to provide instrument hardware and assay troubleshooting training.
Training includes hands-on troubleshooting activities.
Day One:
COURSE INTRODUCTION and TRAINING OVERVIEW
SERVICE TOOLS
COMPONENT and SOFTWARE OVERVIEW
Day Two:
REVIEW
BASIC OPERATION
VACUUM/PRESSURE and FLUIDIC SYSTEM
OPTIC SYSTEM and DATA INTERPRETATION
Day Three:
HEMOGLOBIN SYSTEM
ELECTRONIC and POWER and ELECTRONIC SYSTEMS
SAMPLE LOADER
Day Four and Five:
TROUBLESHOOTING ACTIVITIES
PREVENTATIVE MAINTENACE
INSTALLATION
AbbottLink System
FINAL EXAM
Day Six and Seven:
CD3200 SYSTEM OVERVIEW
8 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Course Objectives
Hazards
The CELL-DYN Ruby and CELL-DYN 3200 Systems have been designed for optimal
operator safety. However, this does not reduce the importance of safety awareness
where hazards exist. Standard warning conventions, including hazard signal words and
symbols are described below:
Signal Word Definition
WARNING Denotes a physical, mechanical, or procedural condition that could
result in moderate to serious personal injury.
CAUTION Denotes a condition or activities that could result in minor injury.
Note Denotes operator or service information.
Safety icons in this manual and on the CELL-DYN System identify potentially dangerous
conditions. Service Personnel must recognize the icons and understand the type and
degree of potential hazard. If text accompanies the icon, it describes the nature of the
hazard and is labeled with WARNING or CAUTION.
Review the Hazard and Safety Information contained in the CELL-DYN RUBY Service and Support
Manual Section: General Data, CELL-DYN Ruby Operator’s Manual, Section; 8 Hazards, CELL-DYN
3200 Service and Support Manual Section: General Data, CELL-DYN 3200 Operator’s Manual, Sec-
tion; 8 Hazards for complete information.
Wear appropriate personal protective equipment such as gloves, lab coat, and protective
eyewear when working in the Lab Environment.
Dispose of all biohazardous materials in accordance with local, state, and federal
regulations governing the treatment of regulated medical waste. Dispose of sharps (e.g.
probes, needles, broken glass, and slides) that are contaminated with potentially
infections materials in an appropriately labeled, puncture-resistant, and leak proof
container.
CELL-DYN Ruby® Field Service Training Workbook 9
204343-101 Dec 2008
Course Objectives
Safety Icons and Hazard Symbols
Safety icons in this manual and on the CELL-DYN System identify potentially dangerous
conditions. Service Personnel must recognize the icons and understand the type and
degree of potential hazard. The following icons may be used with text or in lieu of text. If
text accompanies the icon, it describes the nature of the hazard and is labeled with
WARNING or CAUTION. In some situations, instrument labels refer Service Personnel
to the manual for specific information.
Safety Icon Definition and Descriptions:
Safety Icon Hazard Description
WARNING: Biohazard Identifies an activity or area where potentially infectious materials
may be present. Follow procedures as outlined in “Biological
Hazards” Section of the CELL-DYN Ruby System Operator’s
Manual; Section 8 Hazards.
CAUTION: Electrical Shock Hazard Identifies the possibility of electrical shock if procedural or
engineering controls are not observed.
CAUTION: Class 3B laser light when Warns against direct viewing of the beam or reflections from the
open. Avoid exposure to beam. beam.
Identifies an activity that may present a safety related hazard, and advises the Operator to consult caution/
warning instructions. Examples Include:
CAUTION: Lifting Hazard Identifies an activity where one may be required to lift or move a
heavy object. Obtain assistance when moving and/or use appropriate
lifting devices.
CAUTION: Moving Parts Identifies an activity or area where moving parts are present.
CAUTION: Chemical Hazard Identifies an activity or area where hazardous chemicals are present.
Refer to the Material Safety Data Sheet (MSDS) or package insert for
specific safety information.
WARNING: Splash/Spray Hazard Identifies an area where fluids may be under pressure. Safety glasses
with side shields must be worn when handling or working near
potentially infectious materials.
Some general safety icons not directly related to the CELL-DYN System but identify
potentially dangerous conditions:
Safety Icon Hazard Description
CAUTION: Hot Surface Identifies an area where a hot surface is present or may be present in
case of an instrument malfunction.
WARNING: Probe Stick Hazard Identifies an activity or area where probes may be present.
Avoid placing your hand in the range of a moving probe in order to
minimize the risk of skin puncture.
Electrostatic sensitive devices Identifies an area where electrostatic sensitive devices may be
present. A ground strap must be worn while servicing the system.
Note: Card Cage Ground A protective grounding symbol appears on the System at any electrical
terminal that must be connected to earth ground before any other
connections can be safely made to the equipment.
10 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Course Objectives
Other Related Symbols
Icon Description
The alternating current symbol appears on the System at a
terminal to which or from which an alternative (sine wave)
current or voltage can be applied or supplied.
On an electrical switch, a vertical bar identifies the ON
position.
On an electrical switch, a circle identifies the OFF position.
Hazard Symbol Definition and Descriptions
The labeling of CELL-DYN System reagents/calibrators/controls or liquid consumables
may include one or more of the following hazard symbols. The symbols and/or other
country-specific warnings are used to convey properties of the chemical or chemical
mixture, and to notify the user that precautions should be taken when handling material.
Always consult the Assay-specific Package Insert or Material Safety Data Sheet for
further information.
Hazard Symbol Definition/Description (with Standard Abbreviation)
Indicates that the material is Harmful (Xn) or Irritant (Xi)
Other hazard symbols not directly related to the CELL-DYN System but notify the user
that precautions should be taken when handling material.
Hazard Symbol Definition/Description (with Standard Abbreviation)
Indicates that the material is Highly Flammable (F) or Extremely
Flammable (F+).
Indicates that the material is Toxic (T) or Very Toxic (T+).
Indicates the material is Corrosive (C).
Indicates that the material is Dangerous for the environment (N).
CELL-DYN Ruby® Field Service Training Workbook 11
204343-101 Dec 2008
Course Objectives
Notes Laser Safety
The CELL-DYN Ruby is a Class 1 laser product.
Do not look directly into the laser beam, aperture, or any reflection
of the beam from a mirror-like surface. Do not place any objects
into the beam, or remove the protective covers, or bypass the
interlocks. Do not use controls or adjustments, or perform
procedures other than those specified. Do not remove, damage, or
obliterate the laser warning labels. If any label becomes illegible,
replace it. When the access door, or inner protective cover are
removed, helium-neon laser power up to 10 mW continuous wave
at 632.8 nm in a beam with a 1 mR divergence could be accessible
in the interior of the optics bench. This amount of energy, with
insignificant attenuation with distance, is sufficient to cause eye
damage.
Caution
Use of controls or adjustments or performance of procedures other than those specified
in the CELL-DYN Ruby Service and Support Manual, Advisories and/or Bulletins may
result in hazardous laser light exposure. If the instrument is used or modified in a
manner not specified by the manufacturer, the protection provided by the instrument
may be impaired.
Electrostatic Discharge (ESD)
Many of the electronic components on the CELL-DYN Systems
circuit boards are susceptible to electrostatic discharge (ESD).
Always follow static protective procedures prior to touching and
working on the instrument.
End of Module
12 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 1: Service Tools
This module introduces the principles, and procedures associated with:
• Service Resource Documentation
• Achieving Service Excellence
• General Troubleshooting Principles
STOP
THINK
EVALUATE
PROCEED
Effective Troubleshooting Model
CELL-DYN Ruby® Field Service Training Workbook 1-1
204343-101 Dec 2008
Objectives
Notes
Objectives
After completing this module, you should be able to:
• Identify Web based resources for you to use during FSR
activities
• Identify tangible materials that you will utilize in your role as a
FSR in laboratory sessions in this course and in the field
• Describe the Achieving Service Excellence (ASE) process
• Discuss the Effective Troubleshooting/S.T.E.P. process and
how it is utilized to resolve root cause errors
• Discuss the F.O.R.T. concept of classifying errors into
subsystems
• List the top 5 instrument failures/errors that result in FSR visits
• Identify commonly used parts that are used in troubleshooting
and that have a high failure rate
1-2 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Resources and Materials
Resources and Materials
Web Based Resources
Web based resources contain digital copies of books, procedures, illustrations. Most of
these resources will be found through the Global Service and Support (GSS) website.
Your instructor will guide you to each of these resources on you laptop.
Resource Location Purpose
GSS Website Houses current technical
information.
Training Materials GSS Web/Hematology/Ruby Access to on-line Training
Training documents.
Instrument Service GSS Web Home Page and The TSB and ISA all Product
Advisory (ISA)/Technical GSS Product Page Database is used for the
Service Bulletin (TSB) distribution of Technical
Database Service Bulletins (TSB) and
Instrument Service Advisories
(ISA) to Abbott Personnel
only.
Analyzer TSB Sticker is
located on Front Flow Panel
on the Optic Flow Cell cover.
NOTE: The information in these databases is CONFIDENTAL and for INTERNAL
USE ONLY. No information in this database may be given out (electronic or
hard copy) to any NON-Abbott Personnel without the prior written permission
from Abbott Laboratories.
eSolutions GSS Web/Hematology/Ruby Troubleshooting database.
eSolutions and GSS Web
Home Page
ACE Training Sessions GSS Web Home Page Continuing Education.
CELL-DYN Ruby® Field Service Training Workbook 1-3
204343-101 Dec 2008
Resources and Materials
Classroom Materials
You will also have access to tangible resources that you will be able to use in the
classroom and on the work site. These materials are generally found on the GSS Web.
Some of these resources have been provided for you. Your instructor will distribute the
various diagrams, password Log-in cards and Quick Reference Guides. The STEP
process worksheets will be included with each module where needed.
Resource Location Purpose
11x17 Color Flow GSS Web/Hematology/ Troubleshooting Tool
Diagrams Service and Support Manual/
Troubleshooting/Block
Diagrams
Instructor Provided
11x17 Cable GSS Web/Hematology/ Troubleshooting Tool
Connection Service and Support Manual/
Diagrams Troubleshooting/Block
Diagrams
Instructor Provided
Password Log-in Instructor Provided Log-in codes for accessing
Card non-customer software
menus
Code is entered backwards
Instrument Instructor Provided Commonly used Service
Specific Quick Information
Reference Guides
S.T.E.P. Quick Instructor Provided Troubleshooting Model
Reference Card Reference
1-4 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity 1
Notes
Activity 1
Time to complete: 10 minutes
Activity Instructions
In this activity, the instructor will direct a class review of the ASE
and Effective Troubleshooting (S.T.E.P.) process. An overview of
these process are provided on the Field Service AH-HA pad that
the instructor has provided for you.
Then you will have an opportunity to answer the questions shown
below.
Perform the following:
1. Describe the ASE process and how it is used in the FSR role:
2. Describe the key elements within the four stages of the
S.T.E.P. /Effective Troubleshooting Process
STOP - Identify P__________________
THINK - Gather Meaningful Facts and Data; List the four
Question dimensions:
______________ _____________
______________ ______________
Look for C_______________ (IS/IS NOT)
List C_____________ (what is unique about IS data)
EVALUATE - Test/Check _________ against data
PROCEED - Perform Repair, complete verifications
CELL-DYN Ruby® Field Service Training Workbook 1-5
204343-101 Dec 2008
Activity 2 - Top Failures
Notes
Activity 2 - Top Failures
Instructor Directed Session: 20 minutes
Instructions:
In this activity, you will
• fill out the Common Areas of Failure section of the Ah_Ha
note pad during the instructor led overview.
• complete parts table below
Additions to the tables within the Ah_Ha note pad will occur
throughout the class as you explore these failures in more detail.
Perform the following:
Fill out the Common Failure chart found on the Ah_Ha Field Service
Note Pad during instructor overview.
Complete the Table below during the instructor overview of com-
monly used parts.
Commonly Used Parts
Component Notes
END OF ACTIVITY
1-6 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module Summary
Notes
Module Summary
Now that you’ve completed the Service Tools Module, you should
be able to identify the service resources utilized during service of
the CELL-DYN Ruby System and the type of information contained
or provided by each resource. You should also be able to locate
these reference resources within the various databases, websites
and handouts.
The activities in the upcoming modules will focus on the major
components of the CELL-DYN Ruby subSystems including Power
and Boards, Fluidics, Optics, Robotics, and Temperature, and will
provide you with more opportunities to practice troubleshooting
these subsystems.
CELL-DYN Ruby® Field Service Training Workbook 1-7
204343-101 Dec 2008
Review
Notes
Review
1. Where is the TSB Sticker located on the CELL-DYN Ruby System?
2. What is the current TSB revision level of the CELL-DYN Ruby Systems
in the Classroom?
3. What is the ISA number of the CELL-DYN Ruby Installation
Procedure?
4. Where within the CELL-DYN Ruby Service Resource documentation is
the “Analyzer Setpoints Reference Chart” located?
End of Module
1-8 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 2: Components and Software
This module provides an overview of the CELL-DYN Ruby System hardware and software.
This module introduces the principles, and procedures associated with:
• Component Identification
• Software Menu
• Software Navigation
Example of a CELL-DYN Ruby Monitor
CELL-DYN Ruby® Field Service Training Workbook 2-1
204343-101 Dec 2008
Objectives
Notes
Objectives
After completing this module, you should be able to:
• Remove instrument covers to access internal compo-
nents
• Identify components of the CELL-DYN Ruby System
and their function
• Present assigned components and functions to class-
mates
• Describe the screen areas and functions of the menu
bar commands
• Navigate the software interface to locate and use logs,
views, field service diagnostic procedures and data
reports
• Create a QCID file
• Perform and Instrument Shutdown and power the ana-
lyzer and data module OFF and ON
2-2 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Components
Notes
Activity - Components
Time to complete: 30 minutes
Debrief Session: 45 minutes
Activity Instructions
In this activity, the class will be divided into three groups.
Each group will be assigned a section of the CELL-DYN Ruby
System to locate and identify parts.
In this activity you will:
• Locate each component for the group you are assigned
• groups and components are identified on tables located
on the pages that follow
• Prepare to discuss each components function/purpose
and location with the class during the debrief session
• As directed by instructor, present your groups informa-
tion to the class
• include both location and function of components
• have class participants view the components on their
analyzer during your groups presentation
Following the debrief session presentations, all students will locate
the components found on the Back Instrument Panel along with the
instrument peripherals.
Resources Needed
In this session you will be removing some of the covers from
the instrument. Refer to the CELL-DYN Ruby System
Service and Support Manual R&R A1.01-A1.06 for
instructions on how to remove the covers.
Utilize the Diagrams, you have been provided with, to identify and
locate key components.
During the debrief sessions, use the service notes section to
document additional information, provided by the instructor, within
the tables provided.
CELL-DYN Ruby® Field Service Training Workbook 2-3
204343-101 Dec 2008
Activity - Components
Group 1: Instrument Left Side
Component Function Service Notes
Sample Handler • Controls the Aspiration Tower • SHM boards are interchangeable.
Module #1 Ensure jumper is set appropriately
(SHM #1) per board location.
• Controls Aspiration Probe up/down
stepper motor
Sample Handler • Sample Loader Controller
Module #2
(SHM #2)
Open Tube • Drives open mode wash block up/down
Sampler
Chopper Driver
Vacuum & • Supplies 2 Vacuum and 3 Pressure levels
Pressure
Assembly
Two 28 VDC • Generates Vacuum and Pressure • Pumps cycle ON/OFF every 5
Pumps seconds
Pump Relay • Supplies +28VDC to Vacuum/Pressure • Pump Relay Board is Fused
Module (PRM) Pumps
• Turns Pumps ON and OFF
• Receives control signal from VPM
Multi-Port • Disconnects Vacuum/Pressure lines from
(Quick- the instrument
Disconnect)
coupler
Analyzer Power • Main Power Supply for instrument
Supply (APS) generating +28 and +15.5 VDC
Vacuum • Controls Vacuum/Pressure Levels
Pressure Module • Sends signals to turn vacuum/pressure
(VPM) pumps ON or OFF
Fluid Control • Interface and control functions
Module (FCM) • Amplifies HGB Flow Cell output
Cable • Two CDMs
Distribution • Distributes voltages to solenoids
Module (CDM) 2
• Collects reagent and waste sensor infor-
mation for distribution to FCM
Solenoid Driver • Seven SDMs • Solenoids run on +28VDC and
Module (SDM) 5, • Provide current drive to open and close +15.5VDC
6, and 9 solenoids
2-4 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Components
Group 2: Flow Panel and Nose Cone
Component Function Service Notes
Two Diluent/Sheath • Provides reagent for Rinsing/Dilution/Move-
Reagent Reservoirs ment and hydrodynamic focusing
• Diluent/Sheath (noisy) • Reservoirs are filled using VAC 1
• Diluent/Sheath (quiet) • Delivers/Supplies Diluent/Sheath with pres-
sure
Peristaltic Pump • Stages Sample Dilution for processing
through the Optical Flow Cell:
• RBC/PLT staged 1st
• NOC (if applicable) staged 2nd
• WBC (WOC) staged 3rd
RBC/PLT Mixing • Area for mixing red blood cells and platelets
Chamber to be counted
• Position of input ports create swirling mixing
action when syringe injects fluid in
Optical Flow Cell • Panel removed for adjusting Optical Flow
Access Door Cell
WBC Mixing Chamber/ • Mixing and heating of WBC Lyse and sample • A out of range monitoring
Heater Assembly • Swirling mixing action created when syringe occurs if temperature does
not fall between 20°C and
injects fluid in
40°C
Hemoglobin Flow Cell • Light-tight chamber for hemoglobin measure-
ment
Y-Valve and Motor • Drives Y-valve motor to rotate valve open
Drive Module (MDM) during closed mode of aspiration
Shear Valve • Used to separate blood sample into 3 seg-
ments for dilution and measurement
Hemoglobin Heater • Heats Diluent/Sheath for HGB Dilution (for • A out of range monitoring
use prior to Shear Valve) and HGB/NOC occurs if temperature does
Lyse prior to transfer to HGB Flow Cell not fall between 40°C and
51°C
WBC Lyse Reagent • Provides WBC Lyse to WBC Mixing Chamber
Reservoir • Filled using VAC 1
• Delivered with Pressure 3
Two Ultrasonic Blood • Ensures appropriate sample aspiration condi-
Sensors tion through Shear Valve
(S1, S3) • Short Sample condition detection
CELL-DYN Ruby® Field Service Training Workbook 2-5
204343-101 Dec 2008
Activity - Components
Component Function Service Notes
Optical Short Sample • Ensures appropriate aspiration of
Sensor (S2) blood through closed mode
• Used during Closed Mode only
20 PSI Overpressure • Ensure proper fluid movement of
sensor Diluent/Sheath through the Shear
Valve
Syringes • Inject whole blood and/or reagent to
• RBC/HBG Diluent Syringe their respective cup
• WBC Lyse Syringe
• HBG Lyse Syringe
• Sample Injection Syringe
Normally Open and Closed • Control movement of fluid, vacuum
Solenoids and pressure
Waste Chambers 1, 2, 3, 4 • Waste chambers perform the drain-
ing and collection of waste from the
various components
Mixer Assembly • Mixes blood specimen to ensure
proper cell suspension prior to
closed mode aspiration
Tube Spinner • Rotates specimen tubes to position
barcode label for reading by the
barcode reader
• This allows for positive specimen
identification
Tube Capture Chute • Catches tubes or spilled blood
Barcode Reader • Reads specimen tube bar code
labels
Rack Advance Prawls • Move racks from right to left on
Sample Loader
Cross Transfer Guides • Moves racks in a horizontal plane
• moves racks forward on load side
• moves racks away from the analyzer
on the unload side
Sample Loader Multi-Port • Disconnects Sample Loader pneu-
(Quick Disconnect) Coupler matic lines
Manual Drain Lines • Liquid in an accumulator can be
manually drained via drain line
2-6 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Components
Group 3: Instrument Right Side and Top View
Component Function Service Notes
Data Module and • Personal Computer subsystem is main
Power Switch intelligence of CD Ruby System
• Powers both Data Module and Analyzer ON/OFF
• When the instrument is in Standby Mode, the Data
Module power button is used to power the
Analyzer and Data Module OFF
• Press and hold (4 seconds) then release the
button
• To power ON the system, press and then release
the Data Module power button
Single Board • Data Module communication with analyzer
Computer (SBC) CPU/DCM and interface with peripheral devices
such as Printer, LIS and CD-RW
Backplane PCB • Provides slot for power and communication
transfer between PCBs
Central Processing • Serial communication via High Speed Serial Link
Unit/Device (HSSL) to the Data Module Single Board
Control Module Computer
(CPU/DCM)
ATX Computer • Supplies +3.3VDC, +5VDC, +12VDC voltages to • The link (cable) should be
Power Supply (PS) Data Module Backplane, Hard Disk Drive (HDD), checked before replacing
Floppy Disk Drive (FDD) and Power Distribution the APS
Module (PDM) • Power is now controlled by
the Windows XP software
• “Master” Power Supply. Signals the Analyzer
and the motherboard
Power Supply (APS) to turn ON and turn OFF
High Speed Serial • High Speed Serial Link between analyzer and
Link (HSSL) Data Module
Main Amplifier • Processes optical channel signals between
Module (MAM) pre-amplifiers and SPM PCB
Signal Processor • Detects and counts valid cell pulses
Module (SPM)
Shear Valve Driver • Drives the Shear Valve Motor to rotate ceramics
Assembly to cut blood sample into 3 segments
Cable Distribution • Two CDMs
Module (CDM) 1 • Distributes voltages to solenoids
• Collects reagent and waste sensor information for
distribution to FCM
Motor Processing • Sends power, speed and direction to stepper • Stepper Motors run on
Module (MPM) drivers +28VDC
Solenoid Driver • Seven SDMs • Solenoids run on +28VDC
Module (SDM) 1, • Provide current drive to open and close solenoids and +15.5VDC
2, 3 and 4
CELL-DYN Ruby® Field Service Training Workbook 2-7
204343-101 Dec 2008
Activity - Components
Top View
Component Function Service Notes
Power Distribution • Generates +5VDC, +15VRaw, +15VDC, • Produces the +5V for the logic
Module (PDM) and +12VDC in the Analyzer
The +5V for the computer is
• Distributes power throughout the
produced by the ATX power
analyzer
supply.
• +5V produced by the PDM is
used for system communica-
tions
Temperature Control • Control and drive WBC and HGB Heaters • Enabled during instrument
Module (TCM) prime; disabled during standby
• Takes approx. 6 minutes to
stabilize
• Five LEDs & three potentiome-
ters
Optics Bench • Optical measurement System using up to
• Flow Cell four channels to count, size, and classify
blood cells using MAPSS technology
• PMTs
• Nozzle Assembly • Each channel provides its own distinct
• Laser Tube information relating to cell size or mor-
phology (structure)
• Four Channels:
• 0o - Cellular Size.
• 10o - Cellular Complexity.
• 90o - Cellular Lobularity.
• 90oD - Cell Granularity.
Laser Power Supply • Powers Laser Tube • Located beneath the Optical
Bench
• Receives +28V from APS
• Typical Laser Power Reading
>5.0 mw.
2-8 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Components
All Students will locate the following Rear components:
Component Function Notes
Back Panel of Instrument
Reagent Inlet Ports • Reagent In
Waste Outlet and • Waste Out
External Waste Full
Sensor Connector
Main Power Switch • Turn OFF/ON power to entire instru- • It is not necessary to turn the Sys-
ment tem main power switch OFF under
normal operating conditions.
• For periods of inactivity, there is a
standby mode.
• If the system is idle for four hours, it
automatically goes into Standby
mode.
USB, PS-2, Ethernet • Device connections
Device Connections
Fans • Cooling, air movement • +28VDC Fan 1, 2 & 3
Data Module* rear view
Speaker Plug in from Monitor
Host CPU
USB Ports
AbbottLink Plug in
Bar Code
Reader
*Note: configuration may vary, refer to Service
and Support Manual for most current information.
CELL-DYN Ruby® Field Service Training Workbook 2-9
204343-101 Dec 2008
Activity - Components
All Students will locate the following Miscellaneous Components:
Component Function Notes
Peripherals
17 inch color flat • User interface with touch screen • Monitor Driver is part of Abbott Software
panel monitor capability load
Membrane keyboard • Rubber exterior for user input
Mouse • Cursor movement
Hand-held Bar Code • Manual reading of sample bar
Reader codes
Printer • Data output
Locate Analyzer Status Indicator Lights. Note status indication listed below.
LED Color Status Indication
READY Green The Analyzer is ready to run specimens.
BUSY Yellow The Analyzer is busy.
FAULT Amber The Analyzer is not ready to run specimens.
All Students Perform the following Procedure:
Perform Instrument Shutdown and Power OFF
Refer to the CELL-DYN Ruby System Operator’s Manual Section 5 Operating
Instructions; Subsection: System Priming, Interruption, and Standby for instruction.
END OF ACTIVITY
2-10 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
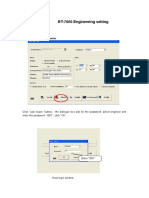
Activity - Software 1
Notes
Activity - Software 1
Instructor Directed Session: 60 minutes
Activity Instructions
In this activity you will:
• Explore the CELL-DYN Ruby software as your
instructor directs you to perform various tasks.
• This will include identifying key areas of the screen,
their function, and how to navigate to various screens.
• locate logs and perform functions within the software
Finally, you will perform case study #1 found after the end of this
activity.
Instructor Directed Review:
If the CELL-DYN Ruby is not ON, Power the System ON
Refer to the CELL-DYN Ruby System Operator’s Manual
Section 5 Operating Instructions; Subsection: System Prim-
ing, Interruption, and Standby for instruction.
CELL-DYN Ruby Virtual Machine Demo
NOTE:The CELL-DYN Ruby Virtual machine is a network
accessible program that allows the user to run the current
version of the CELL-DYN Ruby User Interface software
through their personal computer. The Virtual CELL-DYN
Ruby software looks and behaves like a regular CELL-DYN
Ruby System, however some of the User functions (ones
requiring an analyzer) are not available. Instructions for
accessing the CELL-DYN Ruby Virtual machine are located
on the GSS website home page, under the Expert
Information link.
Use your CELL-DYN Ruby Analyzer to follow an Instructor led
review of the Software
CELL-DYN Ruby® Field Service Training Workbook 2-11
204343-101 Dec 2008
Activity - Software 1
Notes Access Levels
• When the CELL-DYN Ruby data station is powered on the user
will automatically be logged in as “cd” in Windows and as
“Guest” in the application. This is lowest access level.
• Other log-in Options are:
• Admin: no password or default but a password can be
setup
• CSC: password is todays date +5
• FSE: password is the Architect login codes
• CSC and FSE have full access rights
• Guest: no password and no password can be assigned.
NOTE: Some commands may be unavailable (grayed out)
depending on the instrument status or user access
level.
Display Screen
• Refer to the Software Map on the following page and the figure
below during instructor review of Display Screen primary areas
2-12 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Software 1
CELL-DYN Ruby Software MAP
NOTE:For information on the latest Software versions and menus, refer to the CELL-DYN
Ruby Operator’s Manual. The information below is for training purposes only.
Primary Sections:
Title Bar Example Only
Menu Bar
Tool Bar
Operating State
Sampling Mode
QC Status
Status Bar
VIEW
System (Controlled by Tool Bar buttons)
Messages
Next Open Tube Entry
Di
Displays operator entered
Specimen ID or QCID, Specimen Type
and Test Selection for next Specimen
to be sampled in Open Mode
Function Keys
Title Bar
Identifies the View displayed. Displays the last run sequence number and the current date and time.
Menu Bar (Example of Menu structure, available options may vary based on software version.)
File: Access Basic System Commands: Diagnostics: Access diagnostic functions such as:
• Backup and Restore • Diagnostic Views:
• Shutdown and Exit • Check to display 4th tab
Setup: Customize System Operating Conditions such as: • Raw Data & Count Rate Summary
• Patient Sample Setup • HSSL Log
• Units Set Selection • Mechanical Operations
• Customizing Run and Data Views • Digital/Voltages Readings
• Customizing Moving Average View • Auto-Gain Wizard
• Customize Print Report • Setpoints
• QCID Setup • Bar Code Alignment
• Administrative Setup Window • Extended WBC Diag
Calibration: Access Calibration Procedures such as: • SRP/Blood Comparison
• Last Auto-Calibration Date • Electronic Cells Diag
• Quick Precision Check • Reset Admin Password
• Calibration Log Help: • Operator’s Manual
• Auto-Calibration Wizard • About CELL-DYN Ruby (software ver)
• Manual Calibration (FSE login Only) Sign Off: Allows Operator to update OPID
CELL-DYN Ruby® Field Service Training Workbook 2-13
204343-101 Dec 2008
Activity - Software 1
Tool Bar
The Tool bar buttons control the display of the Main View and the associated function keys.
Run View: Display specimen view of the last run sequence number
Orders: Display Pending Orders
Data Log: Display system data log
QC View: Display QC Log
Groups: Displays samples based on groups of criteria
Groups are: FWBC, NRBC/RRBC, Exceptions, and Not Transmitted
• Group criteria cannot be edited and groups cannot be added
Reagent: Display selected tab: Current Reagent, Reagent Log
• Note: the Current Reagent display is a calculated Software indication of reagent status
Maintenance: Display selected tab: Scheduled, As-Needed, Special Protocols, Maintenance Log
• Maintenance Log is a record of maintenance performance dates
Access Help Videos
System: Display selected tab:
• Calibration Log - Record of last calibration date and Record of Precision results
• Event Log - Record of Faults
• Set Point Log - Record of changes to set points
FILTER and SEARCH Functions
Example Only
Advanced tab
contains various
Search Criteria
F3 - Find/Filter
Opens the Find/Filter dialog box, which has two tabs: Find/Filter and Advanced Find/Filter.
Find – locates the earliest matching entry. The number of matches is displayed, along with a Find Next key
that is used to move to the next matching entry.
Filter – displays a new screen with all matching entries. The filtered entries screen is exited by selecting the
Unfilter function key.
2-14 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Software 1
Perform the Following: Notes
Locate (Do NOT perform) the following software functions:
LIS Loopback test
Solenoid operations
Dilution factors: view/edit
Disable analyzer
Enable/disable bar code check digit
Quick Precision Check
Set Default Patient test selection
Create a new QCID - Whole Blood Control
Refer to the CELL-DYN Ruby System Operator’s Manual, Sec-
tion 11 Quality Control, Subsection Quality Control Software,
QCID File Setup for additional information.
Steps:
1. Select Setup from the menu bar and QCID Setup from the pull-
down menu. The QCID Setup: View dialog box appears (the
default view displays QCID: Background).
2. Select the Create button and the QCID Setup: Basics dialog box
opens (The default view displays Control Type: Commercial).
3. Select Whole Blood from the drop down menu in the Control
Type field.
4. Enter a QCID identifier in the New QCID field.
5. Select Continue.
6. Enter the control information (original specimen ID, draw date/
time) in the appropriate fields in the Control Data tab. This infor-
mation is optional for whole blood controls. The default test selec-
tion is CBC+NOC.
7. Click on the QC Limits tab and enter the control means and limits.
8. Click on the Westgard tab and select rules to activate (optional).
9. When all information has been entered, select OK to complete
QCID creation. The QCID Setup: View dialog box opens, reflect-
ing the newly created QCID information.
10. Identify QCID icon
CELL-DYN Ruby® Field Service Training Workbook 2-15
204343-101 Dec 2008
Activity - Software 1
Notes Locate the following information:
The current software version of the system
Record Version _______
The date when the shear valve was last cleaned
Record Date _______
The faults that occurred in the last eight (8) hours
The date of the last calibration
Record Date _______
The lot numbers of the current reagents (using the software)
END OF ACTIVITY
2-16 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Case Study 1
Notes
Case Study 1
Time to complete: 15 minutes
The customer is reporting that a blue screen is shown on their
display and that the instrument is unresponsive to
commands. The following troubleshooting steps were performed
or observed, and the issues remain unresolved:
• Customer has cycled power
• +5VDC Power is present and steady
Using your knowledge of the STEP Process, of normal instrument
operation, and your troubleshooting resources (Troubleshooting
Information Database [eSolutions], TSBs/ISAs, etc.), you have
determined that the Hard Disk Drive (HDD) on the customer’s
instrument has failed.
After replacing the HDD you now need to reinstall the software.
The customer has a back up copy that they have made during
weekly backups.
Use the Instrument Service and Support Manual and ISA database
to identify the procedures that would be necessary for you to
restore the instrument back to proper operation. List the
procedures below:
CELL-DYN Ruby® Field Service Training Workbook 2-17
204343-101 Dec 2008
Case Study 1
Notes
2-18 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Software 2
Notes
Activity - Software 2
Time to complete: 90 minutes
Activity Instructions
In this activity you will perform service procedures that could be
required in the event of a Hard Drive Failure.
Resources Needed
Refer to the CELL-DYN Ruby System Service and Support
Manual, Verification Procedure Section and the ISA Database
for instruction.
Perform the following Procedures:
Backup Procedure (VP-48)
Operating System (OS) Install and Hard Drive Format (VP-47)
Application Software Install (VP-41)
Restore from Backup (VP-49)
Operations Manual Install (VP-46)
Touchscreen Calibration (VP-6)
Printer Driver ISA 170-005 (current revision)
Perform Instrument Shutdown and Power OFF
NOTE: After a software installation has been performed re-
configure the analyzers screen saver.
END OF ACTIVITY
CELL-DYN Ruby® Field Service Training Workbook 2-19
204343-101 Dec 2008
Module Summary
Notes
Module Summary
Now that you’ve completed the Component and Software
Overview Module, you should be able to identify the major
components on the CELL-DYN Ruby System and the role the
component performs during instrument operation.
The activities in the upcoming modules will provide more detail on
how these components fit into the major instrument subsystems
including Power and Boards, Fluidics, Optics, Robotics, and
Temperature, and will provide you with more opportunities to
practice troubleshooting these subsystems.
2-20 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Review
Notes
Review
1. List the two (2) heaters that have been added to the CELL-DYN Ruby
System?
2. List the appropriate step(s) to calibrate the Elo Touchscreen monitor.
3. Where are the SHM boards located?
4. Explain the difference in function between the Optical Short Sample
Sensor (S2) and the Two Ultrasonic Blood Sensors (S1, S3).
5. Describe the difference between the two power switches on the
instrument and how each is properly used.
End of Module
CELL-DYN Ruby® Field Service Training Workbook 2-21
204343-101 Dec 2008
Review
Notes
2-22 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 3: Basic Operation
This module provides an overview of the CELL-DYN Ruby System operation. This module
introduces the principles, and procedures associated with:
• Sample Analysis
• Maintenance
• Normal Operating conditions
• Precision and Calibration
Shear Valve Assembly
Ultrasonic
RBC/PLT Mixing Sensor (S1)
Diluent/Sheath Chamber Short Sample
Reservoir 1 HGB
Diluent/Sheath Heater Assembly Sensor (S2)
Reservoir 2 HGB Flow Cell
Solenoid
WBC Lyse
Reservoir
Waste
Vent Chambers
Chamber Waste Normally HGB Lyse Diluent/Sheath
Chambers Closed Solenoid Syringe Syringe
Sample Transfer WBC Lyse
WBC Mixing
Peristaltic Chamber/WOC Syringe
Pump (staging) Bubble Trap Heater Sample Injection
Syringe
CELL-DYN Ruby Flow Panel Graphic
CELL-DYN Ruby® Field Service Training Workbook 3-1
204343-101 Dec 2008
Objectives
Notes
Objectives
After completing this module, you should be able to:
• Perform Auto-clean procedure
• Perform Shear Valve cleaning
• Perform the Quick Precision Check in Open and Closed
Mode
• Explain why it is important to use fresh, whole blood
when performing a precision check
• Describe normal operation of instrument components
through direct observation of instrument functions while
processing specimens in Open and Closed modes
• Detect abnormal component function during specimen
analysis processes by employing direct observation
• Use the Auto-Calibration Wizard to calibrate the
CELL-DYN Ruby System and to report acceptable QC
results
• Determine situations where it is advisable to perform
calibration and those where it is not
• Identify which parameters can be calibrated
All procedures should be performed in accordance with
specifications outlined in CELL-DYN Ruby Operator’s Manual,
CELL-DYN Ruby Service and Support Manual and/or ISAs and
TSBs.
3-2 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Basic Operation
Basic Operation
The term Basic Operation is often used to refer to processes and procedures required for
performing the day-to-day operation of the CELL-DYN Ruby. However, it does not refer to only
those procedures performed on a daily basis. As presented in this module, Basic Operation
process and procedures include:
• System Priming and Standby
• Instrument Maintenance
• Instrument Configuration
• Calibration
• Quality Control
• Reagent Handling
• Specimen Processing
The overall operation of the CELL-DYN Ruby System is to analyze EDTA-anticoagulated blood
and report hematological parameters. The EDTA-anticoagulated blood specimen consists of both
a liquid and a cellular portion.
• The liquid portion, called Plasma,
consists of various nutrients, proteins,
enzymes, hormones and water.
• The cellular portion consists of three WBC
cellular types:
• White Blood Cells (WBC)
• Red Blood Cells (RBC)
PLT
• Platelets (PLT) RBC
Proper mixing of specimens prior to sample aspiration is essential for obtaining accurate results
on the CELL-DYN Ruby System. Specimens stored at refrigerator temperatures must be brought
to room temperature prior to mixing.
For control or calibrator mixing instructions, refer to the manufacturer’s product insert.
Recommended Volume Requirements in Specimen Collection Tube
Closed Mode: Minimum Specimen Volume > 1.2 mL
Open Mode: Minimum Specimen Volume > 0.5 mL (500μL)
NOTE:0.18 mL (180μL) - In Micro-Specimen Collection Tubes (Non-vacuum)
NOTE:Follow the collection tube manufacturer’s recommendation for minimum
volume in specimen tubes.
CELL-DYN Ruby® Field Service Training Workbook 3-3
204343-101 Dec 2008
Parameters and Reagents
Parameters and Reagents
As with other CELL-DYN Systems the Hematological Parameters are either directly
measured (M), derived (D) or calculated (C).
NOTE: Information on measured, derived and/or calculated parameters can
be found in the CELL-DYN Ruby Operator’s Manual Section 3
Principles of Operation.
White Blood Cell Parameters Red Blood Cell Parameters
M WBC: White Blood Cell Count (WOC) M RBC: Red Blood Cell Count
C NEU: Neutrophil Absolute Count C MCH: Mean Cell Hemoglobin
C LYM: Lymphocyte Absolute Count C MCHC: Mean Cell Hemoglobin Concentration
C MONO: Monocyte Absolute Count C HCT: Hematocrit
C EOS: Eosinophil Absolute Count D MCV: Mean Cell Volume
C BASO: Basophil Absolute Count D RDW: Red Cell Distribution Width
M %N: Neutrophil Percentage of WBCs M HGB: Hemoglobin Concentration
M %L: Lymphocyte Percentage of WBCs Reticulocyte Package
M %M: Monocyte Percentage of WBCs M %R: Percentage of Reticulocytes
M %E: Eosinophil Percentage of WBCs C RETC: Reticulocyte absolute concentration
M %B: Basophil Percentage of WBCs Platelet Parameters
M** PLT: Platelet Count
D MPV: Mean Platelet Volume
C PCT*: Plateletcrit
C PDW*: Platelet Distribution Width
*Clinical significance has not been established for PCT or PDW. Therefore, they are not reportable in the US
**The Platelet Count is directly derived from measured optical data.
3-4 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Parameters and Reagents
Reagent Overview Notes
Instructor Directed Session: 15 minutes
The CELL-DYN Ruby System uses the following reagents:
• Diluent/Sheath
• Acts as the diluting fluid for RBC/PLT, HGB and
NOC (Nuclear Optical Count).
• Maintains the stable cell volume of the red cells
and platelets during counting and sizing.
• Provides an acceptable background count.
• Serves as a sheath fluid for the laminar flow.
• Acts as a rinsing agent for fluidics system.
• Cyanide (CN)-Free HGB/NOC Lyse
• Rapidly lyses the RBCs releasing the hemoglobin
contained in the cell.
• Strips the WBC cytoplasm, leaving the nuclear
membrane intact so the white cell nuclei can be
enumerated.
• Converts the hemoglobin to a stable chromagen.
• Provides an acceptable background count.
• WBC Lyse
• Acts as the diluting fluid for WOC (WBC Optical
Count).
• Osmotically lyses RBC.
• Maintains the light scattering properties of the
WBCs for the duration of the measurement period.
• Provides sufficient wetting action to prevent accu-
mulation of air bubbles in the Optical Flow Cell
system.
• Provides an acceptable background count.
• Reticulocyte Reagent (when Reticulocyte testing is
performed)
Reagents must be stored at room temperature to ensure optimal
performance. All reagents should be protected from direct sunlight,
extreme heat, and freezing during shipment and storage. Reagents
must not be used if they were frozen at any time.
Reagent performance is monitored through Quality Control Sample
analysis, Background counts, and detection sensors.
CELL-DYN Ruby® Field Service Training Workbook 3-5
204343-101 Dec 2008
Parameters and Reagents
Notes
3-6 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Calibration Overview
Notes
Calibration Overview
Controls, Calibrator, and Standard Reference Particles (SRPs) are
reference materials used to test, set, and monitor CELL-DYN Ruby
performance.
Day-to-day verification of System calibration is performed using
CELL-DYN Control products. The frequency of quality control runs
should be determined by each laboratory and conform to the
guidelines established by regulatory agencies.
The CELL-DYN Ruby system should not require frequent
calibration if it is operated and maintained according to the
Operator’s Manual recommendations.
Calibration should be considered as the very last step in a
troubleshooting sequence. Performing unnecessary calibrations
may mask an underlying problem with instrument performance.
When to perform
Scheduled calibration of the CELL-DYN Ruby System should
conform to the guidelines established by regulatory agencies.
Unscheduled calibration is indicated following service adjustments
performed by Abbott Field Service such as major component
changes. Unscheduled calibration is also necessary when
indicated by the results of the Quality Control program.
Calibration may need to be performed under the following
circumstances:
• When there is a complete change of reagents, i.e.,
change in type of reagent from same vendor, or change
to a different vendor.
• When indicated by quality control data.
• After major maintenance and service procedures.
• At least every six months or as directed by the regula-
tory agencies governing the laboratory.
NOTE: Refer to the CELL-DYN Ruby Operator’s Manual
Chapter 6 Calibration Procedures for additional
information.
CELL-DYN Ruby® Field Service Training Workbook 3-7
204343-101 Dec 2008
Calibration Overview
Notes Calibrated Parameters
The following parameters can be calibrated:
• WBC (WOC/NOC), RBC, HGB, MCV, PLT, and MPV.
Calibration Materials
Two types of calibration materials can be used to calibrate the
CELL-DYN Ruby System:
• Commercial Calibrator or Assayed Fresh Whole Blood
Calibration Procedures
Calibration consists of three groups of procedures
• Pre-Calibration Procedures - to verify proper instrument
performance to ensure a successful calibration.
• Calibration Procedures
Two methods of calibration are available on the
CELL-DYN Ruby System:
• Auto-Calibration Wizard
• Manual Calibration
• Post-Calibration Procedures - to confirm calibration.
NOTE: Refer to the CELL-DYN Ruby Operator’s Manual
Chapter 6 Calibration Procedures for additional
information.
3-8 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Basic Operation
Notes
Activity - Basic Operation
Time to complete: 3 hours
Debrief Session: 15 minutes
Activity Instructions
In this activity you will perform the following:
• Prime/Background count
• Auto-clean
• Shear Valve Cleaning
• Quick Precision Check in Open mode
• Quick Precision Check in Closed mode
• Calibration using Auto-cal Wizard
• Observe and Document Instrument “normal” Operation
Instrument Observation should include observing the blood
sample path as the instrument aspirates, separates, stages
and analyzes. Due to the speed of the analyzer, you will need
to do this several times for each component that you observe.
Record your observations in the tables provided on the follow-
ing pages.
Instructor Debrief/Review:
At the conclusion of the activity, your instructor will lead a group
review of “normal” function, calling on students randomly for
their observations.
Resources Needed
In this session utilize the Diagrams you have been provided in
class to identify and locate key components.
Refer to the CELL-DYN Ruby Operator’s Manual Section 5
Operating Instructions, Subsection Specimen Analysis; Section
6: Pre-Calibration Checklist; and Section 9: Service and Main-
tenance for information on the procedures performed in this
activity.
The instructor will provide fresh whole blood and calibrator for
use during this activity. Disposable Pipettes and Red Top
Tubes are also needed.
CELL-DYN Ruby® Field Service Training Workbook 3-9
204343-101 Dec 2008
Activity - Basic Operation
Notes Perform the following:
WARNING: Biohazard. Potential Biohazard, follow biosafety
practices.
CAUTION: Moving Parts.
Perform an Instrument PRIME
Select the F12 – Prime function key to activate prime cycle
and run an Auto Background.
OR
Select Prime task button from the Maintenance, Special Pro-
tocols tab view to activate prime cycle and run an AutoBack-
ground.
NOTE:The Ruby System will not perform a prime operation
until the HGB Heater is up to specification beginning
with Ruby Software version 2.0 and higher.
Review Background results to determine if they are within specifi-
cation. (Refer to the Operator’s Manual, Section 4: Performance Specification
for acceptable ranges)
Are your results within specification?
YES _______
NO _______ List what value is out of range below and describe
what corrective action you will perform.
Perform Auto-clean (Refer to the Operator’s Manual, Section 9: Service and
Maintenance)
Perform Shear Valve Cleaning (Refer to the on-board Software Mainte-
nance Video)
Perform a Quick Precision Check in the OPEN mode. (Refer to the
Operator’s Manual Section 6: Pre-Calibration Checklist)
NOTE:Proper mixing of specimens prior to sample aspiration is
essential for obtaining accurate results on the CELL-DYN Ruby
System. Specimens to be run in the Open Mode must be well
mixed on a mechanical mixer or hand mixed by inversion per
the laboratory’s protocol. Immediately prior to sample
aspiration, mix again by inverting the tube a minimum of 10
times.
For Open Mode only, when specimens have not been run for one
hour or more, a background should be run immediately prior to
running a patient specimen.
3-10 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Basic Operation
Observe Instrument Operation and record requested data within the Tables contained on
the following pages. Complete all tables.
Knowing how the instrument sounds and looks during routine operation is a critical key
to troubleshooting.
OPEN MODE OBSERVATON
Component/Function Note Observations
Blood Sample Path to Open Mode:
Ultrasonic Sensors Note length of blood sample extension going into and out from the
Shear Valve prior to rotation.
Rotation of Shear Valve Describe Speed of rotation (fast, slow, quantify timing, etc.):
Describe Sound of Shear Valve rotation (beeps, mechanical, grinding,
etc.):
What is the Direction of Rotation?
Bubble Mixing Describe Bubbling speed/intensity in RBC/PLT chamber:
List locations where Bubble Mix is used on the Flow Panel:
Draining and filling of the How full does the chamber fill?
RBC/PLT Mix Chamber
Does the RBC/PLT Mix Chamber completely empty during the drain
cycle?
How does it rinse?
HGB Heater and WBC Touch each Heater Assembly and quantify the level of heat present.
Heater Assembly HGB Heater (Temp between 40°C and 50°C):
WBC Heater (Temp between 20°C and 40°C):
(C
(Continued on next page)
CELL-DYN Ruby® Field Service Training Workbook 3-11
204343-101 Dec 2008
Activity - Basic Operation
OPEN MODE OBSERVATON
Component/Function Note Observations
Speed of blood segments RBC+PLT Segment
being delivered to mixing
areas
(Describe the delivery speed of HGB+NOC Segment
the items listed)
WOC Segment
Rotation of Peristaltic Describe Speed of rotation (fast, slow, quantify timing, etc.):
Pump for Staging
Describe Sounds during rotation (beeps, mechanical, grinding, etc.):
List solenoids involved in staging blood through ports 1 and out port 2
through valve 52:
Syringe Movement All Syringe movement (smooth, jerky, rapid, slow, stop/start):
(Describe the movement of the
items listed)
Injection Syringe move during count cycle (smooth, jerky, rapid, slow,
stop/start):
Opening/Closing of Once a solenoid has been closed, press on it with your finger. Was
Solenoids there movement or a clicking sound?
(Continued on next page)
3-12 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Basic Operation
OPEN MODE OBSERVATON
Component/Function Note Observations
Listen to normal Pneumatic pumps-(how long do they run?)
instrument sounds
(Describe the sound of the
items listed) Solenoids opening and closing:
Peri-pump rotation:
Syringe Drive movement:
Shear Valve rotation:
Observe draining and Foaming occurs in what Waste Chamber?
filling of Reagent
Reservoirs and Waste
Chambers
How do the Reagent Reservoirs signal that they are full?
Name the Reagents used on the CELL-DYN Ruby System.
Record your Open precision check results in the table below:
Parameter %CV Limit Your %CV Results Met Specification
Yes No
WOC < 2.4%
NOC < 2.8%
RBC < 1.8%
HGB < 1.4%
MCV < 0.8%
PLT < 3.8%
If any precision results are out of specification describe the corrective action that you
would take to resolve.
CELL-DYN Ruby® Field Service Training Workbook 3-13
204343-101 Dec 2008
Activity - Basic Operation
Perform a Quick Precision Check in the CLOSED mode.
NOTE: To perform a Closed Mode Precision, proper preparation of the specimens is
essential for obtaining accurate results on the CELL-DYN Ruby System. To
prepare specimens for use follow the guidelines stated below:
• Obtain 15 ml of normal whole blood within four (4) hours of draw.
• All specimens must have been properly collected in tubes containing EDTA
anticoagulant.
• Pool, mix, and aliquot into five (5) unused, red top (non anticoagulant) tubes.
• Be certain that all specimens used are brought to room temperature and mixed
well before aspiration.
Observe Instrument Operation and record information in the table below while precision
analysis is running:
CLOSE MODE OBSERVATION
Component/Function Observation
In closed mode, observe rack Do the racks move smoothly or jerkily?
movement, sample mixing, tube
spin and aspiration
How many times are specimen tubes mixed?
How fast is the tube spun?
Does the blood travel through the Ultrasonic Sensors or the
Optical Blood Sensor?
Note length of blood sample extension going into and out from
the Shear Valve prior to rotation:
Record your Closed precision check results in the table below:
Parameter %CV Limit Your %CV Results Met Specification
Yes No
WOC < 2.4%
NOC < 2.8%
RBC < 1.8%
HGB < 1.4%
MCV < 0.8%
PLT < 3.8%
If any precision results are out of specification describe the corrective action that you
would take to resolve.
3-14 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Basic Operation
Perform a Calibration using the Auto-Calibration Wizard, note the Notes
following:
• For Training Purposes Only:
• BEFORE pressing the Finish Bias Check button, review
your data with the instructor.
NOTE:Beginning with Ruby Software version 2.0 and higher
the Bias Check can be performed separately from a
calibration.
Once your calibration has been successfully completed, print a
report
END OF ACTIVITY
CELL-DYN Ruby® Field Service Training Workbook 3-15
204343-101 Dec 2008
Module Summary
Notes
Module Summary
Now that you’ve completed the Basic Operation Module, you
should be able to identify Normal operating conditions on the
CELL-DYN Ruby System. You should also be able to perform
maintenance procedures, sample analysis, open and closed mode
precision and calibration.
3-16 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Review
Notes
Review
1. How many reagents are used on the CELL-DYN Ruby System to
perform a CBC?
List the Reagents and their basic function:
2. During normal operation, while the instrument is idle and in the Ready
state, the vacuum and pressure pumps cycle on/off every _________
seconds.
3. Where is bubble mix used on the CELL-DYN Ruby Flow Panel AND
what does normal bubble-mix look like?
4. What is the HGB Heater operating range?
5. What is the WOC Heater operating range?
6. Describe the process for preparing whole blood for a closed mode
precision.
CELL-DYN Ruby® Field Service Training Workbook 3-17
204343-101 Dec 2008
Review
7. Complete the Table by recording either a Calculated (C), Measured (M) or Derived (D) next
to each parameter.
White Blood Cell Parameters Red Blood Cell Parameters
WBC: White Blood Cell Count (WOC) RBC: Red Blood Cell Count
NEU: Neutrophil Absolute Count MCH: Mean Cell Hemoglobin
LYM: Lymphocyte Absolute Count MCHC: Mean Cell Hemoglobin Concentration
MONO: Monocyte Absolute Count HCT: Hematocrit
EOS: Eosinophil Absolute Count MCV: Mean Cell Volume
BASO: Basophil Absolute Count RDW: Red Cell Distribution Width
%N: Neutrophil Percentage of WBCs HGB: Hemoglobin Concentration
%L: Lymphocyte Percentage of WBCs Reticulocyte Package
%M: Monocyte Percentage of WBCs %R: Percentage of Reticulocytes
%E: Eosinophil Percentage of WBCs RETC: Reticulocyte absolute concentration
%B: Basophil Percentage of WBCs Platelet Parameters
PLT: Platelet Count
MPV: Mean Platelet Volume
PCT*: Plateletcrit
PDW*: Platelet Distribution Width
*Clinical significance has not been established for PCT or PDW. Therefore, they are not reportable in the US.
End of Module
3-18 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 4: Vacuum/Pressure & Fluidics
This module provides an overview of two of the CELL-DYN Ruby System Subsystems. This
module introduces the principles, and procedures associated with:
• Vacuum and Pressure SubSystem
• Fluidics SubSystem
• Normal Operation
• Service Procedures
Vacuum Pressure Assembly
Accumulator
Pressure
Pump
Shear Valve Assembly
Vacuum
Pump
Y-Valve
Open Mode
Probe
Solenoid
Closed Mode
Probe
Flow Panel - Aspiration
CELL-DYN Ruby® Field Service Training Workbook 4-1
204343-101 Dec 2008
Notes
4-2 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Objectives
Notes
Objectives
After completing this module, you should be able to:
• Use the diagram of the CELL-DYN Ruby System Vac-
uum/Pressure Assembly to describe the operation of
the Vacuum/Pressure subsystem and describe how flu-
ids are moved
• List 3 Pressure accumulators, their pressure rating and
function
• List 2 Vacuum Accumulators, their rating, and function
• Remove and Replace the Vacuum Pressure Assembly
• Identify Key Components of the Vacuum/Pressure sys-
tem
• Perform Verification Procedures to assure that the Vac-
uum/Pressure system is working correctly
• Perform Temperature Control Module Adjustment
(VP-36)
• Use the CELL-DYN Ruby System Color Flow Diagram
and blue dye to locate and identify pathways, syringes
and valves in the fluidics subsystem
All procedures should be performed in accordance with
specifications outlined in CELL-DYN Ruby Operator’s Manual,
CELL-DYN Ruby Service and Support Manual and/or ISAs and
TSBs.
CELL-DYN Ruby® Field Service Training Workbook 4-3
204343-101 Dec 2008
Vacuum & Pressure
Vacuum & Pressure
Your instructor will discuss the Vacuum/Pressure Subsystem using the diagram located on the
following page.
The main purpose of the CELL-DYN Ruby’s Vacuum/Pressure Subsystem is to move the fluids
throughout the analyzer.
The components of the Vacuum/Pressure Subsystem are also used to:
• Monitor all levels of pressure and vacuum in the analyzer.
• Control the opening and closing of all solenoids associated with the vacuum and pres-
sure subsystem.
• Interface with the Data Module to display Vacuum/Pressure levels and receive the refer-
ence voltage from the Data Module for control of vacuum and pressure levels.
Components Description/Function
Two DC Pumps • One Vacuum and One Pressure pump.
• +28VDC from Switching Power Supply via Power Distribution Module (PDM).
• ON/OFF Control is performed by PRM via signal from VPM.
• PRM is fused.
• Each pump draws 15 amps at start-up, a 0.1 second delay is performed between
the two pumps to protect from circuit overload.
Three Pressure • Enclosed container that acts as a pneumatic storage device.
Accumulators
• Each container stores a different level of pressure (13, 9 and 4.25 PSI.)
Two Vacuum • Enclosed container that acts as a pneumatic storage device.
Accumulators
• Each container stores a different level of Vacuum (13” and variable).
• An error, “Accumulator wet”, will display if fluid collects in either vacuum accumulator.
• Liquid is detected when the level reaches a set of liquid sensing conductors that
are connected to the VPM.
• Liquid must be evacuated before the error message can be cleared.
• The liquid can be automatically drained.
• Each vacuum accumulator has a drain line at bottom of the bottle. The line is
connected to the system and is used to automatically drain liquid from the
accumulator.
• The liquid can also be manually drained.
• A drain line, located on the left side of the flow panel can be used to manu-
ally drain accumulator.
When troubleshooting Accumulator Wet errors, verify whether or not the accumulator contains
any fluid. If fluid is present, consider the following:
• Vac accumulator #1 wet: inspect WC #3 & #4, exercise valves 32 & 98, inspect tubings.
• Vac accumulator #2 wet: inspect WC #2,exercise valve 18 inspect associated tubing.
• Inspect Reagent Reservoirs for cracks. Inspect associated tubing and valves.
• Verify readings in Digital Voltage Readings screen.
• Use Hemostats to isolate problem.
4-4 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Vacuum & Pressure
The Vacuum and Pressure Subsystem
Pressure Function Vacuum Function
13 PSI • Used to generate other pressures 13” HG • Reagent Fill
• Sample Loader • Waste Fill
• Pressure to Waste Chambers for emptying. • Sample Loader
9 PSI • Laminar Flow Variable • Sample Aspiration
• Pressurizes Diluent/Sheath (noisy) Reservoir 2
• Flushing flow cell
4.25 PSI • Agitate/mix the sample in preparation for staging
• Induced into bottom of each Mixing Chamber
(RBC/PLT, HGB/NOC and WOC)
• Pressurizes Diluent/Sheath (quiet) Reservoir 1
and WBC Lyse Reservoir
• Flushing and Rinsing functions throughout system
Electronics
VPM • Controls Vacuum/Pressure Levels PRM • Pumps ON/OFF
If you suspect a defective VPM Pressure Sensor, check the ohm reading using a digital voltmeter.
• Attach the DVM leads between pins 2 and 4 on the pressure sensor (the pins are located just below the
sensor). A reading of greater then 4.8 kOhms may indicate a bad sensor. If you suspect a bad sensor
replace the board. Most sensors will read in the 4.0 to 4.5 kOhm range.
CELL-DYN Ruby® Field Service Training Workbook 4-5
204343-101 Dec 2008
Activity - Vac/Pres
Activity - Vac/Pres
Time to complete: 30 minutes
Activity Instructions
In this activity you will:
• remove Vacuum Pressure Assembly RR E 1.01 and locate components
• perform Vacuum and Pressure VP-15, VP-4 and VP-16
Resources Needed
The instructor will provide hemostats for you to use during this activity.
In this session you will be performing service procedures. Refer to the CELL-DYN
Ruby System Service and Support Manual Removal and Replacement, and
Verification Procedure Sections for instructions.
Perform the following:
Remove the Vacuum Pressure Assembly (R&R E1.01)
• Notify Instructor once Vacuum Pressure Assembly has been removed.
Locate the components listed below and present a review of the Assemblies Compo-
nents and functions to the instructor:
3 Pressure Accumulators
2 Vacuum Accumulators
Vacuum and Pressure pumps
VPM
PRM
Multi-Port Coupler and Manual Drain Lines
• Answer the review questions at the end of this module while awaiting your groups
turn with the instructor
Perform VP-15 Vacuum & Pressure Retention Verification
Perform VP-4 VPM Reference Voltage Verification/Adjustment
Perform VP-16 Vacuum & Pressure Level Verification/Adjustment
NOTE: The Vacuum & Pressure Recovery diagnostic is only for use on the CD3200
System it does not work on the CELL-DYN Ruby System.
END OF ACTIVITY
4-6 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Fluidics
Fluidics
Your instructor will discuss the Fluidics Subsystem using the flow diagram and flow panel
diagrams previously distributed. It is important to correlate the Flow Panel components to the
aspiration, dilution, staging, measurement and waste pathway for RBC/PLT, WBC and HGB.
The CELL-DYN Ruby’s Fluidic Subsystem components control the flow, pathway, quantity and
type of fluids in use. Key fluidic processes include, specimen aspiration, sample dilution and
mixing, waste management, and flushing.
Aspiration Separation Dilution
Analysis
Measurement Results
of Data
The status sensor subsystem provides the main computer with the status of the system
mechanical, electronic and fluidic functions. The subsystem uses optical sensors, and ultrasonic
sensor and fluid sensors to detect various system conditions.
CELL-DYN Ruby® Field Service Training Workbook 4-7
204343-101 Dec 2008
Fluidics
STEPS EVENT
The sample is aspirated. Blood is aspirated through the Shear Valve using variable vacuum. The variable
vacuum uses two levels of vacuum, one for the Open Mode and one for the
Closed Mode.
• Open Mode is used to aspirate the blood from a collection tube that has
been opened and is held under the open mode probe.
• Closed Mode is used to mix and then aspirate the blood directly from a
closed collection tube by piercing the tube stopper.
Sensors check the integrity of To detect a short sample condition during sample aspiration two ultrasonic
the aspirated sample sensors (S1, S3) and one blood sample detector (S2) are employed in the
sample aspiration flow system.
• S1 controls the leading edge (open & closed mode) during aspiration.
• S3 controls the leading edge (open mode) during transfer. These sen-
sors detect the presence or absence of liquid in the aspiration line, and
they are not adversely affected by the density or viscosity of the sample.
• Blood sample detector (S2) controls the leading edge (closed mode) dur-
ing transfer.
Separation The Shear Valve rotates to isolate three segments of the blood sample:
RBC/PLT = 1.67µL WBC = 20µL HGB = 12µL
Dilution and Mixing To begin the dilution process, the blood segments are picked up at the Shear
Valve by reagents delivered through syringes, the segments are then directed to
their respective dilution/mixing chambers where the final dilution is prepared:
• RBC/PLT is diluted with Diluent/Sheath
• HGB is diluted with Diluent/Sheath and HGB Lyse
• WBC is diluted with WBC Lyse
The input ports in the dilution cups are oriented so that the whole blood and
reagent swirl when injected by the syringes. This swirling action along with
Bubble mix is used to mix the reagent and whole blood, resulting in a
homogeneous final dilution.
Staged for Optical After dilution and mixing, the RBC/PLT, WBC and NOC samples must be staged
Measurement before processing through the Optical Flow Cell. The staging is performed by a
peristaltic pump through valve 5-2:
• RBC/PLT is staged first through valve 5-4
• NOC is second through valve 4-1
• WBC is third through valve 5-5
After staging, the sample dilution is injected into the Optical Flow Cell by the
Sample Injection Syringe.
Laminar Flow and Optical Laminar flow describes the flow properties of two liquids moving at different rates
Measurement of speed in the same direction without intermingling.
Diluent/sheath (Reservoir 2 through valve 6-5) is forced into the outer area of the
flow cell by 9 psi. The pressure hydrodynamically focuses the sample stream
aligning the blood cells in single file through the sensing region.
Flushing and Waste At the end of the measurement cycle, the CELL-DYN Ruby System flushes and
drains the system components. There are four waste chambers that perform the
draining and collection of waste from various components.
• 12” Hg (#1) vacuum level is used to pull fluids into the reagent reservoir
and waste chambers
• 12 psi pressure used to empty chambers
4-8 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Fluidics & Temp
Activity - Fluidics & Temp
Time to complete: 25 minutes
Debrief Session: 10 minutes
Activity Instructions
In this activity you will:
• use dye to trace RBC/PLT, WBC and HGB pathways
• perform Temperature Control Module Adjustment VP-36
• complete Case Study 2
Instructor Debrief/Review:
At the conclusion of the activity, your instructor will lead a group review calling on stu-
dents randomly for their observations.
Resources Needed
In this session utilize the Flow Diagrams you have been provided in class.
The instructor will provide blue dye for you to use during this activity.
Refer to the CELL-DYN Ruby System Service and Support Manual, Verification Pro-
cedure Section for information on performing the procedures listed on the following
page.
CELL-DYN Ruby® Field Service Training Workbook 4-9
204343-101 Dec 2008
Activity - Fluidics & Temp
Notes Perform the following:
WARNING: Splash Spray Hazard.
Aspirate dye and observe as the colored solution proceeds
through the fluidics system.
Observe the pathways for RBC/PLT, WBC and HGB.
Review the pathway using your Color Flow Diagram.
Identify the key valve numbers and syringe(s) used for each
sample stream path and record in the table below.
Sample Key Valves Syringe(s)
Stream Mixing Staging Laminar Flushing
Flow
RBC/PLT
HGB
WBC
Perform VP- 36 Temperature Control Module (TCM)
Adjustment Procedure. Record Voltages Below:
R4 ________ (Reference Voltage)
R7 ________ (For 45oC Setting)
R14 ________ (For 25oC Setting)
NOTE: The TCM controls and drives the WBC and HGB
Heaters.
Locate the following 5 LEDs on the Temperature Control Module
and record LED Status (ON or OFF):
DS1 ________ (ON = HGB heater is energized)
DS2 ________ (ON = WOC heater is energized)
DS3 ________ (ON = HGB Heater temp. is out of range)
DS4 ________ (ON = WOC Heater temp. is out of range)
DS5 ________ (ON = Power Supply 28V is alive)
END OF ACTIVITY
4-10 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Case Study 2
Notes
Case Study 2
A CELL-DYN Ruby System has HGB Heater errors on all
specimen analysis including background counts. The following
troubleshooting steps were performed or observed, and the issues
remain unresolved:
• Customer has cycled power
• Auto Clean was performed
• The tubing to HGB Heater was reseated
• The HGB Heater feels warm to the touch
Using your knowledge of the STEP Process, of normal instrument
operation, and your troubleshooting resources (Troubleshooting
Information Database [eSolutions], TSBs/ISAs, etc.); What
procedures, checks, measurements, etc. would use to isolate the
root cause of this error?
What would you do to resolve this error?
CELL-DYN Ruby® Field Service Training Workbook 4-11
204343-101 Dec 2008
Module Summary
Notes
Module Summary
Now that you’ve completed the Fluidics & Vacuum/Pressure
Module, you should be able to identify the operating conditions of
these subsystems on the CELL-DYN Ruby System. You should
also be able to perform key Service and Support procedures and
use diagrams to relate subsystem function.
4-12 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Review
Notes
Review
1. Why do the vacuum and pressure pumps cycle so frequently?
2. What screen contains the Vacuum/Pressure Readings?
3. True or False
The PRM PCB receives +28 VDC from the PDM J20 to operate
the pumps.
4. What are the 3 Pressure levels?
5. Describe how the pressure system is used to maintain Pressure 3.
6. Where is the Multi-Port (Quick Disconnect) Coupler located on the
Pneumatic Unit?
CELL-DYN Ruby® Field Service Training Workbook 4-13
204343-101 Dec 2008
Review
Notes
7. Which waste chamber has a Bubble Trap to prevent residual waste
from entering the vacuum accumulator?
8. There are five (5) ports on the HGB Flow Cell, which port is used to
deliver sample into the flow cell?
9. The HGB Heater is used to heat which two (2) reagents?
End of Module
4-14 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 5: Optics
This module provides an overview of the CELL-DYN Ruby System Optics Bench and Optical
Measurement Subsystem. This module introduces the principles, and procedures associated
with:
• Optics Bench
• Service Procedures
• Data Interpretation and Flagging
• Moving Average Program
CELL-DYN Ruby Optical Bench
90o Scatter
0o Scatter (Cell Lobularity)
(Cell Size)
10o Scatter 90oD Scatter
(Cell Granularity)
(Cell Complexity)
CELL-DYN Ruby® Field Service Training Workbook 5-1
204343-101 Dec 2008
Notes
5-2 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Objectives
Notes
Objectives
After completing this module, you should be able to:
• Identify key components of the Optics Bench and Opti-
cal Measurement Subsystem
• Demonstrate Optics Bench alignment procedures
• Perform Extended Mode Diagnostics
• Use the Auto-Gain Wizard to adjust gains
• Describe how the CELL-DYN Ruby System, measures
and analyzes a sample. Explain the process for any
WBC or RBC/PLT parameters
• Recognize the measured parameter found within RBC
indice calculations
• Recognize parameters included in Moving Average
Program and locate program data stored within the soft-
ware for the purpose of evaluating instrument perfor-
mance
All procedures should be performed in accordance with
specifications outlined in CELL-DYN Ruby Operator’s Manual,
CELL-DYN Ruby Service and Support Manual and/or ISAs and
TSBs.
CELL-DYN Ruby® Field Service Training Workbook 5-3
204343-101 Dec 2008
Optics
Optics
The purpose of the CELL-DYN Ruby’s Optical Measurement Subsystem is to count, classify and
size blood cells.
Staging
The measurement process begins with the Sample Dilution being “staged” by the Sample
Aspiration Pump through valve 5-2 to an Optical Flow Cell within the Optics Bench.
Sample dilutions are staged in the following order:
• RBC/PLT dilution is staged first through valve 5-4
• NOC dilution is staged second through valve 4-1
• NOC is not ran unless selected/configured by the operator or processing in FWBC mode
• The WBC dilution is staged last through valve 5-5
Analysis
Next, the cells within the sample dilution are injected into the Optics Bench where they are
quantitatively analyzed through the process of Flow Cytometry. In Flow cytometry individual
cells in a single file are passed through a beam of light. A sensor or sensors measure the loss of,
or scattering of, light created by the physical or chemical characteristics of the cells.
The precision of the fluid flow through the Optical Flow Cell is critical to proper cell identification,
as well as proper alignment of the flow cell and bench components with the laser beam.
5-4 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Optics
Key Features Analysis Description
Laminar Flow • Diluent/Sheath enters near the base of the Flow Cell Assembly under a pressure of 9 psi.
• Since the Sample and Diluent/Sheath liquid streams are traveling at different speeds, they
flow alongside one another but do not mix.
Sample Injection • Sample Injection Syringe injects the staged diluted sample into the flow cell.
Hydrodynamic • A cone-shaped flow cell directs the fluid flow through a narrow internal quartz chamber.
Focusing
• The shape of flow cell and flow rate of Diluent/Sheath forces the cells to flow in single file.
Light Beam • A laser beam is positioned by optical bench components to intersect the cells as they pass in
single file through the Optical Flow Cell.
• A helium neon laser is used to generate the beam of light.
• Typically the laser’s power output is >5.0 milliwatts.
• The beam of laser light is vertically polarized.
Light Scatter • As the light strikes the cells it scatters yielding information about the cells characteristics:
• 0o scatter relates to Cellular Size.
• 10o scatter relates to Cellular Complexity.
• 90o scatter relates to Cellular Lobularity.
• 90oD scatter relates to Cell Granularity.
Light Detection • Four Light Detectors collect the light scatter.
• Each of the four light detectors identify light scatter in measures of 0 to 256 light channels
• The more light detected, the higher the channel number recorded, and the more pronounced the
particular cell characteristic.
• Light scatter information is graphically presented in the form of scatterplots and histograms.
• Microspheres (known size) are used to set the detectors (channel sensitivity).
• FL-Cal is used to set gains for RBC Linear MCV Measurement.
• Photo-Diodes detect and amplify the 0o and 10o light scatter.
• Photo-Multiplier Tubes (PMTs) detect and amplify the 90o and 90oD light scatter.
• PMT Pre-amplifiers supply high voltage to PMTs and provide initial amplification of PMT signal.
Laser PS Powers Laser Tube; +1,700 - 3,000 VDC
Laser Beam • The cylindrical lens, forward slit and imaging lens shape and focus the laser beam into a “top
Focusing and hat” profile, which provides a laser beam of uniform intensity.
Alignment
• Mirrors are used to align/position the laser beam.
• The flow cell is also aligned to the laser beam for optimal light scatter.
• Two screws adjust the position of the flow cell:
• The “Y” screw affects the 0o/10o channels.
• The “X” screw affects the 90o/90o depolarized channels.
Moving one adjustment screw can indirectly affect the alignment of other channels.
Measurement • MAM processes signals between pre-amplifiers (PAM), photodiodes and SPM.
(Circuitry)
• SPM detects valid cell pulses, counts valid cell pulses, and captures the peak voltages of
valid cell pulses. Sends data to CPU/DCM.
• CPU/DCM digitizes signals, sorts the pulse signals into one of 256 size channels as list mode
data, and converts the data into a reportable result.
CELL-DYN Ruby® Field Service Training Workbook 5-5
204343-101 Dec 2008
Optics
CELL-DYN Ruby Optical Flow Cell
Note: The Y are the top adjustment screws and the X are the side adjustment screws located on the mirrors.
Note: PMT Dynode Verification and Adjustment (CD3200 Service and Support Manual VP-22)
CELL-DYN Ruby Optics (Laser) Bench Graphic
5-6 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
MEASUREMENT
Laser Bench Alignment Training Aid
Adjustment Tools Setup Action
1 Laser Tube Ruler or other measurement Remove Laser Tube: Laser Tube Positioning:
Positioning device • Disconnect laser connector and short both male pins to • Extend laser body 3/4” beyond front clamp.
instrument chassis. • Oriented laser label up (at top) & Close shutter.
• Remove Laser Clamps & replace Laser. • Plug in laser connector; Turn Inst. power ON.
• Install clamps; tighten until snug, but Laser can still be moved. • Prime analyzer & allow 15 minute warm-up.
• PERORM ACTION. • Fully open laser shutter & verify it is free of debris.
2 Laser Power • Laser Power Meter • Secure power meter detector head into detector mount with Laser Power:
• Detector Mount Assembly locking screw. • Verify Laser Power Reading >3.5mw.
• Place detector mount into tooling holes between the front and • Typically the laser’s power output is >5.0 milliwatts.
rear mirrors (leave in place for next step).
• Set power meter to 20mW scale & turn ON. PERFORM ACTION
3 Verify Laser • Laser Power Meter • Power detector should still be in tooling holes between the front Tube Vertical Polarization:
Tube Vertical • Detector Mount Assembly and rear mirrors. • Rotate Laser tube body to achieve lowest power
Polarization • Polarizer Assembly • Set power meter to 20μw scale. reading (<3mw); Record power reading.
• Allen Wrenches • Place Polarizer Assembly between detector mount & rear mirror. • Tighten mount screws, reverify power reading.
• Slightly Loosen Laser mount screws. PERFORM ACTION. • Power level doesn’t change more than +1 mw after
• Remove all tools from bench when step complete. clamps tightened.
4 Rear Mirror • Alignment Post • Place Alignment Post in front tooling hole between Front Mirror Course Beam Alignment Rear Mirror:
Course • 0.50” Allen Wrench and Cylindrical Lens. • Adjust Rear Mirror to Center laser beam onto alignment
Alignment • PERFORM ACTION. post hole.
• Remove Alignment Post when step complete. • X Screw for Horizontal (left/right).
• Y Screw for Vertical (up/down).
• Laser beam should pass through hole in alignment post.
5 Rear Mirror • Laser Power Meter • Install Power detector into Polarizer assembly. Maximum power through the forward slit:
Adjustment • Detector Mount Assembly • Hang assembly on Forward Slit between slit and Imaging Lens. • Adjust Rear Mirror X screw for maximum beam power.
(X-axis) • 0.50” Allen Wrench • PERFORM ACTION. • Monitor power reading from behind the forward slit.
• Remove all tools from bench when step complete. • > 1/5 Vert. polarization reading.
Post
Mirror
Laser Alignment Tools Laser Power Meter Polarizer Alignment Post Hanging Mount
CELL-DYN Ruby® Field Service Training Workbook 5-7
204343-101 Dec 2008
MEASUREMENT
Laser Bench Alignment Training tool
Adjustment Tools Setup Action
6 Center Beam on • 0.50” Allen Wrench N/A Laser beam centered horizontally on Obscuration Bar
Obscuration Bar • Adjust Front Mirror X screw to center laser beam (white dot) left/
right onto obscuration bar (Y Screw for vertical).
• Re-verify Beam power (see step 5; repeat step 5 as needed).
7 Rear & Front • 0.50” Allen Wrench • MAINTENTANCE VIEW, Select SPECIAL PROTOCOLS, Center Vertically and Horizontally on 90 Degree slit
Mirror Alignment • Syringe EMPTY/FILL OPTICAL FLOW CELL, EMPTY FLOW CELL. • Look for image of flow cell walls projected onto 90D slit (two dots).
(Y-axis) • Digital Volt Meter • Remove PMTs cover and remove pinch tubing from V-56. 1. Adjust REAR Mirror Y screw to vertically center Flow Cell Image
(DVM) • Connect syringe to T fitting at V-54. Inject large air bubble. (dots) on 90D Slit.
• PERFORM ACTION. • Y Screw for Vertical (up/down).
• Reinstall covers, pinch valve tubing V-56 and remove syringe. 2. Verify DVM reading on 0D & 10D Photodiode PCB with Flow Cell
• Select FILL FLOW CELL and run one Prime cycle. in place.
• Set DVM to 300 mV scale and connect to 0D photodiode PCB. • A minimum DMV reading of <100mV.
• Positive lead to TP1 & negative lead to TP2. • Note: A reading of <50mV should be obtained if flow cell
• Verify reading in Specification. is removed from pathway.
• Connect DVM to 10D photodiode PCB TP1 and TP2. • Adjust the front mirror Y for vertical (up/down) as indicated.
• Verify reading in Specification. • Re-verify Beam power (see step 5; repeat step 5 as needed).
• If out of spec., repeat flow cell internal & external cleaning.
8 Optical Flow Cell • Allen Wrenches • Prepare Microsphere Solution: • Refer to CELL-DYN Ruby Service and Support Manual VP-18
Alignment • 7.0 Microspheres • 15 drops 7.0 polymer to 2.0 mL Diluent, Mix. section on Coarse Flow Cell alignment, for instruction.
(Coarse) • Diluent • PERFORM ACTION described in VP-18.
9 Flow Cell • Allen Wrenches • Run microsphere suspension. • Adjust Flow Cell Highest Mean Channel and Lowest CV.
Alignment • EXTENED WBC DIAGNOSTICS, follow on-screen info. • 0/10 use lower (Y) micrometer for tightest pop., and lowest CVs.
• Solution under probe; press START of F8. • 90/90D use (X) micrometer for highest channel and to move
butterfly high and away toward the upper right.
• Fine tuning of flow cell can be achieved by making SMALL
adjustments and reviewing CVs in CALCV screen.
10 Gains and • Microspheres • Complete Gain Adjustment (VP-20). • Refer to CELL-DYN Ruby Service and Support Manual VP-20 for
Verification • Fresh Whole Blood • Run Fresh Whole Blood and verify scattergram output. instruction.
• Control Material • Verify Precision and Control recovery. • Run Whole Blood - look for tight populations, good separation,
proper position of Neutrophil and Lymphocyte Population.
• Verify Precision and Control recovery.
5-8 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Optics
“Normal” WBC Scatterplot Pattern
NEUTROPHILS
MONOCYTES
EOSINOPHILS
LYMPHOCYTES
BASOPHILS
N1 REGION
CELL-DYN Ruby® Field Service Training Workbook 5-9
204343-101 Dec 2008
Activity - Optics
Notes
Activity - Optics
Instructor Demonstration: 60 minutes
Time to complete: 90 minutes
Debrief Session: 15 minutes
Activity Instructions
In this activity each of you will be:
• performing an Optics Bench alignment on a demonstra-
tion bench
• performing validation procedures on the analyzers
Optics Bench
You will have an opportunity later in the class to perform an
alignment on an actual instrument.
First the Instructor will conduct a brief overview of the Bench
Alignment procedure. Refer to the Optics Bench Alignment
Procedure (VP-18) during instructor review.
Resources Needed
In this session you will be performing service procedures.
Refer to the CELL-DYN Ruby System Service and Support
Manual for instruction.
The following items are needed:
1. Optics Bench Alignment Procedure (VP-18) for instruction
NOTE: Review of the CD3000 Series Laser Alignment
training program located on the GSS website is
recommended.
2. Alignment Tools, Microspheres and FL-Cal
3. Proper eye shielding to prevent eye damage from the laser as refer-
enced in the Optics Bench Alignment Procedure (VP-18)
5-10 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Optics
Perform the following: Notes
CAUTION: Class 3B laser light when open. Avoid exposure
to beam.
Perform Optics Bench Alignment Procedure on the demo Optic
Bench (VP-18)
• For Training Purposes Only:
• STOP after Step #4 of the Perform Optical Flow Cell
Alignment (Coarse) section of the procedure
• Notify Instructor for inspection of bench alignment
• After instructor review, if working in a training pair, the
next student should perform VP-18 on the demo bench
up to the step indicated above. Then Notify Instructor
for Review.
• Move to the analyzer and complete VP-18 beginning at Step 1
of Performing Flow Cell Alignment.
• DO NOT adjust the Flow Cell on the analyzer. Notify
Instructor if the Flow Cell is out of alignment
Perform the Auto-Gain Wizard procedure located within the
Systems Gains Verification/Adjustment Procedure (VP-20)
Print the following information:
Normal Patient report containing the 0°/10° and
90°/90°D scatterplots
Print X-B Data
QC View, F5- Moving Average, F8 - Closed Batch Data,
F1-Print
(Use File Print Setup to change paper orientation to Landscape
and File, Print Preview to make sure the printout meets your
labs expectations before selecting F1 – Print.)
NOTE:These printouts will be referenced during the Data
Interpretation and Moving Average instructor led
discussions.
END OF ACTIVITY
CELL-DYN Ruby® Field Service Training Workbook 5-11
204343-101 Dec 2008
Data Interpretation
Data Interpretation
Your instructor will conduct a review of the how the optics counts, classifies and sizes cells. This
will include a review of scatterplots, histograms, test parameters, and flagging.
Multi-Angle Polarized Scatter Separation (MAPSS™)
As previously stated, the CELL-DYN Ruby System uses flow cytometric techniques to analyze
the RBC, PLT, WBC, and NOC populations. It also uses MAPSS™ (Multi-Angle Polarized
Scatter Separation) technology.
MAPSS technology uses various combinations of measurements from four light detectors (0°,
10°, 90°, and 90°D) to classify WBC subpopulations and to provide morphological flagging.
• 0°= size and 10°= complexity
• 90°= lobularity and 90°D= granularity
During the measurement cycle light signals collected by each detector are converted into
electrical signals or pulses. If a pulse falls above the hardware threshold in the 0° and 10°
detectors, the cell counter counts the pulse and stores it for further evaluation. Pulses that fall
below this threshold are not included in the count.
5-12 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Data Interpretation
The pulses are then sorted into light channels ranging from 0 to 256: the more light detected, the
higher the channel number recorded, and the more pronounced the particular cell characteristic.
This data is called list mode data.
For WBC cell subpopulations the system uses all four angles of scatter to distinguish cell types
and classify WBCs into the five subpopulations as shown in the graphic on the proceeding page:
1. First, the data is used to discriminate between Mononuclear and Polymorphonuclear
cells (90°/10°).
2. Then between Polymorphonuclear cells: Eosinophils and Neutrophils (90°D/90°).
3. And lastly between Mononuclear cells: Monocytes and Lymphocytes (0°/10°) and
Basophils.*
4. Optical light scatter is plotted as a scatterplot graph. A scatterplot displays each cell
as a single dot plotted where X and Y axes intersect. The dots are generally color
coded to represent a specific cell population or sub-population.
* Basophils appear among Mononuclear rather than Polymorphonuclear cells because their granules dissolve
in the WBC Lyse reagent; on Scatterplots they appear as degranulated cells.
NOTE:The Red Blood Cell parameters, including Reticulocytes, are determined using three
angles (0°,10° and 90°) of light scatter. Reticulocytes 10° scatter is similar to the
scatter for a mature RBC, but Retics exhibit greater 90° scatter. Platelet parameters
are determined using two angles of light scatter (0°and 10°).
Algorithms, programmed within the software, then determine the WBC count and the percent of
cells in each subpopulation. Once the WBC count is determined, the absolute number of cells in
each subpopulation is calculated by multiplying that WBC count by the percentage.
The Optically measured parameters are:
• WBC Count and five part WBC Cell differential % (Lymphocytes, Monocytes, Neutrophils, Baso-
phils and Eosinophils)
• RBC Count
• PLT Count
• An algorithm analyzes the Histogram to eliminate interference and determine the lower and upper
thresholds for the count. Once thresholds have been determined, the PLT count is derived from 10° data.
Histogram
The CELL-DYN Ruby System generates Histograms from the channel data stored in List Mode.
Histograms represent the frequency and size of cells. The X axis is the size (volume) and the Y
axis is the frequency (count). The CELL-DYN Ruby generates Histograms for WBC, RBC, and
PLT data.
CELL-DYN Ruby® Field Service Training Workbook 5-13
204343-101 Dec 2008
Data Interpretation
Operational Messages and Data Flagging
Operational messages and data flags appear on the Run View screen, on printed reports
and can be transmitted to a laboratory computer system. Messages are divided into the
following categories:
System Messages:
• Fault Conditions
• Status Conditions
System messages are displayed in the view when the instrument detects an
inappropriate condition during specimen processing. When necessary, data is
suppressed.
Parameter (data) Flagging Messages:
• Dispersional Data Alerts
• Suspect Parameter Flags
• Suspect Population Flags
• Interpretive Messages
A parameter flag is a signal or communication to alert the operator that the analyzed
sample does not meet “normal” criteria based on system programmed and/or user-
entered criteria. When there is an increased amount of flagging on “normal” samples, or
abnormal samples are not flagged appropriately, as determined by a review of stained
blood smears, then Customer Support and/or Service may be called to correct the
situation.
For additional information refer to the CELL-DYN Ruby System Operator’s Manual,
Section 3: Principles of Operation, Subsection: Messages and Data Flagging.
5-14 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Data Interpretation
Data Flags: Notes
Some of the more common data flags encountered are:
• Var Lym – variant lymphocytes present
• IG - Immature granulocytes present
• Band - immature Neutrophils present
• NRBC - Nucleated red blood cells present
• Blast - large immature blood cells present
Other flags include the following:
• DFLT – is an indication that there is not a clear separa-
tion between one or more cellular populations.
• FWBC- is an indication that Fragile WBC’s are present
causing an inaccurate WBC count. In such
cases the customer is advised to run the speci-
men in CBC+NOC mode.
• NOC: Nucleated Optical Count is another means of
counting WBC’s using the dilution left over after
HGB measurement.
The CELL-DYN Ruby System utilizes the nuclear
membrane left intact by the 15 second HGB lyse
from the HGB measurement. The leftover sample
is then passed through the Optical Flow Cell and
the nuclei of the cells are counted. Since there is a
1:1 ratio between the WBC count and WBC nuclei,
a correct WBC count is reported
• RRBC- is an indication that lyse resistant RBC’s may be
erroneously affecting the WBC count falsely ele-
vating it. The customer is advised to run the
specimen in CBC+RRBC mode to provide an
additional 15 seconds to lyse the resistant red
blood cells.
CELL-DYN Ruby® Field Service Training Workbook 5-15
204343-101 Dec 2008
Data Interpretation
Notes Interpretive Messages:
Interpretive messages are printed only when the Interpretive
Report option is selected on the Setup, Customize Printed Report
dialog box. The messages appear only on the Chartable Page
graphics report and are generated when the numeric limits entered
in the Patient Limit Sets are exceeded.
• Patient Limit Sets contain specified numerical lower and
upper limits for each parameter.
A listing of possible Interpretive Messages may be found in the
CELL-DYN Ruby Operator’s Manual, Section 3: Principles of
Operation, subsection: Operational Messages and Data Flag-
ging.
Results that fall outside the range of the selected limit set are
displayed in color.
• Yellow indicates that the result exceeded the lower limit.
• Purple indicates that the result exceeded the upper
limit.
• Purple and Yellow results are underlined on the graphic
printouts.
• Results that exceed a parameter’s linear range are indi-
cated by >>>> in place of the result.
• Results that have been determined to require laboratory
validation are indicated by an asterisk [*] next to the
result.
• Results that do not have sufficient data to calculate
values are represented by -------.
Example Data Flags and Colored Results
5-16 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Data Interpretation
Troubleshooting Data Flags
There are several programs are available for monitoring system performance during routine
analysis of patient specimens such as:
• Quality Control File Statistics
• Moving Average Programs including X-B
NOTE:The Moving Average programs on the CELL-DYN Ruby automatically and
continuously monitor instrument performance. This allows identification of
potential problems and more efficient troubleshooting. The programs track the
results of various parameters in the patient population analyzed on the System.
For additional information refer to the CELL-DYN Ruby Operator’s Manual
Section 11 Quality Control.
When troubleshooting data flagging issues, consider the following:
Verify Instrument Function including the following areas:
Fluidics (verify precision, observe dye to verify proper fluid flow, etc.)
Background Counts
Vacuum/Pressure Readings
Voltage Readings
Raw Data Summary
Verify Optics Bench %CV readings and Mean Channels. Clean and Align as indicated.
Note the following:
4095 is the maximum DAC setting on any one angle of scatter
Laser Power must not be less than 3.5mw
Offset values should all be < 1.0
Verify Sample Integrity. Consider the following:
Specimen Stability (Suspect Population Flags may be seen on samples processed more
than 4 hours after collection time.)
Specimen Collection and Mixing
Interfering Substances may be present in specimen
Verify Reagent Integrity.
Verify Site Environmental conditions. Consider the following:
Clearance
Power
Temperature
CELL-DYN Ruby® Field Service Training Workbook 5-17
204343-101 Dec 2008
Data Interpretation
Notes
5-18 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Case Study 3
Case Study 3
A CELL-DYN Ruby has elevated Band/IG Flagging. The Customer has verified that the
flagging is NOT correlating with Manual Blood Smears or an alternate analyzer. The
following troubleshooting steps were performed or observed, and the issues remain
unresolved:
• Customer has cycled power
• Auto Clean was performed
• 7.0 Latex CVs are reporting as follows:
• 0° = 6.2%
• 10° = 5.5%
• 90D° = 14.0%
• 90° = 13.0%
Using your knowledge of the STEP Process, of normal instrument operation, and your
troubleshooting resources (Troubleshooting Information Database [eSolutions], TSBs/
ISAs, etc.); What procedures, checks, measurements, etc. would use to isolate the root
cause of this error?
What would you do to resolve this error?
CELL-DYN Ruby® Field Service Training Workbook 5-19
204343-101 Dec 2008
Case Study 3
Indices
Indices are a group of tests that provide clinically relevant information about the size,
shape and content of Red Blood Cells. They are used for diagnosing anemia.
HCT – Hematocrit: HCT = (RBC X MCV) / 10
Ratio of the volume of Red Blood Cells to plasma. Expressed as a %. Hematocrit is a
calculated parameter.
MCV – Mean Cell Volume
Indicates volume or average size of Red Blood Cells in a specimen. MCV is derived from
directly measured RBC size data from the 0°, 10°, and 90° optical detectors.
The average of the three measurements is the volume for each cell. The measured data
is used to plot the RBC histogram that is used to determine the MCV.
FL CAL is a stabilized RBC preparation that is used to standardize and check the optics
bench of Ruby and 3200. FL CAL is run with known target channels for a known size to
set-up the gains for the RBC Linear MCV measurement. Unless the appropriate gains
are used, the MCV signal will not be linear.
NOTE:FL CAL target channels are subject to change. Always refer to the GSS
website for the latest information.
NOTE: MCVs use Sphere Technology on the CELL-DYN Ruby System. Traditionally
the MCV results form Sphere Technology run higher than those determined
from traditional Impedance Technology.The degree of difference is generally
higher on abnormal samples than normal samples.
MCH – Mean Cell Hemoglobin: MCH = (HGB / RBC) X 10
Expresses the average amount of Hemoglobin in each RBC. MCH is a calculated
parameter.
MCHC – Mean Cell Hemoglobin Concentration: MCHC = (HGB/HCT) x 100
Indicates the overall Hemoglobin concentration in an RBC mass. In other words, the
average “fullness” of each RBC. MCHC is a calculated parameter.
NOTE: The term “fullness” is relative to the average size of an RBC. It indicates how
much of the RBC contains Hemoglobin.
RDW – Red Cell Distribution Width
RDW is a measure of the differences in Red Blood Cell sizes. The bigger the variation in
size, the bigger the RDW value. RDW is represented as a percentage. The RDW is
derived from the RBC histogram after the MCV is determined using the 20th and 80th
percentiles. Basically it represents the width of an RBC Histogram.
5-20 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Case Study 3
Indice Troubleshooting Matrix:
The CELL-DYN Ruby’s X-B Program monitors MCV, MCH, MCHC Indice results. When
the X-B results are out of control, data should be reviewed for shifts and trends in the
results.
If two or more X-B batches are “OUT” that is indicative of systematic error. Systematic
errors require a stepwise troubleshooting approach to isolate the source of the problem.
Since two of the RBC indices included in the X-B program are calculated parameters,
their inter-relationships can be used to assist in troubleshooting. The table below
correlates the X-B Pattern to the relationship of the measured and derived parameters:
X-B Pattern Relationship to Measured and Derived Parameters
If the
If the HGB is: If the MCV is:
RBC Count is:
Calculation
MCH will be: Low High High Low N/A N/A HGB ÷ RBC x 10
MCHC will be: Low High High Low Low High HGB ÷ HCT x 100
or
(HGB x 1000)÷ (RBC x MCV)
MCV will be: N/A N/A N/A N/A High Low Derived from RBC optical data
When troubleshooting RBC Indice issues troubleshoot the measured or derived
parameter and consider the following:
• A change in the MCV value will affect the following indices:
• MCV and MCHC
• MCHC has the MCV in the denominator of the calculation
• A change in the RBC value will affect the following indices:
• MCH and MCHC
• MCH and MCHC has RBC in the denominator of the calculation
• A change in the HGB value will affect the following indices:
• MCH and MCHC
• MCH and MCHC has HGB as the numerator of the calculation
CELL-DYN Ruby® Field Service Training Workbook 5-21
204343-101 Dec 2008
Case Study 3
Notes Moving Average Programs
The Moving Average programs on the CELL-DYN Ruby are
software programs designed to automatically and continuously
monitor instrument performance through statistical analysis of a
batch(s) of patient results for their associated parameters. This
allows identification of potential problems and more efficient
troubleshooting.
The Moving Average Programs and their associated parameters
and measurements include the following:
• X-B Program: MCV, MCH, MCHC
• WBC Program: WBC, %N, %L, %M, %E, %B and popu-
lation statistics (mean) for neutrophils and lymphocytes
• RBC/PLT Program: RBC, RDW, HGB, HCT, MCH,
MCHC, MCV, PLT, and population statistics (mean) for
linear RBC, PLT, and RBC
• RETC Program: %R and population statistics (mean)
for RETC
The Moving Average Programs calculate and monitor means for
their associated parameters in batches of 20 samples. The
calculated mean for each new batch is compared to the target
value and its action limits.
• Upper and lower acceptance limits determine which
patient results are used in a batch. These limits are set
widely to exclude only grossly abnormal specimens.
• The target value, which is the expected mean of the
parameter results, is analogous to the assay value for a
commercial control. Target values are derived from the
patient population analyzed on the System.
• The action limit, which is the acceptable limit of varia-
tion around the target value of the mean for a batch,
expressed as a percentage
Population statistics are used by Abbott field personnel to evaluate
fluidics or other system problems.
NOTE: For additional information on Moving Average
Programs refer to the CELL-DYN Ruby Operator’s
Manual Section 11 Quality Control.
To view Moving Average data go to QC View, F5- Moving Average
and use the F8 - Closed Batch Data view to examine the batch
means.
5-22 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module Summary
Notes
Module Summary
Now that you’ve completed the Optics Module, you should be able
to identify the operating conditions of the Optics Bench and
measurement subsystems on the CELL-DYN Ruby System. You
should also be able to:
• perform key Service and Support procedures
• use diagrams to relate subsystem function
• identify the measured parameter used within RBC
indice calculations
• recognize parameters included in Moving Average Pro-
gram and locate Moving Average program data within
the software for the purpose of evaluating instrument
performance
CELL-DYN Ruby® Field Service Training Workbook 5-23
204343-101 Dec 2008
Review
Review
1. Name the four angles of scatter that are measured on the CELL-DYN Ruby System and
what cell characteristic they are looking at.
a. ______________________________________________________
b. ______________________________________________________
c. ______________________________________________________
d. _______________________________________________________
2. If the RBC Count is increased, the MCH will be ___________ and the MCV will
be____________.
3. If the HGB measurement is low, the MCH will be ___________ and the MCHC will
be____________.
4. If a customer reports that the CELL-DYN Ruby System is reporting numerous Band flags
that are not confirmed by a blood smear. Where would you begin your troubleshooting?
5. What order are samples staged into the Optical Flow Cell?
6. What is an acceptable Laser Power Reading on the CELL-DYN Ruby System?
7. List the software steps required to view the Moving Average Program results.
End of Module
5-24 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 6: Hemoglobin
This module provides an overview of the CELL-DYN Ruby System Hemoglobin (HGB)
Subsystem. This module introduces the principles, and procedures associated with:
• Theory of Operation
• Hemoglobin Components
• Service Procedures
Hemoglobin Flow Cell
CELL-DYN Ruby® Field Service Training Workbook 6-1
204343-101 Dec 2008
Notes
6-2 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Objectives
Notes
Objectives
After completing this module, you should be able to:
• Describe how the CELL-DYN Ruby System measures
Hemoglobin concentration
• Perform HGB Current Verification/Adjustment (VP-5)
• Complete provided case studies with an acceptable reso-
lution
All procedures should be performed in accordance with
specifications outlined in CELL-DYN Ruby Operator’s Manual,
CELL-DYN Ruby Service and Support Manual and/or ISAs and
TSBs.
CELL-DYN Ruby® Field Service Training Workbook 6-3
204343-101 Dec 2008
Hemoglobin
Hemoglobin
The CELL-DYN Ruby System uses a Colorimetric process for analyzing HGB Concentration.
STEPS EVENT
Heater A Hemoglobin Heater (set to 45°C ±0.5°C) Heats the Diluent/Sheath that is used in
preparing the HGB dilution.
Sample Dilution To Make the Hemoglobin Dilution:
• The Diluent/Sheath syringe pushes the HGB sample from the Shear Valve
with Diluent/Sheath into the HGB Mixing Chamber.
• The HGB Lyse Syringe injects HGB Lyse into the HGB Mixing Chamber.
• The System bubble mixes the fluids. During mixing, the HGB reagent lyses
the RBCs and reacts with the hemoglobin to form a stable chromagen. The
nominal temperature of the dilution is approximately 32°C (89.6°F).
• The HGB Mixing Chamber is also the HGB Flow Cell. The HGB sample
remains in the mixing chamber until sample measurement.
Measurement The HGB Flow Cell is a light tight enclosure. It uses an LED (Light Emitting Diode)
monochromatic light at 555nm to pass through the solution.
A photo detector collects the light that passes through.
The Higher the concentration of solution, the more light absorbed, so the lower the
reading.
Readings Five separate readings are made on each sample. The lowest and highest readings
are eliminated, and the three remaining readings are averaged to give a final result.
• The sample enters through the top front port of the HGB Flow Cell
Five readings are taken on a Diluent/Sheath blank. The lowest and highest readings
are eliminated, and the three remaining readings are averaged to give a final result.
• Diluent/Sheath is supplied through valves 9-4 and 9-5.
The system compares the two readings (reference and sample) to determine the
HGB concentration of the sample.
Raw Data The sample and hemoglobin reference readings are displayed on the RAW DATA
SUMMARY screen. When viewing those readings, note the following:
• Bubbles in the flow cell can yield a very low, or very high reading.
• Average Sample Raw Data reading is ~700.
• The Reference Reading is 2050 + 200.
• On a background count, both the sample and reference readings should be
similar numbers.
Be sure to press STOP before exiting RAW DATA SUMMARY Screen.
Electronics The FCM (Flow Control Module) supplies the 5V drive voltage for the LED located in
the HGB Flow Cell. The output signal from the HGB Flow Cell photo detector is
amplified by the FCM and sent to the CPU/DCM.
6-4 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Hemoglobin
Hemoglobin Flow Cell Graphic
Hemoglobin Circuitry
CELL-DYN Ruby® Field Service Training Workbook 6-5
204343-101 Dec 2008
Activity - Hemoglobin
Notes
Activity - Hemoglobin
Time to complete: 15 minutes
Activity Instructions
In this activity you will:
• perform Hemoglobin Current Verification/Adjustment
Then you will practice using your knowledge by performing a case
study that follows this activity.
It is important to know how the CELL-DYN Ruby System measures
Hemoglobin and how to verify proper function.
Finally, you will complete a case study that will test your knowledge
of hemoglobin measurement on the CELL-DYN Ruby System.
Note the following:
• Customers commonly use a “RULE OF THREE” to check
to see if their Complete Blood Counts results are valid.
• The RULE OF THREE states that HGB X 3 = HCT (+/-3).
• The rule only applies to normal patients with a normal
MCV and MCH value.
• When referring to a failure of this rule, customers gener-
ally state their H&Hs (Hemoglobin and Hematocrit) are
not matching.
• When Troubleshooting H&Hs not matching remember
that Hemoglobin is directly measured and Hematocrit is
calculated.
• Also when troubleshooting H&Hs not matching, verify the
HGB Reference reading is within specification.
Resources Needed
In this session you will be performing service procedures.
Refer to the CELL-DYN Ruby System Service and Support
Manual Verification Procedures for instructions.
Perform the following:
VP-5 Hemoglobin Current Verification/Adjustment
END OF ACTIVITY
6-6 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Case Study 4
Notes
Case Study 4
How do you know if the Hemoglobin measurement is occurring
correctly? List at least 3 ways:
1.
2.
3.
CELL-DYN Ruby® Field Service Training Workbook 6-7
204343-101 Dec 2008
Module Summary
Notes
Module Summary
Now that you’ve completed the Hemoglobin Module, you should be
able to identify the operating conditions of the Hemoglobin
Subsystem. You should also be able to perform routine Service
and Support procedures and repair.
6-8 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Review
Notes
Review
1. Where are the HGB Reference Readings located and what is a normal
reading?
2. What reagent is used during the HGB Reference Read?
3. According to the Cable Connection Diagram, what board supplies LED
current the HGB Flow Cell?
4. The higher the sample reading the ______________ the concentration
of HGB in the sample.
What is a typical Sample Reading?
5. The sample enters the HGB Flow Cell through which line (top or side)?
6. Where are the voltage readings for the Hemoglobin Flow Cell located?
End of Module
CELL-DYN Ruby® Field Service Training Workbook 6-9
204343-101 Dec 2008
Review
Notes
6-10 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 7: Electronics and Power
This module provides an overview of two of the CELL-DYN Ruby System SubSystems. This
module introduces the principles, and procedures associated with:
• Electronics SubSystem
• Power SubSystem
• Maintenance
• Service Procedures
Laser Power Fluid Control Module Solenoid Drive Modules
Supply
CELL-DYN Ruby Left Side Internal View Cable Distribution
Module 2
CELL-DYN Ruby® Field Service Training Workbook 7-1
204343-101 Dec 2008
Objectives
Notes
Objectives
After completing this module, you should be able to:
• Use cable the Cable Connection Diagram to locate key
PCBs (Printed Circuit Boards) and record a basic func-
tion for each
• Use the Cable Power Block Diagram to locate the 4
power supply assemblies and list the voltages gener-
ated by each
• Verify system voltage by using VP-1
All procedures should be performed in accordance with
specifications outlined in CELL-DYN Ruby Operator’s Manual,
CELL-DYN Ruby Service and Support Manual and/or ISAs and
TSBs.
7-2 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Electronics
Notes
Activity - Electronics
Time to complete: 25 minutes
Debrief Session: 10 minutes
Activity Instructions
In this activity you will:
• locate key PCBs (Printed Circuit Boards)
• review PCB function using the Cable connection dia-
gram
To understand the overall system operation, it is important to
understand the devices that control, monitor, and power the
functional subsystems.
Circuit Boards can be classified by their functionality, location
and/or the analyzer subsystem for which they belong.
Board classifications include:
• Miscellaneous Boards
• Measurement and Motion Control Boards
• Motor and Valve Control Boards
• Sensor Processing Boards
Communication between boards take place along data and control
busses.
During the module debrief, your instructor will call upon you to
demonstrate the location of an assigned board and it’s function.
Resources Needed
In this session you will be locating components. Refer to the
CELL-DYN Ruby Service and Support Manual, General Data
Section for additional information.
CELL-DYN Ruby® Field Service Training Workbook 7-3
204343-101 Dec 2008
Activity - Electronics
Perform the following:
Locate Printed Circuit Boards (PCBs) identified in the following tables and review their
function using the Cable Connection Diagram:
Component Function Notes
Measurement and Motion Control Boards
CPU/DCM PCB • Serial communication via High Speed Load HSSL com when board
Serial Link (HSSL) to the Data Module replaced (VP-51)
Single Board Computer
Signal Processor • Detects and counts valid cell pulses Failure often represents as a loss of
Module (SPM) • Sends optical data to CPU/DCM for signal and/or unable to adjust gain.
processing
Main Amplifier • Processes optical channel signals
Module (MAM) between pre-amplifiers and SPM PCB
• output signals from the four optical
channels are amplified and sent to
SPM
PMT Pre- • The pre-amplifiers provide electrical
Amplifiers pulses that represent the light scat-
tered when a cell passes through the
laser beam
• PMT preamplifier supplies the high
voltage (VDYN) to the PMT
• Photo-multiplier tube channels (90° or
90°D), gain can be changed by vary-
ing the dynode voltage (supply voltage
to the PMT) on the preamplifier mod-
ule, or by changing the gain on the
Main Amplifier Module (MAM)
Photo-Diode • A photo-diode in each of the preampli-
PCBs fiers provide initial detection and
amplification of the forward scatter
channels
Motor Processor • Sends power, speed and direction to
Module (MPM) stepper drivers
Solenoid Control/ • Seven SDMs
Solenoid Drive • Provide drive and current to open and
Modules (SDM) close solenoids
• LEDs
Pump Relay • Supplies 28VDC to Vacuum/Pressure
Module (PRM) Pumps
• Turns Pumps ON and OFF
7-4 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Electronics
Component Function Notes
Measurement and Motion Control Boards (continued)
Shear Valve Driver • Motion control of the Shear Valve Assembly
Module
Motor Drive Module • Drives Y-valve motor to rotate valve open
(MDM) during closed mode of aspiration
Sample Handler • Performs the same functions as the MPM SHM boards are
Module (SHM1 and and stepper drivers for the aspiration probe interchangeable. Ensure jumper
SHM2) up/down motor is set properly per board
• Drives the DC barcode spin motor location.
• Controls the Aspiration Tower
• Sample Loader Controller
Miscellaneous Boards
Cable Distribution • Two CDMs
Module (CDM) • Distribute +28vdc and +15.5vdc voltages to
solenoids
• Collects reagent and waste sensor informa-
tion for distribution to FCM
Vacuum Pressure • Controls Vacuum/Pressure Levels
Module (VPM) • Sends signals to turn vacuum/pressure
pumps ON or OFF
CELL-DYN Ruby® Field Service Training Workbook 7-5
204343-101 Dec 2008
Activity - Electronics
Component Function Notes
Sensor Interface Boards
Flow Control Module • Interface and control functions No LEDs
(FCM) • HGB Voltage
Temperature Control • Control and drive WBC and HGB Heaters 5 LEDs:
Module (TCM) • Takes approximately 6 minutes to stabilize • DS1: HGB heater is energized
• During Standby the heater elements are when LED is ON
disabled • DS2: WOC heater is energized
when LED is ON
• DS3: HGB Heater temp is out of
range when LED is ON
• DS4: WOC Heater temp. is out of
range when LED is ON
• DS5: Power Supply 28V is alive
with LED is ON
Reagent Sensor • In-line Reagent Fluid Detection
Board (RSB)
Sample Handler • Provides the LED drive and reads the out-
Module2 (SHM2) puts of the photo-detectors of the optical
sensors in the Sample Loader
• Controls five three-way valves
Shear Valve Sensor • Senses ceramic rotation
Board (SVSB)
END OF ACTIVITY
7-6 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Case Study 5
Notes
Case Study 5
You have just received a service call for a CELL-DYN Ruby
System because it is generating error message AIM 0840 Vacuum
Accumulator #1 Wet.
The following troubleshooting steps were performed or observed,
and the issue remains unresolved:
• Customer cycled power
• Customer performed drain accumulator in Special Pro-
tocols and noted no fluid being pushed out through the
waste line during drain accumulator protocol
• Customer tried to manually drain accumulator with a
syringe and no fluid is being pulled out
Using your knowledge of the STEP Process, of normal instrument
operation, and your troubleshooting resources (Troubleshooting
Information Database [eSolutions], TSBs/ISAs, etc.); What
procedures, checks measurements, etc. would you use to isolate
the root cause of this error?
CELL-DYN Ruby® Field Service Training Workbook 7-7
204343-101 Dec 2008
Power Distribution
Power Distribution
Your instructor will discuss the Power Distribution Subsystem using the diagram located below.
The Power Distribution subsystem supplies the Analyzer voltages used for measurement, fluidic
control and mechanical motion control. It consists of:
• Analyzer Power Supply (APS)
• ATX Computer Power Supply (CPS)
• Laser Power Supply (LPS)
• Power Distribution Module (PDM)
CELL-DYN Ruby Power Diagram
7-8 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Power
Activity - Power
Time to complete: 40 minutes
Debrief Session: 10 minutes
Activity Instructions
In this activity you will:
• locate the Power Supply Assemblies and Power Distribution Module
• perform System Voltage Verification (VP-1)
• trace power from the power supply to primary components using the power
block and record information in provided tables
When troubleshooting possible power issues, it is important to have a thorough
understanding of where a particular voltage is utilized and the origin of that voltage.
During the module debrief, your instructor will call upon you to identify the function of
various generated power voltages and to relate these to normal vs. abnormal function.
Resources Needed
In this session you will be locating components. Refer to the CELL-DYN Ruby
Service and Support Manual, General Data Section and Verification Procedure
section for additional information and instruction.
You will also need your Power Block Diagram.
Perform the following:
CAUTION: Electrical Shock Hazard. Follow electrical safety practices.
Locate the following power components:
APS
PDM
ATX CPS
LPS
Perform System Voltage Verification (VP-1)
CELL-DYN Ruby® Field Service Training Workbook 7-9
204343-101 Dec 2008
Activity - Power
Use the Power Block diagram to trace the power distribution to primary instrument com-
ponents. Then record that component and/or function in the table below underneath the
proper voltage and power supply. Two have been provided as an example.
Power Supply Voltages and Distribution Service Notes
Analyzer Power +15.5 VDC
Supply (APS) • Solenoid Activate
+28 VDC
• Solenoid Hold (regulated)
Power Distribution +5 VDC
Module (PDM)
+/- 15VRAW
+/- 15VDC
+/- 12VDC
Power Supply Voltages Distribution Service Notes
ATX Computer +3.3 VDC 5 LEDs on Backplane
Power Supply
(ATX CPS)
+5VDC
+/- 12VDC
Laser Power 1700-3000 VDC
Supply (LPS)
END OF ACTIVITY
7-10 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Case Study 6
Notes
Case Study 6
The Instrument will not power ON, there are no fans running and
the monitor has the dialog box, “check video cable connection”.
The following troubleshooting steps were performed or observed,
and the issues remain unresolved:
• Customer verified the power switch on back of analyzer
is in the ON position
• Customer checked power cord connection between the
instrument and wall outlet
• Customer plugged in an electrical device into wall out-
let. Electrical device is working
Using your knowledge of the STEP Process, of normal instrument
operation, and your troubleshooting resources (Troubleshooting
Information Database [eSolutions], TSBs/ISAs, etc.); What
procedures, checks, measurements, etc. would use to isolate the
root cause of this error?
What would you do to resolve this error?
CELL-DYN Ruby® Field Service Training Workbook 7-11
204343-101 Dec 2008
Case Study 7
Notes
Case Study 7
The instrument you have been called to repair is generating HSSL
Com Communications errors only while performing Extended
Auto-Cleans. Customer cycles power but the issue reoccurs
everytime she performs the extended auto-clean.
Using your knowledge of the STEP Process, of normal instrument
operation, and your troubleshooting resources (Troubleshooting
Information Database [eSolutions], TSBs/ISAs, etc.); What
procedures, checks, measurements, etc. would use to isolate the
root cause of this error?
What seems to be the problem?
7-12 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module Summary
Notes
Module Summary
Now that you’ve completed the Electronic and Power Module, you
should be able to locate key components and identify Normal
operating conditions on the CELL-DYN Ruby System. You should
also be able to perform routine service procedures and system
repairs.
CELL-DYN Ruby® Field Service Training Workbook 7-13
204343-101 Dec 2008
Review
Review
1. If the +5vdc signal were missing on the ATX CPS what type of errors or symptoms would be
seen on the analyzer?
2. What voltages does the APS generate?
3. Temperature Control Module receives ________ from the PDM via connector J15.
4. What voltages does the ATX CPS generate?
5. The _________ PCBs are used to distribute the +28vdc and +15.5vdc from the PDM PCB
that are used to drive the solenoids in the system.
6. The purpose of the______________ PCB is to turn the vacuum and pressure pumps ON/
OFF.
7. Which SHM PCB provides the LED drive and reads the outputs of the photo-detectors of the
optical sensors in the Sample Loader?
8. Describe the function of the SPM PCB.
End of Module
7-14 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 8: Sample Loader
This module provides an overview of the CELL-DYN Ruby Sample Loader. This module
introduces the principles, and procedures associated with:
• Maintenance
• Service Procedures
• Normal Operation
CELL-DYN RUBY Sample Loader and Flow Panel
CELL-DYN Ruby® Field Service Training Workbook 8-1
204343-101 Dec 2008
Objectives
Notes
Objectives
After completing this module, you should be able to:
• Identify Sample Loader components
• Perform Removal and Replacement Procedure F1.01
• Remove the Sample Loader without disconnecting it
from the main analyzer
• Perform VP-22 through VP-31
All procedures should be performed in accordance with
specifications outlined in CELL-DYN Ruby Operator’s Manual,
CELL-DYN Ruby Service and Support Manual and/or ISAs and
TSBs.
8-2 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Sample Loader
Sample Loader
The CELL-DYN Ruby Sample Loader works in conjunction with the Aspiration Tower, which is
physically mounted to the Sample Loader’s base plate.
The Aspiration Tower is intended to detect tube type, spin tube to read bar code label, pierce
tube to vent/aspirate and wash needle after aspiration. The unit works in conjunction with the
sample aspiration subsystem and aspirates the blood sample.
The Sample Loader will maneuver the rack(s) laterally and longitudinally in order to process the
sample tube(s). Additionally, the Sample Loader will detect tube(s) in the rack, mix the sample,
and read rack/tube bar code labels.
The Sample Loader electronics provide communications with, and control of, the various
electronic and mechanical components in the Sample Loader.
The Pneumatics Control Subsystem provides electronic control of the valves supplying pressure
and vacuum to the air cylinders controlling the cross transfer assemblies (sweep arms), rack
advance pawls, and mixer bladders.
Analyzer
Unload Side
Sample Loader
Load Side
Rack Advance
5th Aspiration Position
4th
Mixing Position
3rd Mixing Position
TOP VIEW
4th Tube Sensor 3rd Tube Sensor
CELL-DYN Ruby® Field Service Training Workbook 8-3
204343-101 Dec 2008
Sample Loader
Component Description
Guide System There are two guide systems: GS1 and GS2 that perform the functions of piercing,
Assemblies tube spinning, and cleaning.
• GS1 guide system controls the vent/aspirate needle
• GS2 guide system controls the bar code spinner, wash block, and tube
height flag.
Both guide systems move vertically along two shafts, and are connected together
by a sliding shaft.
Rack Movement • Racks have an orientation groove to prevent them from being incorrectly
placed in the Sample Loader
• The rack at the processing station is mechanically locked in position after each
index step by a spring-loaded detent pin on the Sample Loader wall that
engages a detent in each tube position in the rack. This ensures correct posi-
tioning and avoids the possibility of accidental movement.
• An air cylinder drives a rod with spring-loaded pawls (fingers) mounted along
the length. These fingers engage grooves in the side of the rack through slots
in the rear wall.
• Cross transfer assemblies provide lateral (Y-axis) movement of the racks. The
movement caused by these cross transfer assemblies occurs on the load
(right) side and the unload (left) side of the Sample Loader. An air cylinder is
used to drive the sweep arms together in an equal arc simultaneously.
• Sensors:
• Load Side Empty Sensor
• Unload Side Fourth Rack Sensor
• Unload Side Fifth Rack Sensor
Tube Sensors • Two (2) optical sensors mounted on a PCB to detect the presence of tubes in
the rack.
• The sensors are numbered 4 and 3 (left to right) and indicate the number of
steps that it takes the index pawl to advance to reach each sensor.
• Tube sensors are adjustable.
• The detection of sample tube(s) by these sensors will determine if the mixer
head is lowered to pick up the tubes for mixing. They are also used to deter-
mine an alarm status.
Lateral Indexing
Unload Side Cross Transfer Arms Load Side Cross Transfer Arms
8-4 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Sample Loader
EVENT
Mixing 1. Vacuum is applied:
• and the mixer head is lowered over the sample tubes by the mixer lift air cylinder.
• to the bladders to release the sample tube(s).
2. Pressure is applied to:
• bladders to inflate and capture the sample tube(s).
• mixer lift air cylinder and the mixer head and tubes are raised to clear the rack.
3. The motor:
• rotates the mixer head through at least 15 inversions.
• returns the mixer head to the vertical position.
4. The molded flag on the mixer head body trips an optical sensor, verifying the vertical
position.
When the mixing sequence is completed, the rack indexes one step and the process repeats.
Assuming the sequence starts with the first tube in the rack, each tube receives at least 30
inversions (15 inversions times two mixing positions).
Sample Loader Bladders
CELL-DYN Ruby® Field Service Training Workbook 8-5
204343-101 Dec 2008
Sample Loader
Sample Loader Pneumatics
8-6 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Sample Loader
EVENT
Aspiration Tower • During Sample aspiration GS2 solenoid stop (plunger) and GS1 sensors:
• move down for a pre-determined period of time
• stop moving when the spin cone makes contact with the top of the tube
• GS1 moves the vent/aspirate needle into the tube to a distance dictated by the
type of tube being processed. This pierces the cap and vents the tube to atmo-
spheric pressure and allows sample aspiration to take place.
• after sample aspiration concludes, GS1 moves the vent/aspirate needle up and
the outside of the needle is washed. GS1 continues upward to home position,
while the GS2 solenoid stop (plunger) engages the bottom of GS2 preventing it
from moving down.
• GS1 moves the vent/aspirate needle into the wash block and the vent needle is
rinsed.
• Vacuum #2 (Closed) is applied to aspirate the sample.
• Spin Cone rotates specimen for barcode read to occur.
• Tube height sensors (S1 and S2) are checked to determine the type of tube being pro-
cessed (Standard BD or Sarstedt).
• Diluent/Sheath is used to back flush through the end of the needle, and the wash block
vacuum port collects the waste.
Sample Loader Aspiration Tower
CELL-DYN Ruby® Field Service Training Workbook 8-7
204343-101 Dec 2008
Activity - Sample Loader
Notes
Activity - Sample Loader
Time to complete: 45 minutes
Debrief Session: 10 minutes
Activity Instructions
In this activity you will:
• observe the function of the Sample Loader
• locate and identify components
• perform key R&R and VP procedures
Then you will practice using your knowledge by performing a case
study that follows this activity.
During the module debrief, your instructor will call upon you to
demonstrate the location of an assigned board and it’s function.
Resources Needed
In this session you will be locating components. Refer to the
CELL-DYN Ruby Operator’s Manual, Section 1 Use or
Function and/or the CELL-DYN Ruby Service and Support
Manual, General Data Section for additional information.
Perform the following:
Open the front cabinet and remove covers as directed by your
instructor
Run QC material through the analyzer. During the processing,
observe the following:
Sample Detection
Sample Bar Code Read
Sample Aspiration
Probe Rinse
Specimen replacement
Perform R&R procedure F1.01 Sample Loader
• Remove the Sample Loader without disconnecting it
from the analyzer
As directed by your instructor, perform R&R procedure H1.01
Syringe Drive Assembly
8-8 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Sample Loader
Remove the service panels beneath the loader
Notes
Identify the following components:
Internal Barcode reader
Detent pin assembly
Mixer Head Assembly with home flag/sensor/release
mechanism
• Common source of failure following customer mainte-
nance; realign
Tube detect sensor
Indexer Assemblies with prawls
• Leaks onto prawls can cause them to stick
Removable Cross Transfer Assemblies
Rack Position Sensing
Interlocking Loader Slide Rails
Quick Disconnect Coupler
Pneumatic Control Subsystem with five 3-way valve
bank
• Must be replaced as a bank
Tube Spinner
• Jams and creates bar code read errors
Perform the following VP’s:
VP-22 Aspiration/Vent Needle Verification
VP-23 Tower Unit Stop Solenoid Verification
VP-24 Bar Code Spin Assembly Verification
VP-25 Tube Height Sensors (S1/S2) Verification
VP-26 Mixer Up/Down Verification
VP-27 Mixer Head Rotation Verification
VP-28 Mixer Bladders Verification
VP-29 Rack Advance & Tube Sensors Verification
VP-30 Cross Transfer Arms & Rack Sensors
Verification
VP-31 Mixer Bladders Pressure Verification/Adjustment
END OF ACTIVITY
CELL-DYN Ruby® Field Service Training Workbook 8-9
204343-101 Dec 2008
Case Study 8
Notes
Case Study 8
You have just received a service call for a CELL-DYN Ruby
System because the sweep arm assembly on the Sample
Loader is not pushing racks back on the load side.
The following troubleshooting steps were performed or observed,
and the issues remain unresolved:
• Power to the analyzer has been cycled
• Customer has switched between the open and closed
mode
• Customer has extended the sweep arms manually with
no obstruction found
• Customer has cleaned the sample loader base plate
Using your knowledge of the STEP Process, of normal instrument
operation, and your troubleshooting resources (Troubleshooting
Information Database [eSolutions], TSBs/ISAs, etc.); What
procedures, checks, measurements, etc. would use to isolate the
root cause of this error?
What would you do to try and resolve this error?
8-10 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module Summary
Notes
Module Summary
Now that you’ve completed the Sample Loader Module, you should
be able to locate key components and identify Normal operating
conditions on the CELL-DYN Ruby System. You should also be
able to perform routine service procedures and system repairs.
CELL-DYN Ruby® Field Service Training Workbook 8-11
204343-101 Dec 2008
Review
Notes
Review
1. The Sample Loader uses both vacuum and pressure to fill and empty
the bladders. TRUE FALSE
2. How much pressure is used to inflate the mixer bladders?
What provides it?
3. The GS2 guide system controls what systems on the Sample Loader?
4. Where should you measure the pressure for the Bladder Assembly?
5. What valve on the Sample Loader is used for Rack Advancement?
6. What valve on the Sampler Loader is used to raise the mixer head
assembly?
7. The Sample Loader employs five 3-way valves to drive the air
cylinders and mixer bladders. What PCB controls these valves?
End of Module
8-12 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 9: Troubleshooting Activities
This module provides an opportunity to practice all that you have learned in troubleshooting
situations:
STOP
THINK
EVALUATE
PROCEED
Effective Troubleshooting Model
CELL-DYN Ruby® Field Service Training Workbook 9-1
204343-101 Dec 2008
Troubleshooting Run A & B-Fluidics
Troubleshooting Run A & B-Fluidics
This exercise will be focused upon practicing your skills in determining the root cause of
an instrument error and in using the troubleshooting process.
You will complete two errors that will be related to the fluidics subsystem.
Upon encountering an error state you will follow the Effective Troubleshooting S.T.E.P.
process. Both you and your partner will work through the S.T.E.P. worksheet to isolate
and identify the most likely cause. After you have done this, you will perform any
necessary procedures to return the instrument to a fully functional state.
WARNING: Biohazard. Potential Biohazard, follow biosafety practices.
CAUTION: Moving Parts.
WARNING: Splash/Spray Hazard
In order to complete this exercise you will do the following:
1. Upon detection of an error state, stop. Document the error on the top of the S.T.E.P. work-
sheet.
2. Complete a S.T.E.P. worksheet for each error (A and B) following the Effective Troubleshoot-
ing guidelines throughout your repair.
3. After identifying the root cause of the error, perform the repair, then perform the required ver-
ification procedures and return the instrument to a “Ready” state.
4. Be prepared to share your process with the class. Each group will have a different system
error and will discuss how they arrived at the solution and their process as documented on
the S.T.E.P. worksheet.
9-2 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Troubleshooting Run A & B-Fluidics
The S.T.E.P. Worksheet
STOP Identify the PROBLEM. (List error code or describe problem situation)
THINK List Meaningful DATA such as Abnormal Observations, Test Results, Voltage Readings, LEDs,
Lot numbers, etc.:
Look for COMPARISONS to another system, component, assay, analyzer etc.
Identify assay(s), system(s) and/or similar component(s) where the problem IS and IS NOT.
Problem IS occurring here Problem IS NOT occurring here
List CLUES. (What is unique about the PROBLEM (IS data)?)
• Look for patterns, differences, symptoms, procedure/checks results, etc.
Categorize probable area of failure based on gathered data. Is the Problem related to:
OPERATOR REAGENT ENVIRONMENT ANALYZER (Circle One)
Which instrument system is the most likely area of failure?(check all that apply)
___Fluidics ____ Optics ____ Robotics ____ Temperature Control ____ Power ___Other
EVALUATE Identify possible CAUSES and TEST the cause against the Meaningful Data and Clues. The
cause must explain ALL the data or it is not the root cause of the problem.
List the step(s) used to isolate the cause of the failure?
(Diagnostic Tests, Precision Run, Swap boards, etc.)
P ROCEED List the step(s) used to verify the failure had been resolved?
(List Procedures, Diagnostic tests, etc.)
CELL-DYN Ruby® Field Service Training Workbook 9-3
204343-101 Dec 2008
Troubleshooting Run A & B-Fluidics
Notes
9-4 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Troubleshooting Run A & B-Fluidics
The S.T.E.P. Worksheet
STOP Identify the PROBLEM. (List error code or describe problem situation)
THINK List Meaningful DATA such as Abnormal Observations, Test Results, Voltage Readings, LEDs,
Lot numbers, etc.:
Look for COMPARISONS to another system, component, assay, analyzer etc.
Identify assay(s), system(s) and/or similar component(s) where the problem IS and IS NOT.
Problem IS occurring here Problem IS NOT occurring here
List CLUES. (What is unique about the PROBLEM (IS data)?)
• Look for patterns, differences, symptoms, procedure/checks results, etc.
Categorize probable area of failure based on gathered data. Is the Problem related to:
OPERATOR REAGENT ENVIRONMENT ANALYZER (Circle One)
Which instrument system is the most likely area of failure?(check all that apply)
___Fluidics ____ Optics ____ Robotics ____ Temperature Control ____ Power ___Other
EVALUATE Identify possible CAUSES and TEST the cause against the Meaningful Data and Clues. The
cause must explain ALL the data or it is not the root cause of the problem.
List the step(s) used to isolate the cause of the failure?
(Diagnostic Tests, Precision Run, Swap boards, etc.)
P ROCEED List the step(s) used to verify the failure had been resolved?
(List Procedures, Diagnostic tests, etc.)
CELL-DYN Ruby® Field Service Training Workbook 9-5
204343-101 Dec 2008
Troubleshooting Run A & B-Fluidics
Notes
9-6 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Troubleshooting Run Optics
Troubleshooting Run Optics
This exercise will be focused upon practicing your skills in determining the root cause of
an instrument error and in using the troubleshooting process.
The error in this activity will be related to the optics subsystem. Upon encountering an
error state you will follow the Effective Troubleshooting S.T.E.P. process. Both you and
your partner will work through the S.T.E.P. worksheet to isolate and identify the most likely
cause. After you have done this, you will perform any necessary procedures to return the
instrument to a fully functional state.
WARNING: Biohazard. Potential Biohazard, follow biosafety practices.
CAUTION: Class 3B laser light when open. Avoid exposure to beam.
In order to complete this exercise you will do the following:
1. Upon detection of an error state, stop. Document the error on the top of the S.T.E.P. work-
sheet.
2. Complete the S.T.E.P. worksheet following the Effective Troubleshooting guidelines through-
out your repair.
3. After identifying the root cause of the error, perform the repair, then perform the required ver-
ification procedures and return the instrument to a “Ready” state.
4. Be prepared to share your process with the class. Each group will have a different system
error and will discuss how they arrived at the solution and their process as documented on
the S.T.E.P. worksheet.
CELL-DYN Ruby® Field Service Training Workbook 9-7
204343-101 Dec 2008
Troubleshooting Run Optics
Notes
9-8 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Troubleshooting Run Optics
The S.T.E.P. Worksheet
STOP Identify the PROBLEM. (List error code or describe problem situation)
THINK List Meaningful DATA such as Abnormal Observations, Test Results, Voltage Readings, LEDs,
Lot numbers, etc.:
Look for COMPARISONS to another system, component, assay, analyzer etc.
Identify assay(s), system(s) and/or similar component(s) where the problem IS and IS NOT.
Problem IS occurring here Problem IS NOT occurring here
List CLUES. (What is unique about the PROBLEM (IS data)?)
• Look for patterns, differences, symptoms, procedure/checks results, etc.
Categorize probable area of failure based on gathered data. Is the Problem related to:
OPERATOR REAGENT ENVIRONMENT ANALYZER (Circle One)
Which instrument system is the most likely area of failure?(check all that apply)
___Fluidics ____ Optics ____ Robotics ____ Temperature Control ____ Power ___Other
EVALUATE Identify possible CAUSES and TEST the cause against the Meaningful Data and Clues. The
cause must explain ALL the data or it is not the root cause of the problem.
List the step(s) used to isolate the cause of the failure?
(Diagnostic Tests, Precision Run, Swap boards, etc.)
P ROCEED List the step(s) used to verify the failure had been resolved?
(List Procedures, Diagnostic tests, etc.)
CELL-DYN Ruby® Field Service Training Workbook 9-9
204343-101 Dec 2008
Troubleshooting Run Optics
Notes
9-10 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Troubleshooting Run Temperature, Power and/or
Troubleshooting Run Temperature,
Power and/or Electronics
This exercise will be focused upon practicing your skills in determining the root cause of
an instrument error and in using the troubleshooting process.
The error in this activity will be related to the temperature, power and/or electronic
subsystems. Upon encountering an error state you will follow the Effective
Troubleshooting S.T.E.P. process. Both you and your partner will work through the
S.T.E.P. worksheet to isolate and identify the most likely cause. After you have done this,
you will perform any necessary procedures to return the instrument to a fully functional
state.
WARNING: Biohazard. Potential Biohazard, follow biosafety practices.
CAUTION: Electrical Shock Hazard. Follow electrical safety practices.
In order to complete this exercise you will do the following:
1. Upon detection of an error state, stop. Document the error on the top of the S.T.E.P. work-
sheet.
2. Complete the S.T.E.P. worksheet following the Effective Troubleshooting guidelines through-
out your repair.
3. After identifying the root cause of the error, perform the repair, then perform the required ver-
ification procedures and return the instrument to a “Ready” state.
4. Be prepared to share your process with the class. Each group will have a different system
error and will discuss how they arrived at the solution and their process as documented on
the S.T.E.P. worksheet.
CELL-DYN Ruby® Field Service Training Workbook 9-11
204343-101 Dec 2008
Troubleshooting Run Temperature, Power and/or Electronics
Notes
9-12 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Troubleshooting Run Temperature, Power and/or
The S.T.E.P. Worksheet
STOP Identify the PROBLEM. (List error code or describe problem situation)
THINK List Meaningful DATA such as Abnormal Observations, Test Results, Voltage Readings, LEDs,
Lot numbers, etc.:
Look for COMPARISONS to another system, component, assay, analyzer etc.
Identify assay(s), system(s) and/or similar component(s) where the problem IS and IS NOT.
Problem IS occurring here Problem IS NOT occurring here
List CLUES. (What is unique about the PROBLEM (IS data)?)
• Look for patterns, differences, symptoms, procedure/checks results, etc.
Categorize probable area of failure based on gathered data. Is the Problem related to:
OPERATOR REAGENT ENVIRONMENT ANALYZER (Circle One)
Which instrument system is the most likely area of failure?(check all that apply)
___Fluidics ____ Optics ____ Robotics ____ Temperature Control ____ Power ___Other
EVALUATE Identify possible CAUSES and TEST the cause against the Meaningful Data and Clues. The
cause must explain ALL the data or it is not the root cause of the problem.
List the step(s) used to isolate the cause of the failure?
(Diagnostic Tests, Precision Run, Swap boards, etc.)
P ROCEED List the step(s) used to verify the failure had been resolved?
(List Procedures, Diagnostic tests, etc.)
CELL-DYN Ruby® Field Service Training Workbook 9-13
204343-101 Dec 2008
Troubleshooting Run Temperature, Power and/or Electronics
Notes
9-14 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Troubleshooting Run Sample Loader and Tower
Troubleshooting Run
Sample Loader and Tower
This exercise will be focused upon practicing your skills in determining the root cause of
an instrument error and in using the troubleshooting process.
The error in this activity will be related to the Sample Loader and tower subsystem. Upon
encountering an error state you will follow the Effective Troubleshooting S.T.E.P. process.
Both you and your partner will work through the S.T.E.P. worksheet to isolate and identify
the most likely cause. After you have done this, you will perform any necessary
procedures to return the instrument to a fully functional state.
WARNING: Biohazard. Potential Biohazard, follow biosafety practices.
CAUTION: Moving Parts.
In order to complete this exercise you will do the following:
1. Upon detection of an error state, stop. Document the error on the top of the S.T.E.P. work-
sheet.
2. Complete the S.T.E.P. worksheet following the Effective Troubleshooting guidelines through-
out your repair.
3. After identifying the root cause of the error, perform the repair, then perform the required ver-
ification procedures and return the instrument to a “Ready” state.
4. Be prepared to share your process with the class. Each group will have a different system
error and will discuss how they arrived at the solution and their process as documented on
the S.T.E.P. worksheet.
CELL-DYN Ruby® Field Service Training Workbook 9-15
204343-101 Dec 2008
Troubleshooting Run Sample Loader and Tower
Notes
9-16 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Troubleshooting Run Sample Loader and Tower
The S.T.E.P. Worksheet
STOP Identify the PROBLEM. (List error code or describe problem situation)
THINK List Meaningful DATA such as Abnormal Observations, Test Results, Voltage Readings, LEDs,
Lot numbers, etc.:
Look for COMPARISONS to another system, component, assay, analyzer etc.
Identify assay(s), system(s) and/or similar component(s) where the problem IS and IS NOT.
Problem IS occurring here Problem IS NOT occurring here
List CLUES. (What is unique about the PROBLEM (IS data)?)
• Look for patterns, differences, symptoms, procedure/checks results, etc.
Categorize probable area of failure based on gathered data. Is the Problem related to:
OPERATOR REAGENT ENVIRONMENT ANALYZER (Circle One)
Which instrument system is the most likely area of failure?(check all that apply)
___Fluidics ____ Optics ____ Robotics ____ Temperature Control ____ Power ___Other
EVALUATE Identify possible CAUSES and TEST the cause against the Meaningful Data and Clues. The
cause must explain ALL the data or it is not the root cause of the problem.
List the step(s) used to isolate the cause of the failure?
(Diagnostic Tests, Precision Run, Swap boards, etc.)
P ROCEED List the step(s) used to verify the failure had been resolved?
(List Procedures, Diagnostic tests, etc.)
CELL-DYN Ruby® Field Service Training Workbook 9-17
204343-101 Dec 2008
Troubleshooting Run Sample Loader and Tower
Notes
9-18 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Troubleshooting Run Sample Loader and Tower
Troubleshooting
The following tables contain general tips for troubleshooting failures on a
CELL-DYN Ruby System.
NOTE: When troubleshooting refer to the CELL-DYN Ruby Service and Support
Manual, Technical Service Bulletins, Instrument Service Advisories,
Troubleshooting Information database, and/or the CELL-DYN Operator’s
Manual
PROBLEM Areas of Investigation and possible causes
Sample Loader Faults • Clean sensors, racks, plate, sweep arms and cover.
• Verify that the racks can move right to left without binding:
• Perform adjustments.
• Verify function of Detent pin; remove and clean as needed.
• Use Mechanical Operations to exercise suspect components.
• Verify Sweep arms operation; Clean and adjust as needed:
• Perform VP-30.
• Inspect tubing connections on Manifold Assembly.
• Possible SHM2 failure - replace (Swap with SHM1, set jumpers).
Sample Loader Mixing • Verify Mixhead Home Sensor clean; adjust as needed.
Errors
• Ensure Mixhead Lifter Shaft is clean & dry (NO lubrication).
• Check SL Valves and tubing for obstructions; flush as needed.
Aspiration or Short Sample • Verify Vacuum 2 reading in open, closed and retic modes.
Errors
• Verify VP-4; VPM Reference Voltage in Specification.
• Verify Sample pathway Integrity:
• Check for possible obstruction in Y Valve (unscrew and flush as needed).
• Verify Tubing Integrity (not flared or loose) and all connections tight.
• Check Valve Operation.
• Inspect Ultrasonic Sensor for chipped or broken connectors; replace.
Reagent Detection Errors • Inspect connectors on top of reservoir; clean and adjust as needed.
• Clean Reagent Reservoir - electrodes may be dirty or have build-up.
• Verify Vacuum 1 level within specification.
Blue screen on display and • Possible Hard Drive Failure; Verify voltage on PDM in specification.
unresponsive commands.
• Possible Software Corruption; Reinstall Software.
• Loss of +5 volts:
• Verify voltage at ATX PS.
• Perform VP-01 and verify incoming (wall) power within specification.
HSSL Com errors, Loss of • HSSL Cable loose or not secure.
communication,
• Possible PDM failure:
unresponsive
• +5 Volts low or lacking.
• +15 volts low on test points E1 and E2.
• Perform VP-01 and verify incoming (wall) power within specification.
(Continued)
CELL-DYN Ruby® Field Service Training Workbook 9-19
204343-101 Dec 2008
Troubleshooting Run Sample Loader and Tower
PROBLEM Areas of Investigation and possible causes
Flagging Issues • Verify Sample Staging:
• Integrity of Peri-Pump and Peri-Pump tubing.
• Integrity of fluid pathway (Crimped Tubing, Plaque build-up, etc.).
• Verify valve operation along Count fluid pathway.
• Clean and/or debubble Flow Cell.
• Inspect Optical injection nozzle for protein build up in bottom of the
injection port (clean if indicated).
• Verify Integrity of Sample Injection and Diluent/Sheath Syringe.
• Verify Bench Alignment; check CVs and perform Gain Optimization.
MCV Bias or mismatch • Verify Sample and Reagent Integrity; ensure correct Dil/Sheath in use.
• Verify Integrity of Sample Injection and Diluent/Sheath Syringe
• Verify Sample Staging:
• Integrity of Peri-Pump and Peri-Pump tubing.
• Integrity of RBC/PLT fluid pathway (Crimped Tubing, Plaque build-up, etc.).
• Verify valve operation along RBC/PLT Count fluid pathway.
• Clean and/or debubble Flow Cell.
• Verify RBC Mixing Chamber Function (bubble mix, draining.)
• Verify Bench Alignment:
• Perform RBC and FL CAL Gain Adjustment.
• Confirm Sample Stream is not wavering.
• Inspect 9PSI accumulator, connectors, and pathway to flow cell.
• Possible clogged Millipore filter.
• Circuitry:
• Verify MAM reference voltage (VP-01).
• Verify CPU/DCM 10 volt reference (VP-02).
Accumulator Wet • Fluid present in accumulator:
• Vacuum accumulator #1 wet: inspect WC #3 & #4. Exercise valves 32 & 98
and inspect associated tubings and fittings.
• Vacuum accumulator #2 wet: inspect WC #2. Exercise valve 18 and inspect
associated tubing and fittings.
• Check for obstructed or crimped tubing connected to Reagent reservoirs
and verify proper seating of dil/sheath filter.
• Exercise valves 61 & 62 and massage associated tubings.
• Inspect fittings for obstructions; flush as needed.
• Reseat tubing in NCV 65 & 66 and verify valve operation.
• Inspect Reagent Reservoir; Possibly Cracked/Leaking.
• Use Hemostats to locate source of leak.
• No fluid present in accumulator:
• Remove any salt build-up on sensors.
• Possible VPM Board failure - replace.
• Possible VPM Board failure - replace.
(Continued)
9-20 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Troubleshooting Run Sample Loader and Tower
PROBLEM Areas of Investigation and possible causes
HGB Result issues • Dirty Flow Cell, Low voltage reading:
• Verify Reference reading: 2050 + 200.
• If the reference reading is <1850, clean the HGB Flow Cell.
• On a background count, Sample & Reference readings should be similar
numbers.
• Very low (<300) reading can be an indication of bubbles.
• Low HGB Voltage:
• Access the VOLTAGE READINGS screen and note the HGB output. It
should be 5.0 ±0.2. Adjust as needed (VP-5).
• FCM failure.
• Verify Solenoid Operation 9-4, 5-1 and 9-5.
• Syringe failure:
• Clean the syringe, check for leaks, replace if needed.
• Verify Syringe Drive Motor Operation.
• Verify Reagent integrity (HGB/NOC):
• Replace as indicated.
• HGB result is displayed as <<<<:
• WBC result exceeds the linearity (>>>>) so HGB result is displayed
as <<<< to indicate possible interference with the HGB due to the
elevated WBC result.
CELL-DYN Ruby® Field Service Training Workbook 9-21
204343-101 Dec 2008
Module Summary
Module Summary
Now that you’ve completed the Troubleshooting Module, you should be able to perform
routine service procedures and system repairs.
End of Module
9-22 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 10: Miscellaneous
This module provides an overview of the CELL-DYN Ruby System Preventative Maintenance
and Installation Procedures. It also provides an overview of AbbottLink and its uses on the
CELL-DYN Ruby System. The module includes the following topics:
• Preventative Maintenance
• Installation
• AbbottLink
• Reticulocyte Package
CELL-DYN Ruby System
CELL-DYN Ruby® Field Service Training Workbook 10-1
204343-101 Dec 2008
Objectives
Notes
Objectives
After completing this module, you should be able to:
• Locate Installation and Preventative Maintenance pro-
cedures
• Perform procedures associated with CELL-DYN Ruby
Installation and Preventative Maintenance
• Log into the AbbottLink
• Navigate to a specific instrument within AbbottLink
• Review the information located on the AbbottLink
Dashboard
• Review historical data searches for message history,
maintenance data and error message history using
AbbottLink
• Retrieve logs to/for troubleshooting or reporting pur-
poses using AbbottLink
All procedures should be performed in accordance with
specifications outlined in CELL-DYN Ruby Operator’s Manual,
CELL-DYN Ruby Service and Support Manual and/or ISAs and
TSBs. AbbottLink procedures should adhere to service and
support AbbottLink policies and procedures.
10-2 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - PM and Install
Activity - PM and Install
Time to complete: 60 minutes
Activity Instructions
In this activity the instructor will debrief the procedures associated with CELL-DYN Ruby
System Installation and Preventative Maintenance.
In this activity you will,
• locate installation and PM checklists
• locate normally closed valves
• perform Open and Closed mode carryover studies
Resources Needed
In this session you will be performing service procedures.
Refer to the CELL-DYN Ruby ISA Database for the Installation and Preventative
Maintenance checklists.
Perform the following:
Locate and Print the Installation Checklist (ISA 170-003)
Locate and Print the Preventative Maintenance Checklist
Instructor Review - Installation
Installation Checklist contains system requirements that must be met prior to system
installation or whenever system is relocated. It includes procedures such as:
• Electrical and Spatial Specification
• Connecting Waste and Reagent tubing
• Installing Pinch valve and peristaltic pump tubing
• Removing Locking Screws
• Removing Packing material and installing components
Review Printer Driver ISA
CELL-DYN Ruby® Field Service Training Workbook 10-3
204343-101 Dec 2008
Activity - PM and Install
Notes Perform the Following:
Locate 6 Normally Closed valves and Floss tubing
Perform Carryover Check on Open Mode
• Locate three normal and three background specimen
results together in data log
Perform Carryover Check on Closed Mode
• Locate three normal and three background specimen
results together in data log
Instructor Review - PM
The Preventative Maintenance checklist contains
recommendations for optimal performance of the CELL-DYN Ruby
System.
Any deviation or change from these suggestions due to or based
on local, country, or area operating procedures and business
needs must be documented and approved locally.
END OF ACTIVITY
10-4 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Case Study 9
Notes
Case Study 9
You have just received a service call on a CELL-DYN Ruby System
because the instrument will not initialize.
The customer states the system locked-up while running and upon
reboot the system will not initialize. Error code HSSL Time-out
2100 on CELL-DYN Ruby System is being generated and system
appears to be locked up.
The Display is ON and the fans appear to be running. The
customer did note that shortly before the issue was noticed the lab
lost power on all systems not connected to emergency back-up
generators.
Upon speaking with the customer you learn that the customer has
a 2nd CELL-DYN Ruby System that is hooked up to emergency
power and a UPS/Line Conditioner. This CELL-DYN Ruby is not
experiencing any issues.
Additional troubleshooting steps performed by customer are listed
below. The issue remains unresolved:
• Tried cycling power to the analyzer two times
• Tried plugging the instrument into a different outlet
• Reseated HSSL Cable on back of system
• Had facilities test outlet states recovering 110VAC
Using your knowledge of the STEP Process, of normal instrument
operation, and your troubleshooting resources (Troubleshooting
Information Database [eSolutions], TSBs/ISAs, etc.); What
procedures, checks measurements, etc. would you use to isolate
the root cause of this error?
CELL-DYN Ruby® Field Service Training Workbook 10-5
204343-101 Dec 2008
Activity - AbbottLink
Notes
Activity - AbbottLink
Time to complete: 60 minutes
Activity Instructions
In this activity you will:
• navigate through the AbbottLink application
• retrieve instrument reports using your laptop computer
These exercises will allow you to practice navigating to a specific
instrument, review the instrument Dashboard, search Historical
data, and locate instrument logs that are available for upload.
Resources Needed
Your AbbottLink ID and Laptop Computer.
Refer to the AbbottLink resources located on the GSS website for
additional information.
Note: If you experience any issues in accessing AbbottLink, you may
contact the AbbottLink administrator by calling 1-800-527-2547 ext 6307,
or by e-mail at abbottlink.administration@abbott.com
Exercise 1: Navigation and Dashboard
Perform the following:
Access AbbottLink
• Type in the AbbottLink address:
Internet: https://abbottlinkuser.abbott.com
Intranet: http://abbottlinkuser.web.abbott.com
• Login using your AbbottLink ID (or one provided by the
instructor)
10-6 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - AbbottLink
Access the Home tab Notes
• While in the Home tab you can browse to the instru-
ment serial number of your choice
Bring up the Device Search screen (Select Search button)
Select the Model drop down box and select CELL-DYN Ruby
then click the Filter Button
Locate the instrument serial number RD-005 and click on the
instrument serial number hyperlink
• The instrument Dashboard for the selected instrument
will display.
Review the instrument Dashboard view below. Identify each sec-
tion listed below:
Location: Where the instrument is physically located
Contacts: Area Support contact information
Data Window: Displays a snapshot of the real-time data that is
collected from the instrument.
The Historical Data link at the bottom of the Data window is
used to access message history.
Action Window: Displays a short list of logs and files that are
available for retrieval from the instrument. The complete list of
logs and files is listed by clicking the View All link in the bottom
right corner of the window.
Files Window: Displays links to each of the last 5 files
retrieved from the instrument. The complete list of files that
have been recently retrieved can be accessed by clicking View
All.
Jump To: Displays a drop down list of items that you can
quickly move to by clicking the desired selection.
Note: Historical Data is stored on the server for 120 days
CELL-DYN Ruby® Field Service Training Workbook 10-7
204343-101 Dec 2008
Activity - AbbottLink
Notes Exercise 2: Historical Data and Logs
Historical data searches are helpful in locating instrument
generated message history, maintenance data, error message
history, and other stored information that can be used for
troubleshooting or reporting purposes. Logs can be useful in
troubleshooting instrument issues. The following logs are available
for upload using AbbottLink:
• Calibration log
• Event Log
• Maintenance Log
• QC Log
• Reagent Log
• Set Point Log
Perform the following:
From the Ruby Instrument SN RD-005 Dashboard, Select Histori-
cal Data from the data window or from the Jump To menu
• Historical Data Item screen appears
In the Data Item menu, scroll down and select Instrument Notifi-
cation Text
Select >=
Type the following: AIM=2000
Note: This allows you to narrow the search criteria to look at
hardware errors
Select Filter button to display all hardware issues
List any hardware issues that are found in Historical Data
__________________________________________
__________________________________________
__________________________________________
__________________________________________
__________________________________________
Pick one (1) instrument issue and use for classroom discussion
10-8 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - AbbottLink
Return to the Dashboard for Ruby Instrument SN RD-005, click on Notes
the View all hyperlink in the Actions Box
• This shows a list of all the logs that can be uploaded to
a computer or CD
Select the CELL-DYN Ruby – Retrieve Event Log by clicking on
the hyperlink, then select execute button in the dialog box that
pops up on the screen.
Note: This action will bring up the instrument Dashboard
Screen again
From the Instrument Dashboard screen look for the Uploaded
Files box and select View all
The Uploaded Files screen will display. Click on the hyperlink
Instrument/CELL-DYN Ruby/XXXXX/EventLog.csv
You have the option to save it to your computer or CD. You can
either click on the Open button to view the file or click on the Save
button to store it onto a CD
END OF ACTIVITY
CELL-DYN Ruby® Field Service Training Workbook 10-9
204343-101 Dec 2008
Reticulocyte Package
Notes
Reticulocyte Package
The Reticulocyte Package software is designed to configure the
instrument to process stained, diluted specimens. This enables the
operator of the CELL-DYN Ruby System to analyze a whole blood
specimen for reticulocytes.
Reticulocytes
Reticulocytes are defined by the Clinical Laboratory and Standards
Institute (CLSI) as transitional red cells, between nucleated red
cells and the so-called mature erythrocytes. Unlike a mature RBC,
reticulocytes contain ribosomal RNA.
Instrument
The Reticulocyte Package is enabled by selecting the Retic test
selection in the Next Open Tube Entry (NOTE) region and
acknowledging the message to run the reticulocyte method startup
script.
The Reticulocyte assay is performed in the WOC channel of the
instrument. During Optical Measurement, 0o, 10o, and 90o scatter
are collected for up to 30,000 events.
• Reticulocytes have 10o scatter that are similar to the
scatter for mature RBC, but differ from them by exhibit-
ing greater 90o scatter.
Reticulocytes are reported in percent (%R). The instrument will
automatically calculate an Reticulocyte Absolute value
(RETC = %R x RBC) if an RBC concentration is entered using the
F12 – RBC Source function key. If no RBC value is entered, only
%R will be reported.
The reticulocyte background (RETC_Background) count should be
included in the daily start-up procedures to check for particulate
matter in the reticulocyte reagent and the CELL-DYN Ruby System
when reticuloctye analysis is performed. An acceptable reticulocyte
background is <100.
The Reticulocyte Package is disabled by selecting either of the test
selections: CBC, CBC + NOC, CBC + RRBC in the Next Open
Tube Entry (NOTE) region and acknowledging the message to run
the reticulocyte method cleanup script.
NOTE:The cleanup script takes approximately three minutes
to return the Analyzer Status to Ready state.
10-10 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Reticulocyte Package
Specimen Preparation Notes
The Reticulocyte specimen is prepared by the operator using
reticulocyte reagent to produce a diluted, stained sample.
Specimens may be run up to 8 hours after collection time when
stored at room temperature.
To prepare a specimen for analysis:
• Manually dispense 20 μL of blood into a tube of
CELL-DYN Reticulocyte Reagent.
• Cap and incubate the stained specimen mixture accord-
ing to the reagent package insert. General guidelines
are included below:
• Incubate the stained reticulocyte specimens on
a rotator or in a rack, after fully inverting the
stained specimens 5 times.
• The stained reticulocyte specimens must incu-
bate at room temperature for at least 15 minutes
but no more than 2 hours prior to processing.
• The stained sample is then ready to be aspirated in the
Open Mode.
NOTE: For additional information on Reticulocyte analysis
on the CELL-DYN Ruby System refer to the
CELL-DYN Ruby Operator’s Manual Section 12
Reticulocyte Package.
Reticulocyte Reagent
• Contains New Methylene Blue stain.
• Stains reticular RNA and stabilizes the RBCs while
decreasing the interference from WBCs and PLTs.
• Stored at room temperature and in the dark.
Quality Assurance
The frequency of quality control runs should be determined by
each laboratory. Control specimens should be run and results
confirmed before reporting any patient results.
A reticulocyte moving average program is available on the
CELL-DYN Ruby as a quality assurance and troubleshooting tool.
It is recommended that an Extended Autoclean be conducted on a
weekly basis if the laboratory is running the Reticulocyte Package.
CELL-DYN Ruby® Field Service Training Workbook 10-11
204343-101 Dec 2008
Reticulocyte Package
Notes
10-12 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Reticulocyte
Notes
(OPTIONAL)
Activity - Reticulocyte
Time to complete: 60 minutes
Activity Instructions
THIS ACTIVITY IS OPTIONAL AND WILL BE PERFORMED AT THE
DISCRETION OF THE INSTRUCTOR.
In this activity you will be running a Reticulocyte Specimen.
Resources Needed
In this session you will be performing Specimen analysis pro-
cedures.
Refer to the CELL-DYN Ruby Operator’s Manual Section 11
Reticulocyte Package for instruction.
Perform the following:
Prepare Reticulocyte Specimen
Remove the specimen from the refrigerator and warm
to room temperature.
Mix the capped specimen by inversion at least 5 times.
Label one tube of reticulocyte reagent for each
specimen.
Pipette 20 μL of the specimen and dispense into the
appropriately labeled reticulocyte reagent tube and cap.
Invert the tube at least 5 times and incubate for a
minimum of 15 minutes at room temperature.
Run a CBC on the remaining EDTA Specimen used above
Verify the Analyzer is in the Ready state, and that you
are in the Run View screen.
In the NOTE region enter a specimen ID. Record the
specimen ID here___________.
Run the specimen using the CBC test selection.
CELL-DYN Ruby® Field Service Training Workbook 10-13
204343-101 Dec 2008
Activity - Reticulocyte
Notes Enable Reticulocyte Analysis
In the NOTE region of the Run View screen, select the
RETIC Test Selection.
Select OK to run the Reticulocyte Method Start Up
Script and enable reticulocyte processing.
Run a RETC_Background
In the NOTE region of the Run View screen, select the
QCID lookup icon. From the pull-down menu, select
RETC_background.
Remove the cap from fresh tube of reticulocyte reagent.
Place the tube under the aspiration probe, immerse the
probe in the reagent, and press the touch plate.
Listen for an audible beep and remove the tube from
under the aspiration probe, and replace the cap on the
reagent tube.
Review the results for the background count.
Run Reticulocyte Specimen
In the NOTE region of the Run View screen, enter the
same specimen ID as used before.
Select the F12 – RBC Source function key to open the
RBC Source Selection for Reticulocyte Absolute
dialog box.
Choose the RBC Source: "RBC from existing
specimen". Select OK.
Mix the reticulocyte specimen by inversion at least 5
times.
Remove the cap and place the specimen tube under
the aspiration probe.
Immerse the probe in the specimen tube. Press the
touch plate.
Listen for an audible beep. Remove the specimen and
replace the cap.
Review the results and print out a hard copy if desired.
Disable Reticulocyte Analysis
In the NOTE region of the Run View screen, select
CBC, and acknowledge the message to run the
reticulocyte method cleanup script.
END OF ACTIVITY
10-14 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module Summary
Notes
Module Summary
Now that you’ve completed the Miscellaneous Module, you should
be able perform routine Service and Support procedures and repair
including Installation, Preventative Maintenance and the
Reticulocyte Package. You should also be able to access reports
using AbbottLink.
CELL-DYN Ruby® Field Service Training Workbook 10-15
204343-101 Dec 2008
Review
Notes
Review
1. In the AbbottLink Dashboard screen which window displays a short list
of logs and files that are available for retrieval from the instrument?
2. Which AbbottLink log would you review to monitor how many times a
system has seen incomplete aspiration errors?
3. How is the reticulocyte reagent stored?
4. Explain how to run a reticulocyte background count.
5. What is an acceptable reticulocyte background count result?
10-16 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Practical Exam
Practical Exam
This exercise will be focused upon accessing your skills in determining the root cause of
an instrument error and in using the troubleshooting process.
Instructor will provide one or more System Troubleshooting Case Studies.
Begin each activity by processing specimen.
Upon encountering an error state follow the Effective Troubleshooting S.T.E.P. process.
Work through the S.T.E.P. worksheet to isolate and identify the most likely cause. After
you have done this, you will perform any necessary procedures to return the instrument
to a fully functional state.
WARNING: Biohazard. Potential Biohazard, follow biosafety practices.
CAUTION: Moving Parts.
In order to complete this exercise you will do the following:
1. Upon detection of an error state, stop. Document the error on the top of the S.T.E.P. work-
sheet.
2. Complete the S.T.E.P. worksheet following the Effective Troubleshooting guidelines through-
out your repair.
3. After identifying the root cause of the error, perform the repair, then perform the required ver-
ification procedures and return the instrument to a “Ready” state.
4. Be prepared to share your process with the instructor.
Upon Completion of ALL practical exams, when instructed:
Perform Clean for Ship (View on-board video)
Place reagent lines in DI water
Remove Peri-Pump tubing from pump, and Tubing from the 6 NC valves
Remove Shear Valve Center Ceramic
CELL-DYN Ruby® Field Service Training Workbook 10-17
204343-101 Dec 2008
Practical Exam
Notes
10-18 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Practical Exam
The S.T.E.P. Worksheet
STOP Identify the PROBLEM. (List error code or describe problem situation)
THINK List Meaningful DATA such as Abnormal Observations, Test Results, Voltage Readings, LEDs,
Lot numbers, etc.:
Look for COMPARISONS to another system, component, assay, analyzer etc.
Identify assay(s), system(s) and/or similar component(s) where the problem IS and IS NOT.
Problem IS occurring here Problem IS NOT occurring here
List CLUES. (What is unique about the PROBLEM (IS data)?)
• Look for patterns, differences, symptoms, procedure/checks results, etc.
Categorize probable area of failure based on gathered data. Is the Problem related to:
OPERATOR REAGENT ENVIRONMENT ANALYZER (Circle One)
Which instrument system is the most likely area of failure?(check all that apply)
___Fluidics ____ Optics ____ Robotics ____ Temperature Control ____ Power ___Other
EVALUATE Identify possible CAUSES and TEST the cause against the Meaningful Data and Clues. The
cause must explain ALL the data or it is not the root cause of the problem.
List the step(s) used to isolate the cause of the failure?
(Diagnostic Tests, Precision Run, Swap boards, etc.)
P ROCEED List the step(s) used to verify the failure had been resolved?
(List Procedures, Diagnostic tests, etc.)
CELL-DYN Ruby® Field Service Training Workbook 10-19
204343-101 Dec 2008
Practical Exam
Notes
End of Module
10-20 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 11: CELL-DYN 3200 System
Comparison
This module provides an overview of the different components, systems, and subsystems that
comprise the CELL-DYN® 3200 System when it is compared to the CELL-DYN Ruby System.
This module introduces the principles, and procedures associated with the CELL-DYN 3200:
• Software
• Basic Operation, Setup and Maintenance
• Service Procedures
• Preventative Maintenance and Installation
CELL-DYN 3200 System
CELL-DYN Ruby® Field Service Training Workbook 11-1
204343-101 Dec 2008
Objectives
Notes
Objectives
After completing this module, you should be able to:
• List the top 5 CELL-DYN® 3200 instrument failures/
errors that result in FSR visits
• Identify commonly used parts that are used in trouble-
shooting
• Identify the major systems, subsystems and component
differences of a CELL-DYN 3200 System vs. a
CELL-DYN Ruby System
• Perform basic operation, setup, and maintenance pro-
cedures on a CELL-DYN 3200 System
• Perform and interpret diagnostic procedures on a
CELL-DYN 3200 System
• Perform removal and replacement procedures on a
CELL-DYN 3200 System
• Troubleshoot to identify root cause of failure on a
CELL-DYN 3200 System
• Repair the CELL-DYN 3200 instrument
• Perform installation and preventative maintenance pro-
cedures on a CELL-DYN 3200 System
All procedures should be performed in accordance with
specifications outlined in CELL-DYN 3200 Operator’s Manual,
CELL-DYN 3200 Service and Support Manual and/or ISAs and
TSBs.
For comparison information, including similarities and
differences, between the CELL-DYN Ruby and CELL-DYN
3200 Systems refer to the current version of ISA 170-001.
11-2 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity -Top Failure
Notes
Activity -Top Failure
Time to complete: 30 minutes
Activity Instructions
Throughout this training course, the instructor will provide
information on the most commonly reported Instrument failure/
errors, the most commonly used parts, and how to identify and
repair these situations.
Resources Needed
Employee Ah_Ha Note pad.
Perform the following:
Record the top 5 common errors and commonly used parts as
revealed by the instructor on the provided Ah_Ha Note pad.
Reported Errors
Part Usage
CELL-DYN Ruby® Field Service Training Workbook 11-3
204343-101 Dec 2008
Activity - Basic Operation
Notes
Activity - Basic Operation
Instructor Directed Session: 20 minutes
Time to complete: 1.5 Hours
Activity Instructions
In this activity you will:
• Explore the CELL-DYN® 3200 software as your
instructor directs you to perform various tasks.
• This will include identifying key areas of the screen,
their function, and how to navigate to various screens.
• locate logs and perform functions within the software
• perform service verification procedures
• run controls and observe Instrument “normal” Operation
Resources Needed
In this session utilize the Quick Reference Diagram you have been
provided in class to aid in Software Navigation.
Instructor will provide samples or controls for precision runs.
Refer to the CELL-DYN 3200 System Operator’s Manual,
Section 5 Operating Instructions, Subsection Software
Flowcharts and the CELL-DYN 3200 System Service and
Support Manual, Troubleshooting Section, Subsection Service
Commands for additional information.
For instruction on setting up a QC File refer to the CELL-DYN
3200 System Operator’s Manual, Section 5 Operating
Instructions, Subsection QC Setup Menu.
For instruction on performing Verification Procedures refer to
the CELL-DYN 3200 System Service and Support Manual,
Verification Procedures Section.
11-4 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Basic Operation
Instructor Directed Review:
Review of Menu Structure and General Navigation
NOTE:After power ON, the CELL-DYN® 3200 System will automatically go to an
Initialized state unlike the CELL-DYN Ruby System.
Main Menu MAIN MENU
RETIC QUALITY SPECIAL
SET UP RUN/PRIME DATA LOG CALIBRATION DIAGNOSTICS
DATA LOG CONTROL PROTOCOLS
DIAGNOSTICS MENU
Diagnostic Menu (partial list) Ready
EXECUTION CNT RATE RAW DATA
FAULT REPORT CLEAR FAULTS MORE MAIN
TIMES SUMMARY SUMMARY
MOTOR SOLENOID PUMP DRAIN
INITIALIZATION MORE MAIN
OPERATION OPERATION OPERATION ACCUMULATOR
WOC CNT RBC/PLT NOC CNT
PRINT RETURN
RATE CNT RATE RATE
VACUUM PRESSURE VACUUM PRESSURE
DIAGNOSTICS
ON/OFF ON/OFF TEST TEST
SOFTWARE DIGITAL VOLTAGE GAIN
MORE PRINT MAIN
VERSIONS READINGS READINGS ADJUSTMENT
Navigate to the following screens:
Voltage Reading Screen
Gain Settings
X-B Data
Error log
Setup a QC file entitled “Data 1”
CELL-DYN Ruby® Field Service Training Workbook 11-5
204343-101 Dec 2008
Activity - Basic Operation
Notes Perform the following Procedures:
WARNING: Biohazard. Potential Biohazard, follow biosafety
practices.
CAUTION: Moving Parts.
Hemoglobin Adjustment Procedure (VP-5)
Backup Procedure (VP-64)
NOTE:It is recommended that this procedure be performed
routinely following instrument service activity.
Hard Drive Formatting (VP-61)
• For Training Purposes Only:
• DO NOT remove the Hard Drive
• At the CREATE DOS PARTITION Step, Select Option
#3 Delete Partition
NOTE: When installing the software, the system takes time to
rebuild the logs. The system may appear it is not
doing anything but be patient as this process takes
time (wait ~5 minutes)
Restore Setup (VP-65)
Access the following hidden screens (use Quick reference Guide):
Dilution Factors
Set Point Entry Screen
Run Controls in the OPEN Mode:
Observe Instrument Operation
List the observed differences when compared to the
CELL-DYN RUBY System for Open Mode operation.
__________________________________________________________
__________________________________________________________
__________________________________________________________
__________________________________________________________
__________________________________________________________
__________________________________________________________
__________________________________________________________
11-6 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Basic Operation
Run Controls in the CLOSED Mode: Notes
Observe Instrument Operation
List the observed differences when compared to
CELL-DYN RUBY System for Closed Mode operation.
__________________________________________________________
__________________________________________________________
__________________________________________________________
__________________________________________________________
__________________________________________________________
__________________________________________________________
__________________________________________________________
__________________________________________________________
__________________________________________________________
END OF ACTIVITY
CELL-DYN Ruby® Field Service Training Workbook 11-7
204343-101 Dec 2008
Activity - Basic Operation
Notes
11-8 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Electronics and Power
Electronics and Power
In this section, your instructor will discuss the similarities and differences in the Electronics and
Power Distribution Subsystems between the CELL-DYN® 3200 and CELL-DYN Ruby Systems.
The electronic subsystem devices control, monitor, and drive many of the instrument compo-
nents. There are many individual printed circuit boards (PCBs) and electronic assemblies
located throughout the analyzer. Many of the PCBs are the same as those on the CELL-DYN
Ruby System and are interchangeable between the two systems. The table below provides a
quick comparison between the electronics of the two systems.
• Same = Interchangeable between CELL-DYN Ruby and CELL-DYN 3200
• New, Different, or “name”= System specific
• None = Not present on that system
CELL-DYN CELL-DYN
Ruby 3200
Component Notes
CPU/DCM Same Same
Signal Processor Module (SPM) Same Same
Main Amplifier Module (MAM) Same Same
Solenoid Driver Module (SDM) Same Same
Cable Distribution Module (CDM) Same Same
Vacuum Pressure Module (VPM) New Different
Flow Control Module (FCM) Same Same
Motor Processor Module (MPM) Same Same
Temperature Control Module (TCM) New None
Pump Relay Module (PRM) New Different
Y-Valve Driver (MDM) Same Same
Shear Valve Driver Module Same Same
Pre-Amplifier Boards Same Same
(0, 10, 90 and 90D)
Sampler Handler Module Same Same
(SHM1 and SHM2)
Power Distribution Module (PDM) New Different
Power Supply Switching Linear
PC Power Supply New None
CELL-DYN Ruby® Field Service Training Workbook 11-9
204343-101 Dec 2008
Electronics and Power
The Power Distribution subsystem supplies the Analyzer voltages used for measurement, fluidic
control and mechanical motion control. It consists of:
• Linear Power Supply Module
• DC power to analyzer components including Fans, motors, pumps and solenoids
• Splits out AC power
• Power Distribution Module (PDM)
• Routes 5V logic to analyzer
• Routes voltage to destinations throughout the analyzer
• Laser Power Supply Module (LPS)
• Power for Helium-Neon Laser
Power Diagram
AC Line
Pow er Sw itch
MAIN POWER SUPPLY PDM J2 + 5, GND-PC MB
+ 12, -12, GND, PF-PC MB
J13 J3
+ 5, -15, GND-MAM , SPM, CPU
J4
+ 5V + 5, + 24, GND-MDM
J5
+ 12V
+ 5, + 12, -12, + 24-M PM
J5 -12V J1 J6
Filter + 24V Spare
(CORCOM) J7
GND + 5, + 15, -15, GND-VPM, FCM
J8
+ 15V + 5. + 12, GND-Hard Drive
Fuse J10
J4 -15V + 5. + 12, GND-Floppy Drive
J9
(Rear) GND J11
+ 5, + 15, -15, GND-SHM1
AC Voltage J12
Selector + 5, + 15, -15, GND-SHM2
J13
Transformer
J4 115VAC + 2400VDC
LASER P/S LASER
(Top)
115VAC, Rear Fan
J7
115VAC, Side Fan
J8
115 VAC, PRM
J2
CELL-DYN® 3200 Power Diagram
11-10 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Power
Notes
Activity - Power
Time to complete: 20 minutes
Activity Instructions
In this activity you will:
• locate electronic logic and power components
• perform System Voltage Verification
• perform troubleshooting case study on analyzer
Then you will have an opportunity to perform service procedures,
along with your training partner, to become familiar with normal
operation of the Electronics and Power subsystems.
Resources Needed
Refer to the CELL-DYN® 3200 System Service and Support
Manual, General Data Section, Subsection Power Distribution
Subsystem or Electronics Subsystem for additional information
on the operation of these subsystems.
In this session you will be removing some of the covers from
the instrument. Refer to the CELL-DYN 3200 System Service
and Support Manual R&R A1.01-A1.06 for instructions on
how to remove the covers and the Verification Procedures
section for instructions on the performance of those
procedures.
You should also utilize the Diagrams, you have been provided with,
to identify and locate key components.
Perform the following:
CAUTION: Electrical Shock Hazard. Follow electrical safety
practices.
Perform System Voltage Verification (VP-1)
CELL-DYN Ruby® Field Service Training Workbook 11-11
204343-101 Dec 2008
Case Study 10
Notes
Case Study 10
Notify your instructor that you are ready to perform Case Study 10
on a CELL-DYN 3200 analyzer
A CELL-DYN® 3200 System, is reporting intermittent Data
Station Time Out / No response /Communication Errors.
The following has been performed on the system but has not
resolved the error:
• Power has been cycled
Using your knowledge of the STEP Process, of normal instrument
operation, and your troubleshooting resources (Troubleshooting
Information Database [eSolutions], IP’s, TSBs/ISAs, etc.) locate
the potential cause of the error on your system.
Record your troubleshooting processes below:
Performed the following Instrument checks, procedures or obser-
vation. Record below:
Identified the following clues:
Replaced the following parts:
Located and resolved the problem immediately through use of e-
solutions or Service Manual. Record source of
error:___________________
END OF ACTIVITY
11-12 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Review 1
Notes
Review 1
1. How do you access Hidden screens on the CELL-DYN® 3200
System?
2. X-B files are accessed under which menu?
3. Compare the CELL-DYN Ruby Power Supplies with the CELL-DYN
3200 Power Supplies?
4. Are the PRM Boards interchangeable between the CELL-DYN Ruby
and CELL-DYN 3200 Systems?
5. What is the function and location of the MAM Board on the CELL-DYN
3200 System?
6. Under what menu is the HGB Voltage Reading Screen located?
CELL-DYN Ruby® Field Service Training Workbook 11-13
204343-101 Dec 2008
Review 1
Notes
11-14 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Vacuum/Pressure & Fluidics
Vacuum/Pressure & Fluidics
In this section, your instructor will discuss the similarities and differences in the Vacuum/Pressure
and Fluidic Subsystems when comparing a CELL-DYN® 3200 and CELL-DYN Ruby System.
The CELL-DYN 3200 System Fluidic and Vacuum/Pressure Subsystems function the same as
the CELL-DYN Ruby System. However, slight differences in components can be observed.
Some of the Differences are highlighted in the table below:
• Same = Interchangeable between CELL-DYN Ruby and CELL-DYN 3200
• New, Different, or “name”= System specific
• None = Not present on that system
CELL-DYN CELL-DYN
Ruby 3200
Component Notes
Vacuum and Pressure Pumps DC AC
Pump Relay Module Different Different
Pneumatic Supply Lines to Instrument Quick Manifold
Disconnect
Manifold Vacuum Accumulator Drain Lines New None
Second Ultrasonic Sensor (after Shear Valve) New None
Open/Closed Aspiration Tubing Lengths Equal Longer
Closed Aspiration Probe Different Different
Open Tube Sample Location Center Right
Y-Valve Location Different Different
WOC Lyse Reservoir New None
HGB/NOC Lyse Check Valve New None
Pneumatic Bubble Mix New None
Location of Sample inlet into HGB Mixing Chamber Top Side
Separation of HGB Sample and Lyse Lines into New (two One line
lines)
HGB Mixing Chamber/Flow Cell
Mixing Chambers Heaters New None
Vent Accumulator Aerosol Filter Check Valve Filter
Waste Chamber 2 and 4 Bubble Traps None Traps
Separation of WOC and HGB Lines into Waste New Combined
Chamber 3 (prevent foaming)
CELL-DYN Ruby® Field Service Training Workbook 11-15
204343-101 Dec 2008
Vacuum/Pressure & Fluidics
Sample Aspiration
• During sample aspiration, whole blood is aspirated by vacuum.
• A mechanical Y-Valve sets the pathway for either Open or Closed Mode sampling.
• The whole blood sample is pulled from the open or closed pathway through the shear
valve by the vacuum in WC2 (Waste Chamber 2).
• Due to the differences in tubing length, the vacuum level is approximately 3.3"
Hg in the Open Mode and approximately 4.5" Hg in the Closed Mode.
• In Closed Mode, the 2nd sensor detects the leading edge of the sample before the
shear valve makes the cut.
• The sensor is an optical sensor and is only used in CLOSED mode.
NOTE:The Closed Aspiration probe is different than the CELL-DYN Ruby probe although
it looks the same and appears to be the same length. There are two major
differences:
• The sample port is smaller on the CELL-DYN® 3200 Probe.
• The inner diameter and wall is slightly smaller on the CELL-DYN 3200
Probe.
• In OPEN mode, the ultrasonic sensor senses the leading and trailing edge of the
sample, and the 2nd sensor is not used.
• The Ultrasonic Sensor monitors the whole blood sample entering the shear
valve.
• The sensor detects the presence or absence of liquid in the aspiration line,
and is not adversely affected by the density or viscosity of the sample.
• The Ultrasonic Sensor monitors aspiration and is used to detect a short sam-
ple situation.
11-16 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Fluidics
Activity - Fluidics
Time to complete: 90 minutes
Activity Instructions
In this activity you will:
• prime/Background count
• replace Shear Valve Driver
• remove and replace Optics Bench
• slide out Pneumatic Assembly and locate components
• perform Vacuum Pressure verification procedures
Resources Needed
Refer to the CELL-DYN® 3200 System Service and Support Manual, General Data
Section and Verification Procedures for additional information on the operation of
these subsystems and performance of the service procedures.
You should also utilize the Diagrams, you have been provided with, to identify fluidic
pathways.
Perform the following:
CAUTION: Electrical Shock Hazard. Follow electrical safety practices.
WARNING: Splash Spray Hazard.
PRIME and Run Background count
Remove Optics Bench (R&R C1.01)
CELL-DYN Ruby® Field Service Training Workbook 11-17
204343-101 Dec 2008
Activity - Fluidics
Remove and Replace Shear Valve Driver Assembly (R&R B1.01)
• FOLLOW PROCEDURE IN SERVICE AND SUPPORT MANUAL
NOTE:Perform Daily Shutdown to place instrument in Standby so ceramics can be
easily removed.
OBSERVE the following:
Shear Valve Spacer
Center ceramic alignment
Inspect the metal pin on the center ceramic
Replace Optics Bench (R&R C1.01)
Slide out Pneumatic Assembly (R&R E1.01) to allow for viewing of subsystem compo-
nents:
AC Pump
AC Pump Relay
Tubing Connections
Perform VP-17 Vacuum Pressure Recovery
Perform VP-15 Vacuum & Pressure Retention Verification
NOTE: VP-4 VPM Reference Voltage Verification/Adjustment will not be performed
as part of this Fluidic service activity as it was performed in a prior activity.
Perform VP-16 Vacuum & Pressure Level Verification/Adjustment
END OF ACTIVITY
11-18 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Troubleshooting Run Fluidics
Troubleshooting Run Fluidics
This exercise will be focused upon practicing your skills in determining the root cause of
an instrument error and in using the troubleshooting process.
The error in this activity will be related to the fluidics subsystem. Upon encountering an
error state you will follow the Effective Troubleshooting S.T.E.P. process. Both you and
your partner will work through the S.T.E.P. worksheet to isolate and identify the most likely
cause. After you have done this, you will perform any necessary procedures to return the
instrument to a fully functional state.
WARNING: Biohazard. Potential Biohazard, follow biosafety practices.
WARNING: Splash/Spray Hazard.
In order to complete this exercise you will do the following:
1. Upon detection of an error state, stop. Document the error on the top of the S.T.E.P.
worksheet.
2. Complete the S.T.E.P. worksheet following the Effective Troubleshooting guidelines through-
out your repair.
3. After identifying the root cause of the error, perform the repair, then perform the required ver-
ification procedures and return the instrument to a “Ready” state.
4. Be prepared to share your process with the class. Each group will have a different system
error and will discuss how they arrived at the solution and their process as documented on
the S.T.E.P. worksheet.
CELL-DYN Ruby® Field Service Training Workbook 11-19
204343-101 Dec 2008
Troubleshooting Run Fluidics
Notes
11-20 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Troubleshooting Run Fluidics
The S.T.E.P. Worksheet
STOP Identify the PROBLEM. (List error code or describe problem situation)
THINK List Meaningful DATA such as Abnormal Observations, Test Results, Voltage Readings, LEDs,
Lot numbers, etc.:
Look for COMPARISONS to another system, component, assay, analyzer etc.
Identify assay(s), system(s) and/or similar component(s) where the problem IS and IS NOT.
Problem IS occurring here Problem IS NOT occurring here
List CLUES. (What is unique about the PROBLEM (IS data)?)
• Look for patterns, differences, symptoms, procedure/checks results, etc.
Categorize probable area of failure based on gathered data. Is the Problem related to:
OPERATOR REAGENT ENVIRONMENT ANALYZER (Circle One)
Which instrument system is the most likely area of failure?(check all that apply)
___Fluidics ____ Optics ____ Robotics ____ Temperature Control ____ Power ___Other
EVALUATE Identify possible CAUSES and TEST the cause against the Meaningful Data and Clues. The
cause must explain ALL the data or it is not the root cause of the problem.
List the step(s) used to isolate the cause of the failure?
(Diagnostic Tests, Precision Run, Swap boards, etc.)
P ROCEED List the step(s) used to verify the failure had been resolved?
(List Procedures, Diagnostic tests, etc.)
CELL-DYN Ruby® Field Service Training Workbook 11-21
204343-101 Dec 2008
Troubleshooting Run Fluidics
Notes
11-22 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Sample Loader
Sample Loader
In this section, your instructor will discuss the similarities and differences in the Sample Loader
Subsystem when comparing a CELL-DYN® 3200 and CELL-DYN Ruby System. Some of these
Differences are highlighted in the table below:
• Same = Interchangeable between CELL-DYN Ruby and CELL-DYN 3200
• New, Different, or “name”= System specific
• None = Not present on that system
CELL-DYN CELL-DYN
Ruby 3200
Component Notes
Interlocking Sample Loader Side Yes Not Present
Rails
Sample Loader Pneumatic Line Quick Individual
connection Disconnect Connections
Tube Sensor PCB Adjustable/ Adjustable/
Facing Rear Facing Front
Open/Closed Aspiration Tubing Equal length Longer Open
Lengths Tubing
Serviceability Access Panels 2 Panels None
Major S/L Assemblies Attached to Yes N/A
Platen from Underneath
Straight Rack Advance Arm and Yes N/A
Pawl Assembly Movement (w/Delrin
guide for stabilization)
Flat Contoured Sweep Arms with Yes N/A
Integrated Gears and Sensor Flags
Replaceable Sweep Arm Bearing Yes N/A
Blocks
Mix Head Home Sensor Optical Reed
Integrated Home Sensor Flag on Yes N/A
Mix Head
Mix Head Tube Inversion Inward Outward
Tube Capture Chute Yes None
Mix Head Release Mechanism for Yes None
Rotating Unit Outward and Cleaning
Mix Motor 0.9 Degree per Step (less Yes N/A
noise)
Location of SHM PCBs Left side of Front Panel
Instrument
CELL-DYN Ruby® Field Service Training Workbook 11-23
204343-101 Dec 2008
Sample Loader
The Pneumatics Control Subsystem provides electronic control of the valves supplying pressure
and vacuum to the air cylinders controlling the cross transfer assemblies (sweep arms), rack
advance pawls, and mixer bladders.
CELL-DYN® 3200 Sample Loader Pneumatic Graphic
11-24 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Sample Loader
Notes
Activity - Sample Loader
Time to complete: 45 minutes
Activity Instructions
In this activity the instructor will assist the class in locating the
Sample Loader components on a CELL-DYN® 3200 System.
Then you will have an opportunity to perform service procedures,
along with your training partner, to become familiar with repair of
the Sample Loader Subsystem.
Resources Needed
Refer to the CELL-DYN 3200 System Service and Support
Manual, General Data Section for additional information on the
operation of this subsystems.
CELL-DYN Ruby® Field Service Training Workbook 11-25
204343-101 Dec 2008
Activity - Sample Loader
Perform the following:
WARNING: Biohazard. Potential Biohazard, follow biosafety practices.
CAUTION: Moving Parts.
Run 5 samples through the Sample Loader and observe it’s operation. Record observa-
tions in table below:
Component Observation and Data Notes
Mix Head # times sample inverted:
Pressure used for Bladder inflation:
List the sensors that verify the specimens tubes were
lifted and dropped by the mix head assembly:
Pneumatic Driver Output Board for Pneumatic Valve:
Subsystem
Valve controlling pressure for Mix Head lift:
Rack Movement Location of sensor that detects when load side empty:
How is processing completion detected?
Moves racks forward (load side):
Moves racks horizontally:
Specimen What sensors are responsible for sensing tube height
and where are they located:
How many tube sensors are there?
Aspiration
11-26 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Case Study 11
Notes
Case Study 11
During the morning run on the CELL-DYN® 3200 System, the
operator notices the Pusher arms are not retracting/extending
properly.
The following troubleshooting procedures were performed without
resolution of the problem:
• Power cycled
• Cleaned Sample Loader and arms
Using your knowledge of the STEP Process, of normal instrument
operation, and your troubleshooting resources (Troubleshooting
Information Database [eSolutions], IP’s, TSBs/ISAs, etc.) answer
the following questions.
Record your troubleshooting processes below:
List whatever procedure(s) you would perform next:
What clues can you identify from the data provided or procedures
you would perform?
What comparisons can you make?
CELL-DYN Ruby® Field Service Training Workbook 11-27
204343-101 Dec 2008
Case Study 11
Notes
11-28 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - Optics
Activity - Optics
Time to complete: 5 hours
Activity Instructions
In this activity you will perform the listed service procedures related to Optics Bench
performance and Data Interpretation/Flagging troubleshooting on a CELL-DYN® 3200
System.
Resources Needed
In this session you will be performing service procedures. Refer to the CELL-DYN
3200 System Service and Support Manual Removal and Replacement and
Verification Procedure Sections for instructions.
Refer to CELL-DYN 3200 Operator’s Manual Section 6 Calibration for additional
information and/or instruction on the instrument calibration procedures.
Perform the following Procedures:
WARNING: Biohazard. Potential Biohazard, follow biosafety practices.
CAUTION: Class 3B laser light when open. Avoid exposure to beam.
Laser Tube replacement and Optics Bench Alignment (VP-18)
Optic Gain Verification/Adjustment (VP-23 through VP-27)
VP-23 WBC Gain Verification/Adjustment
VP-24 WBC OPTI-CAL Verification/Adjustment
VP-25 NOC Gain Adjustment
VP-26 RBC/PLT 0° Gain Verification/Adjustment
VP-27 RBC/PLT 10° Gain Verification/Adjustment
Linear RBC Gain Verification/Adjustment (VP-68)
CELL-DYN Ruby® Field Service Training Workbook 11-29
204343-101 Dec 2008
Activity - Optics
Replace all covers
Auto-Cal Procedure — Open Mode
Perform Pre-calibration Procedures
Perform Auto-Calibration
• Use commercial calibrator or Assayed Whole Blood specimen
• Review, Print, and Activate proposed calibration factors
Perform Post Calibration Verification
NOTE:Calibration should be considered the last step in the troubleshooting sequence
when investigating data problems. Performing unnecessary calibration may
mask an underlying instrument problem.
Cal Procedure — Closed Mode
Complete Mode to Mode Calibration Difference Worksheet
Run Controls to verify instrument performance
Perform Clean for Ship
END OF ACTIVITY
11-30 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Case Study 12
Case Study 12
During the performance of a service call on a CELL-DYN® 3200 System, the Optical Flow
Cell was adjusted to specification. A WOC gain adjustment was performed and the 90D
DAC setting is reporting at 4095 and the 90° is reporting at 1032. The CV’s for the four
channels are reporting: 0°=4.1%, 10°=3.4%, 90°=12.1%, 90°D=10.8%.
The results looked similar to the information shown below
NEU:.010 %N:.222
EOS: 2.49 %E: 53.8
Using your knowledge of the STEP Process, of normal instrument operation, and your
troubleshooting resources (Troubleshooting Information Database [eSolutions],
TSBs/ISAs, etc.), list whatever procedure(s) you would perform next?
CELL-DYN Ruby® Field Service Training Workbook 11-31
204343-101 Dec 2008
Case Study 12
Notes
11-32 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Activity - PM & Install
Notes
Activity - PM & Install
Time to complete: 30 minutes
Activity Instructions
In this activity the instructor will debrief the procedures associated
with CELL-DYN® 3200 System Installation and Preventative
Maintenance.
Resources Needed
In this session you will be performing service procedures.
Refer to the CELL-DYN 3200 ISA Database for the Installa-
tion and Preventative Maintenance checklists.
Perform the following:
Locate and Print the current revision of the Installation Checklist
(ISA 80-026)
Locate and Print the current revision of the recommended Preven-
tive Maintenance Checklist (ISA 80-004)
Locate review the Pre-Calibration Procedures Pre-Calibration
Checklist located in the CELL-DYN 3200 Operator’s Manual Sec-
tion 6 Calibration Procedures and Manual Calibration Worksheets
CELL-DYN Ruby® Field Service Training Workbook 11-33
204343-101 Dec 2008
Activity - PM & Install
Notes Instructor Review - Installation and Preventative Maintenance
The Installation Checklist contains system requirements that must
be met prior to system installation or whenever system is
relocated.
The Preventative Maintenance checklist contains
recommendations for optimal performance of the CELL-DYN®
3200 System.
The instrument is designed to be very stable when operated
according to the recommendations in the Operator’s Manual and
should not require frequent calibration by the Operator.
END OF ACTIVITY
11-34 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Reticulocyte Package
Notes
Reticulocyte Package
The Reticulocyte Package software is designed to configure the
instrument to process stained, diluted specimens. This enables the
operator of the CELL-DYN® 3200 System to analyze a whole blood
specimen for reticulocytes.
Instrument
The Reticulocyte Package must be enabled in order to process
reticulocyte specimens. To enable the analyzer perform the
following steps:
• From the MAIN MENU, press the [SET UP] key followed by
the [OPERATION SET UP] key.
• From the OPERATION SET UP MENU screen, press the
[TURN ON RETIC PKG] key to enable the Reticulocyte Pack-
age.
• The RETIC MAIN menu screen displays and the software to
analyze reticulocytes is now enabled on the CELL-DYN 3200
System.
Each menu and function is modified when used in the Reticulocyte
Package. Therefore, some of the soft keys that are displayed in the
Standard Hematology Mode will be available from the different
Reticulocyte screens, and some will not.
• A Reticulocyte Data Log and a Reticulocyte QC Log are
available for samples and controls run within the Reticulocyte
Package.
Similar to the CELL-DYN Ruby System, the prepared specimen
run with the Reticulocyte Package on the CELL-DYN 3200 System
will measure results as a reticulocyte percentage. The reticulocyte
absolute number is automatically calculated when the RBC value
is made available from the Standard Hematology Data Log or
entered by the operator.
A reticulocyte background count should be included in the daily
start-up procedures on the CELL-DYN 3200 System when
reticuloctye analysis is performed. An acceptable reticulocyte
background is <100.
The CELL-DYN 3200 System can be returned to the Standard
Hematology function by pressing [TURN OFF RETIC PKG] from
the OPERATION SET UP MENU screen.
CELL-DYN Ruby® Field Service Training Workbook 11-35
204343-101 Dec 2008
Reticulocyte Package
Notes Specimen Preparation
Similar to the CELL-DYN Ruby System, the Reticulocyte specimen
is prepared by the operator using reticulocyte reagent to produce a
diluted, stained sample.
NOTE: For additional information on Reticulocyte analysis
on the CELL-DYN® 3200 System refer to the
CELL-DYN 3200 Operator’s Manual Section 14
Reticulocyte Package.
11-36 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module Summary
Notes
Module Summary
Now that you’ve completed the CELL-DYN® 3200 comparison
Module, you should be able to identify the similarities and
differences between the CELL-DYN Ruby System and CELL-DYN
3200 System. This includes software, operating conditions,
subsystems and components. You should also be able to perform
key Service and Support procedures and use diagrams to relate
subsystem function.
CELL-DYN Ruby® Field Service Training Workbook 11-37
204343-101 Dec 2008
Review 2
Notes
Review 2
1. How long should the Vacuum/Pressure Pumps “run” on the
CELL-DYN® 3200 System?
2. Which sensor is used to detect a short sample condition in the Closed
Mode?
Open Mode?
3. What valve is responsible for staging the HGB/NOC Sample to the
Optical Flow Cell?
4. Which level of Vacuum is responsible for aspiration in the Closed
Mode?
Open Mode?
5. What angles of scatter are used to differentiate Eosinophils from
Neutrophils?
6. List a couple of instrument symptoms that could indicate that the
Optics Bench is out of alignment?
End of Module
11-38 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Appendix
CELL-DYN Ruby® Field Service Training Workbook Appendix-1
204343-101 Dec 2008
Notes
Appendix-2 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 1 Review
Notes
Module 1 Review
1. Where is the TSB Sticker located on the CELL-DYN Ruby System?
Flow Panel.
2. What is the current TSB revision level of the CELL-DYN Ruby Systems
in the Classroom?
Student should record TSB revision from TSB Sticker located on
Training Instruments.
3. What is the ISA number of the CELL-DYN Ruby Installation
Procedure?
Current Revision of ISA 170-003.
4. Where within the CELL-DYN Ruby Service Resource documentation is
the “Analyzer Setpoints Reference Chart” located?
CELL-DYN Ruby Service and Support Manual, Troubleshooting Sec-
tion.
CELL-DYN Ruby® Field Service Training Workbook Appendix-3
204343-101 Dec 2008
Module 1 Review
Notes
Appendix-4 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 2 Case Study 1
Notes
Module 2 Case Study 1
The customer is reporting that a blue screen is shown on their
display and that the instrument is unresponsive to commands. The
following troubleshooting steps were performed or observed, and
the issues remain unresolved:
• Customer has cycled power
• +5VDC Power is present and steady
Using your knowledge of the STEP Process, of normal instrument
operation, and your troubleshooting resources (e-solutions, TSBs/
ISAs, etc.), you have determined that the Hard Disk Drive (HDD)
on the customer’s instrument has failed.
After replacing the HDD you now need to reinstall the software.
The customer has a back up copy that they have made during
weekly backups.
1. Use the Instrument Service and Support Manual and ISA database to
identify the procedures that would be necessary for you to restore the
instrument back to proper operation. List the procedures below:
Backup Procedure (VP-48).
Operating System (OS) Install and Hard Drive Format (VP-47).
Application Software Install (VP-41).
Restore from Backup (VP-49).
Operations Manual Install (VP-46).
Touchscreen Calibration (VP-6).
CELL-DYN Ruby® Field Service Training Workbook Appendix-5
204343-101 Dec 2008
Module 2 Case Study 1
Notes
Appendix-6 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 2 Review
Notes
Module 2 Review
1. List the two (2) heaters that have been added to the CELL-DYN Ruby
System?
HGB and WOC Heaters.
2. List the appropriate step(s) to calibrate the Elo Touchscreen monitor.
From the Windows application screen, locate the Elo icon in the lower
right corner. Click on the Elo icon to display the menu. Select Align
from the menu to begin calibration.
3. Where are the SHM boards located?
On the right side of the instrument behind the access cover.
4. Explain the difference in function between the Optical Short Sample
Sensor (S2) and the Two Ultrasonic Blood Sensors (S1, S3).
To detect a short sample condition during sample aspiration and
transfer two ultrasonic sensors (S1, S3) and one blood sample detec-
tor (S2) are employed in the sample aspiration flow system.
• S1 controls the leading edge (open & closed mode) during
aspiration.
• S3 controls the leading edge (open mode) during transfer.
These sensors detect the presence or absence of liquid in the
aspiration line, and they are not adversely affected by the
density or viscosity of the sample.
• Blood sample detector (S2) controls the leading edge (closed
mode) during transfer.
CELL-DYN Ruby® Field Service Training Workbook Appendix-7
204343-101 Dec 2008
Module 2 Review
Notes 5. Describe the difference between the two power switches on the
instrument and how each is properly used.
The System main power switch, located on the back of the Analyzer,
should be left ON during normal operating conditions.
With the System main power switch in the ON position, the Data Mod-
ule Power button (spring-loaded momentary type) is used to power the
Analyzer and Display ON.
The Display and Printer have their own power switches and should be
left ON as long as the main power switch to the System is ON. Power
to the Display and printer should be turned OFF when the System
main power switch is turned OFF.
The Application Programs “shutdown” menu option should be used to
turn the Analyzer OFF.
It is not necessary to turn the System main power switch OFF under
normal operating conditions. The System main power switch (rear
panel) should be turned OFF when a malfunction is suspected, when
when the system will be moved, or when the system will be inactive
for an extended period of time (greater than 2 weeks), or as deemed
necessary by an Abbott representative.
Appendix-8 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 3 Review
Notes
Module 3 Review
1. How many reagents are used on the CELL-DYN Ruby System to
perform a CBC?
Three (3) .
List the Reagents and their basic function:
Diluent/Sheath
• Maintain the stable diluted cell volume of each red cell and
platelet during the count and sizing portion of the
measurement cycle.
• Serve as a sheath fluid for the hydrodynamic focusing
process.
• Serve as a rinsing agent for the fluidics system.
CN-Free HGB/NOC Lyse
• Rapidly lyse the red blood cells and minimize the resultant
cellular debris.
• Strip the white cell cytoplasm leaving the nuclear membrane
intact so the white cell nuclei can be enumerated.
• Convert hemoglobin to a stable chromagen complex that is
measurable at 555 nm.
WBC Lyse
• Act as the diluent for the WBC.
• Osmotically lyse the red cells.
• Maintain the right scattering properties of the WBC for the
duration of the measurement period.
• Provide sufficient wetting action to prevent accumulation of
air bubbles in the WBC flow system.
• Serve as a rinsing agent for the WBC Mixing Chamber.
• Act as a diluent for Reticulocytes.
2. During normal operation, while the instrument is idle and in the Ready
state, the vacuum and pressure pumps cycle on/off every 5 seconds.
(unless Sample Loader lines are clamped).
CELL-DYN Ruby® Field Service Training Workbook Appendix-9
204343-101 Dec 2008
Module 3 Review
3. Where is bubble mix used on the CELL-DYN Ruby Flow Panel AND what does normal
bubble-mix look like?
RBC/PLT, HGB/NOC and WOC Mixing Chambers.
4.25 psi.
4. What is the HGB Heater operating range?
Between 40°C and 51°C.
5. What is the WOC Heater operating range?
Between 20°C and 40°C.
6. Describe the process for preparing whole blood for a closed mode precision.
To prepare specimens for use follow the guidelines stated below:
• Obtain 15 ml of normal whole blood within four (4) hours of draw.
• All specimens must have been properly collected in tubes containing EDTA
anticoagulant.
• Pool, mix, and aliquot into five (5) unused, red top (non anticoagulant) tubes.
• Be certain that all specimens used are brought to room temperature and mixed well
before aspiration.
7. Complete the Table by recording either a Calculated (C), Measured (M) or Derived (D) next
to each parameter.
White Blood Cell Parameters Red Blood Cell Parameters
M WBC: White Blood Cell Count (WOC) M RBC: Red Blood Cell Count
C NEU: Neutrophil Absolute Count C MCH: Mean Cell Hemoglobin
C LYM: Lymphocyte Absolute Count C MCHC: Mean Cell Hemoglobin Concentration
C MONO: Monocyte Absolute Count C HCT: Hematocrit
C EOS: Eosinophil Absolute Count D MCV: Mean Cell Volume
C BASO: Basophil Absolute Count D RDW: Red Cell Distribution Width
M %N: Neutrophil Percentage of WBCs M HGB: Hemoglobin Concentration
M %L: Lymphocyte Percentage of WBCs Reticulocyte Package
M %M: Monocyte Percentage of WBCs M %R: Percentage of Reticulocytes
M %E: Eosinophil Percentage of WBCs C RETC: Reticulocyte absolute concentration
M %B: Basophil Percentage of WBCs Platelet Parameters
**M PLT: Platelet Count
D MPV: Mean Platelet Volume
C PCT*: Plateletcrit
C PDW*: Platelet Distribution Width
*Clinical significance has not been established for PCT or PDW. Therefore, they are not reportable in the US
**The Platelet count is directly derived from measured optical data.
Appendix-10 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 4 Case Study 2
Notes
Module 4 Case Study 2
A CELL-DYN Ruby System has HGB Heater errors on all
specimen analysis including background counts. The following
troubleshooting steps were performed or observed, and the issues
remain unresolved:
• Customer has cycled power
• Auto Clean was performed
• The tubing to HGB Heater was reseated
• The HGB Heater feels warm to the touch
Using your knowledge of the STEP Process, of normal instrument
operation, and your troubleshooting resources (Troubleshooting
Information Database [eSolutions], TSBs/ISAs, etc.); What
procedures, checks, measurements, etc. would use to isolate the
root cause of this error?
Have class list checks and measurements. This may include VP-36
TCM Adjustment, Running Background, Checking Heater Tempera-
ture.
Review Cable Connection Diagram.
What would you do to resolve this error?
VP-36 TCM Adjustment, possible board replacement if board has
failed.
CELL-DYN Ruby® Field Service Training Workbook Appendix-11
204343-101 Dec 2008
Module 4 Case Study 2
Notes
Appendix-12 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 4 Review
Notes
Module 4 Review
1. Why do the vacuum and pressure pumps cycle so frequently?
while the instrument is idle and in the Ready state, the vacuum and
pressure pumps cycle on/off every 5 seconds.
2. What screen contains the Vacuum/Pressure Readings?
Select Diagnostics, Digital/Voltage Readings.
3. True or False----The PRM PCB receives +28 VDC from the PDM J20
to operate the pumps.
True.
4. What are the 3 Pressure levels?
13 psi, 9 psi and 4.25 psi.
5. Describe how the pressure system is used to maintain Pressure 3.
When Pressure 3 falls below the threshold stored and maintained by
the VPM, Solenoid 3 opens to allow pressure from Accumulator 1 to
fill Accumulator 3. When pressure in Accumulator 3 reaches the
specified level, Solenoid 3 is closed.
6. Where is the Multi-Port (Quick Disconnect) Coupler located on the
Pneumatic Unit?
Located on the Left Side of the instrument, next to the Pneumatic
Unit.
7. Which waste chamber has a Bubble Trap to prevent residual waste
from entering the vacuum accumulator?
Waste Chamber 3.
CELL-DYN Ruby® Field Service Training Workbook Appendix-13
204343-101 Dec 2008
Module 4 Review
Notes 8. There are five (5) ports on the HGB Flow Cell, which port is used to
deliver sample into the flow cell?
Top front port.
9. The HGB Heater is used to heat which two (2) reagents?
Diluent/Sheath and HGB Lyse.
Appendix-14 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 5 Case Study 3
Notes
Module 5 Case Study 3
A CELL-DYN Ruby has elevated Band/IG Flagging. The Customer
has verified that the flagging is not correlating with Manual Blood
Smears and an alternate analyzer. The following troubleshooting
steps were performed or observed, and the issues remain
unresolved:
• Customer has cycled power
• Auto Clean was performed
• 7.0 Latex CVs are reporting as follows:
• 0° = 6.2%
• 10° = 5.5%
• 90D° = 14.0%
• 90° = 13.0%
Using your knowledge of the STEP Process, of normal instrument
operation, and your troubleshooting resources (Troubleshooting
Information Database [eSolutions], TSBs/ISAs, etc.); What
procedures, checks, measurements, etc. would use to isolate the
root cause of this error?
Check gain settings and CVs.
Review Moving Average data and Scatterplot.
What would you do to resolve this error?
Adjust Optical Flow Cell Alignment and perform gain adjustment.
CELL-DYN Ruby® Field Service Training Workbook Appendix-15
204343-101 Dec 2008
Module 5 Case Study 3
Notes
Appendix-16 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 5 Review
Notes
Module 5 Review
1. Name the four angles of scatter that are measured on the CELL-DYN
Ruby System and what cell characteristic they are looking at.
a. 0° scatter is mainly representative of particle SIZE
b. 10° scatter is mainly representative of particle COMPLEXITY
c. 90D° scatter is mainly representative of particle GRANULARITY
d. 90° scatter is mainly representative of particle LOBULARITY
2. If the RBC Count is increased, the MCH will be Low and the MCV
will be not affected.
3. If the HGB measurement is low, the MCH will be Low and the
MCHC will be Low.
4. If a customer reports that the CELL-DYN Ruby System is reporting
numerous Band flags that are not confirmed by a blood smear. Where
would you begin your troubleshooting?
Check %CVs and Mean Channels. Review X-B Data.
Verify Fluidic System Integrity.
Adjust Flow Cell and perform Gain Adjustment as Indicated.
5. What order are samples staged into the Optical Flow Cell?
RBC/PLT, NOC and WOC.
The RBC/PLT is staged first through 54, if applicable, the NOC is
staged second through 41 and the WBC (WOC) is staged last
through 55.
6. What is an acceptable Laser Power Reading on the CELL-DYN Ruby
System?
A laser power reading above 3.5 mw.
CELL-DYN Ruby® Field Service Training Workbook Appendix-17
204343-101 Dec 2008
Module 5 Review
Notes 7. List the software steps required to view the Moving Average Program
results.
QC View, F5- Moving Average.
Use the F8 - Closed Batch Data view to examine the batch means.
View the Levey-Jennings graphs of the batches and determine
whether the results are acceptable.
Appendix-18 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 6 Case Study 4
Notes
Module 6 Case Study 4
How do you know if the Hemoglobin measurement is occurring
correctly? List at least 3 ways:
1st are my H&H results matching by x 3 rule.
2nd What are my reference and sample readings:
• What is normal? 2050+/-200.
• What are typically normal patient readings? 700-1000.
• What could very low sample or reference readings around
1-300 mean? Bubbles in HGB Flow Cell / Fluidic Issue.
• If results are zero what could be the problem? Bad Hgb Flow
Cell or FCM board.
3rd check digital voltage reading screen, verify HGB output is set at
5.1v? (Most commonly 5v is set too low. As HGB Flow cell ages the
voltage to HGB Flow cell must be increased to maintain proper mea-
surement) How would you adjust? VP-5.
CELL-DYN Ruby® Field Service Training Workbook Appendix-19
204343-101 Dec 2008
Module 6 Case Study 4
Notes
Appendix-20 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 6 Review
Notes
Module 6 Review
1. Where are the HGB Reference Readings located and what is a normal
reading?
Select Diagnostics, Diagnostic Views, click on Raw Data Summary.
Verify that HGB reference and sample readings are within 2050 ±200.
The difference between the HGB sample and reference readings
must be <20 counts after a background cycle.
2. What reagent is used during the HGB Reference Read?
Diluent/Sheath.
3. According to the Cable Connection Diagram, what board supplies LED
current the HGB Flow Cell?
FCM.
4. The higher the sample reading the lower the concentration of
HGB in the sample.
What is a typical Sample Reading?
• Average Sample Raw Data reading is ~700.
• Reference Reading is 2050 + 200.
• On a background count, both the sample and reference
readings should be similar numbers.
5. The sample enters the HGB Flow Cell through which line (top or side)?
Top front port.
6. Where are the voltage readings for the Hemoglobin Flow Cell located?
From the Diagnostics menu, select:
• Digital / Voltage Readings.
• HGB output (click on box to left).
• Stream.
The Password for Operator ID: FSE is required to gain access to the
Digital /Voltage Readings screen.
CELL-DYN Ruby® Field Service Training Workbook Appendix-21
204343-101 Dec 2008
Module 6 Review
Notes
Appendix-22 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 7 Case Study 5
Notes
Module 7 Case Study 5
You have just received a service call for a CELL-DYN Ruby
System because it is generating error message AIM 0840 Vacuum
Accumulator #1 Wet.
The following troubleshooting steps were performed or observed,
and the issue remains unresolved:
• Customer cycled power
• Customer performed drain accumulator in Special Pro-
tocols and noted no fluid being pushed out through the
waste line during drain accumulator protocol.
• Customer tried to manually drain accumulator with a
syringe and no fluid is being pulled out.
Using your knowledge of the STEP Process, of normal instrument
operation, and your troubleshooting resources (Troubleshooting
Information Database [eSolutions], TSBs/ISAs, etc.); What
procedures, checks measurements, etc. would you use to isolate
the root cause of this error?
Flush Lines, Drain Accumulator.
Discriminate between Pneumatic, electronic or plumbing problem:
• isolate potential air leak using hemostats.
• check solenoid function.
• check for obstructions.
• check VPM functionality.
CELL-DYN Ruby® Field Service Training Workbook Appendix-23
204343-101 Dec 2008
Module 7 Case Study 5
Notes
Appendix-24 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 7 Case Study 6
Notes
Module 7 Case Study 6
Instrument will not power on, there are no fans running and the
monitor has the dialog box, “check video cable connection”.
The following troubleshooting steps were performed or observed,
and the issues remain unresolved:
• Customer verified the power switch on back of analyzer
is in the ON position
• Customer checked power cord connection between the
instrument and wall outlet
• Customer plugged in an electrical device into wall out-
let. Electrical device is working
Using your knowledge of the STEP Process, of normal instrument
operation, and your troubleshooting resources (Troubleshooting
Information Database [eSolutions], TSBs/ISAs, etc.); What
procedures, checks, measurements, etc. would use to isolate the
root cause of this error?
Press the Data Module power button and check to see if the unit
boots up.
Verify outlet voltage is between 100 – 240 VAC and 47 – 63 Hz.
Perform VP-1 System Voltage Verification.
Verify that +5VDC is present on the ATX CPS.
What would you do to resolve this error?
Replace power supply that has no output voltage.
CELL-DYN Ruby® Field Service Training Workbook Appendix-25
204343-101 Dec 2008
Module 7 Case Study 7
Notes
Module 7 Case Study 7
The instrument you have been called to repair is generating HSSL
Com Communications errors only while performing Extended
Auto-Cleans. Customer cycles power but the issue reoccurs
everytime she performs the extended auto-clean.
Using your knowledge of the STEP Process, of normal instrument
operation, and your troubleshooting resources (Troubleshooting
Information Database [eSolutions], TSBs/ISAs, etc.); What
procedures, checks, measurements, etc. would use to isolate the
root cause of this error?
Check incoming power to ensure meeting specifications.
Perform VP-1 System Voltage Verification, issue could be a result of
a failure within the power system.
What seems to be the problem?
Possible PDM failure.
Appendix-26 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 7 Review
Notes
Module 7 Review
1. If the +5vdc signal were missing on the ATX CPS what type of errors or
symptoms would be seen on the analyzer?
Failure to receive this +5vdc signal will render the analyzer inoperable
(no voltages).
2. What voltages does the APS generate?
+15.5VDC and +28VDC.
3. Temperature Control Module receives +28 VDC from the PDM via
connector J15.
4. What voltages does the ATX CPS generate?
+3.3VDC, +5VDC, and ±12VDC.
5. The CDM PCBs are used to distribute the +28vdc and +15.5vdc from
the PDM PCB that are used to drive the solenoids in the system.
6. The purpose of the Pump Relay Module (PRM) PCB is to turn the
vacuum and pressure pumps ON/OFF.
7. Which SHM PCB provides the LED drive and reads the outputs of the
photo-detectors of the optical sensors in the Sample Loader?
SHM2.
8. Describe the function of the SPM PCB.
The Signal Processor Module (SPM) is the first board involved in the
counting and classification of the raw electronic signals received from
the Main Amplifier Module (MAM). The SPM assesses the data
received from the MAM, detects valid cell pulses, counts them, and
captures peak voltages, then forwards the information to the CPU/
DCM for further processing.
CELL-DYN Ruby® Field Service Training Workbook Appendix-27
204343-101 Dec 2008
Module 7 Review
Notes
Appendix-28 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 8 Case Study 8
Notes
Module 8 Case Study 8
You have just received a service call for a CELL-DYN Ruby
System because the sweep arm assembly on the Sample Loader
is not pushing racks back on the load side.
The following troubleshooting steps were performed or observed,
and the issues remain unresolved:
• Power to the analyzer has been cycled
• Customer has switched between the open and closed
mode
• Customer has extended the sweep arms manually with
no obstruction found.
• Customer has cleaned the sample loader base plate
Using your knowledge of the STEP Process, of normal instrument
operation, and your troubleshooting resources (Troubleshooting
Information Database [eSolutions], TSBs/ISAs, etc.); What
procedures, checks, measurements, etc. would use to isolate the
root cause of this error?
Perform VP-30 to see if there are any other Sample Loader issues.
Check cable connection on Valve 2.
Check to see if voltage is being supplied to Valve 2 during VP-30
operation.
Check tubing connection on the manifold assembly.
May need to replace the valve manifold assembly or replace SHM (2).
CELL-DYN Ruby® Field Service Training Workbook Appendix-29
204343-101 Dec 2008
Module 8 Case Study 8
Notes
Appendix-30 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 8 Review
Notes
Module 8 Review
1. The Sample Loader uses both vacuum and pressure to fill and empty
the bladders. TRUE FALSE
Vacuum is applied and the mixer head is lowered over the sample
tubes by the mixer lift air cylinder. Vacuum is applied to the bladders
to release the sample tube(s).
Pressure is applied to bladders to inflate and capture the sample
tube(s). Pressure is applied to the mixer lift air cylinder and the mixer
head and tubes are raised to clear the rack.
2. How much pressure is used to inflate the mixer bladders? 6.5 psi.
What provides it? regulator.
3. The GS2 guide system controls what systems on the Sample Loader?
The bar code spinner, wash block, and tube height flag.
4. Where should you measure the pressure for the Bladder Assembly?
Remove the plug to the pneumatic line verification port just behind
the tower assembly and attach the vacuum/pressure meter to the tub-
ing.
5. What valve on the Sample Loader is used for Rack Advancement?
Valve 1.
6. What valve on the Sampler Loader is used to raise the mixer head
assembly?
Valve 3.
7. The Sample Loader employs five 3-way valves to drive the air
cylinders and mixer bladders. What PCB controls these valves?
SHM2.
CELL-DYN Ruby® Field Service Training Workbook Appendix-31
204343-101 Dec 2008
Module 8 Review
Notes
Appendix-32 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 10 Case Study 9
Notes
Module 10 Case Study 9
You have just received a service call on a CELL-DYN Ruby System
because the instrument will not initialize.
The customer states the system locked-up while running and upon
reboot the system will not initialize. Error code HSSL Time-out
2100 on CELL-DYN Ruby System is being generated and system
appears to be locked up.
The Display is on and fans appear to be running. Customer did
note that shortly before the issue was noticed the lab lost power on
all systems not connected to emergency back-up generators.
Upon speaking with the customer you learn the customer had a
2nd CELL-DYN Ruby System hooked up to emergency power and
has UPS/Line Conditioner installed not experiencing the issue.
Additional troubleshooting steps performed by customer, and the
issue remains unresolved:
• Tried cycling power to the analyzer two times
• Tried plugging the instrument into a different outlet
• Reseated HSSL Cable on back of system
• Had facilities test outlet states recovering 110VAC
Using your knowledge of the STEP Process, of normal instrument
operation, and your troubleshooting resources (Troubleshooting
Information Database [eSolutions], TSBs/ISAs, etc.); What
procedures, checks measurements, etc. would you use to isolate
the root cause of this error?
Check incoming power to ensure meeting specifications.
Perform VP-1 System Voltage Verification.
CELL-DYN Ruby® Field Service Training Workbook Appendix-33
204343-101 Dec 2008
Module 10 Case Study 9
Notes
Appendix-34 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 10 Review
Notes
Module 10 Review
1. In the Dashboard screen which window displays a short list of logs and
files that are available for retrieval from the instrument?
Action Window.
2. Which log would you review to monitor how many times a system has
seen incomplete aspiration errors?
Event Log.
3. How is the reticulocyte reagent stored?
Stored at room temperature and in the dark.
4. Explain how to run a reticulocyte background count.
Verify the Analyzer is in the Ready state, and that you are in the Run
View screen.
In the NOTE region, select the QCID lookup icon. From the pull-down
menu, select RETC_background.
Remove the cap from fresh tube of reticulocyte reagent.
Place the tube under the aspiration probe, immerse the probe in the
reagent, and press the touch plate.
Listen for an audible beep and remove the tube from under the aspi-
ration probe, and replace the cap on the reagent tube.
Review the results for the background count.
5. What is an acceptable reticulocyte background count result?
An acceptable reticulocyte background is <100.
CELL-DYN Ruby® Field Service Training Workbook Appendix-35
204343-101 Dec 2008
Module 10 Review
Notes
Appendix-36 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 11 Review 1
Notes
Module 11 Review 1
1. How do you access Hidden screens on the CELL-DYN® 3200
System?
Diagnostics F12/F1.
2. X-B files are accessed under which menu?
Quality Control Menu.
3. Compare the CELL-DYN Ruby Power Supplies with the CELL-DYN
3200 Power Supplies?
The CELL-DYN Ruby has 4 power Supplies while the CELL-DYN
3200 has 3.
CELL-DYN RUBY:
• Analyzer Power Supply (APS).
• ATX Computer Power Supply (CPS).
• Laser Power Supply (LPS).
• Power Distribution Module (PDM).
• Generates voltages used in analyzer.
CELL-DYN 3200:
• Linear Power Supply Module (Main PS).
• Laser Power Supply Module (LPS).
• Power Distribution Module (PDM).
• Does not generate voltage only distributes voltage.
4. Are the PRM Boards interchangeable between the CELL-DYN Ruby
and CELL-DYN 3200 Systems?
No, the Pump Relay Module (PRM) boards differ between the
CELL-DYN Ruby and CELL-DYN 3200 Systems and are therefore
not interchangeable.
CELL-DYN Ruby® Field Service Training Workbook Appendix-37
204343-101 Dec 2008
Module 11 Review 1
Notes 5. What is the function and location of the MAM Board on the CELL-DYN
3200 System?
The MAM boards on the CELL-DYN® 3200 and CELL-DYN Ruby
System perform the same function. This consists of:
• Processing optical channel signals between pre-amplifiers
and SPM PCB.
• Sending optical data to the CPU/DCM for processing.
6. Under what menu is the HGB Voltage Reading Screen located?
Diagnostics Menu.
Appendix-38 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 11 Case Study 11
Notes
Module 11 Case Study 11
During the morning run on the CELL-DYN® 3200 System, the
operator notices the Pusher arms are not retracting/extending
properly.
The following troubleshooting procedures were performed without
resolution of the problem:
• Power cycled
• Cleaned Sample Loader and arms
Using your knowledge of the STEP Process, of normal instrument
operation, and your troubleshooting resources (Troubleshooting
Information Database [eSolutions], IP’s, TSBs/ISAs, etc.) answer
the following questions.
Record your troubleshooting processes below:
List whatever procedure(s) you would perform next:
Diagnostics Extend/Retract.
What clues can you identify from the data provided or procedures
you would perform?
Pusher arms are not retracting/extending properly.
Use Service Manual general data to determine which valve controls
sweep arms V2 load side and V5 unload side.
What comparisons can you make?
The Sample Loader is driven primarily off vacuum/pressure. The eas-
iest way to troubleshoot the system is to run the loader with a few
blood samples and observe what is occurring correctly and incorrectly
using cause analysis.
CELL-DYN Ruby® Field Service Training Workbook Appendix-39
204343-101 Dec 2008
Module 11 Case Study 11
Notes
Appendix-40 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 11 Case Study 12
Notes
Module 11 Case Study 12
During the performance of a service call on a CELL-DYN® 3200
System, the Optical Flow Cell was adjusted to specification. A
WOC gain adjustment was performed and the 90D DAC setting is
reporting at 4095 and the 90° is reporting at 1032. The CV’s for the
four channels are reporting: 0°=4.1%, 10°=3.4%, 90°=12.1%,
90°D=10.8%.
The results looked similar to the information shown below
NEU:.010 %N:.222
EOS: 2.49 %E: 53.8
Using your knowledge of the STEP Process, of normal instrument
operation, and your troubleshooting resources (Troubleshooting
Information Database [eSolutions], TSBs/ISAs, etc.), list whatever
procedure(s) you would perform next?
Neutrophils are being falsely identified as Eosinophils. 90D DAC set-
ting 4095 is at highest reading.
Perform VP-22 PMT pre-amplifier adjustment.
CELL-DYN Ruby® Field Service Training Workbook Appendix-41
204343-101 Dec 2008
Module 11 Case Study 12
Notes
Appendix-42 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
Module 11 Review 2
Notes
Module 11 Review 2
1. How long should the Vacuum/Pressure Pumps “run” on the CELL-DYN
3200 System?
Pumps kick on and off intermittently throughout a cycle for a
CELL-DYN® 3200 CS (Closed Sampler) and SL (Sample Loader).
During READY status on a CS the pumps only kick on > 2 min.
For a SL the pumps may cycle very 10-30 seconds in READY status.
2. Which sensor is used to detect a short sample condition in the Closed
Mode?
In the Closed Mode the ultrasonic and an additional optical sensor is
used. The ultrasonic checks for sample integrity to ensure no bubbles
in the line and the optical sensor is a timed stopping point to ensure
enough sample has been aspirated.
Open Mode?
Only the Ultrasonic Sensor is used to detect presence or absence of
blood in the aspiration line.
3. What valve is responsible for staging the HGB/NOC Sample to the
Optical Flow Cell?
Valve 41.
4. Which level of Vacuum is responsible for aspiration in the Closed
Mode?
4.5” Hg Acceptable Range of (4.2-4.8).
Open Mode?
3.3” Hg Acceptable Range of (3.1-3.5).
5. What angles of scatter are used to differentiate Eosinophils from
Neutrophils?
90ºD for Granularity and 90º for Lobularity.
CELL-DYN Ruby® Field Service Training Workbook Appendix-43
204343-101 Dec 2008
Module 11 Review 2
Notes 6. List a couple of instrument symptoms that could indicate that the
Optics Bench is out of alignment?
The appearance of “George” is compressed meaning the lympho-
cytes populations are not falling at approximately 1 x 1 and Neutro-
phils at 2.5 x 2.5.
Also Poor CV’s with 0º>8%, 10º>5%, 90º>18%, and 90ºD>18%.
End of Module
Appendix-44 CELL-DYN Ruby® Field Service Training Workbook
Dec 2008 204343-101
You might also like
- ClimaDocument1 pageClimaАлексПрынзинNo ratings yet
- BioSystems A-15 Analyzer - Service Manual PDFDocument131 pagesBioSystems A-15 Analyzer - Service Manual PDFJay KimNo ratings yet
- RT-7600 EngineeDocument2 pagesRT-7600 EngineeАлексПрынзинNo ratings yet
- BioSystems A 25Document147 pagesBioSystems A 25VictorNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- GE 15 - Week 6 To 7Document33 pagesGE 15 - Week 6 To 7Adrian leeNo ratings yet
- RA 062 Generic Balance MachineDocument6 pagesRA 062 Generic Balance MachineAidan BrittonNo ratings yet
- SAILOR 6249 VHF Survival Craft UserManualDocument58 pagesSAILOR 6249 VHF Survival Craft UserManualRichard Jaye BalisacanNo ratings yet
- SoftMount Gun User Manual - Ver 2 - 0 - WGP-SMG-UM-EL-2 - 0-0519Document81 pagesSoftMount Gun User Manual - Ver 2 - 0 - WGP-SMG-UM-EL-2 - 0-0519Gues001No ratings yet
- Baseline Risk Assessment and Risk Matrix (An Example)Document8 pagesBaseline Risk Assessment and Risk Matrix (An Example)Victor75% (4)
- City of Malolos DRRM Plan 2022 2026Document235 pagesCity of Malolos DRRM Plan 2022 2026arch.stephenbuenaventuraNo ratings yet
- HIRA No 22 Installation Use of Temp Electrical Supplies SBDDocument2 pagesHIRA No 22 Installation Use of Temp Electrical Supplies SBDMobin Thomas AbrahamNo ratings yet
- TRA Strainer Cleaning 01Document7 pagesTRA Strainer Cleaning 01Ijaz Hussain0% (1)
- Disaster Baguio PDFDocument129 pagesDisaster Baguio PDFLee Cel100% (4)
- All Hira 2-1Document156 pagesAll Hira 2-1Mojib. AhmadNo ratings yet
- Blower Units DELTA BLOWER Generation 5Document199 pagesBlower Units DELTA BLOWER Generation 5victorhernandezrega100% (2)
- Safety & Pollution Control in ManufacturingDocument7 pagesSafety & Pollution Control in ManufacturingttNo ratings yet
- Field Safety Considerations and Its Design ImplicationsDocument192 pagesField Safety Considerations and Its Design ImplicationsPRASAD6219100% (1)
- Vulnerable Life Situation PerspectiveDocument13 pagesVulnerable Life Situation Perspectivecitylink set6pasig100% (1)
- Swiss Re Mind The Risk PDFDocument39 pagesSwiss Re Mind The Risk PDFlifeadventureNo ratings yet
- Toolbox Talks Office SafetyDocument1 pageToolbox Talks Office Safetyfandhy AnwarNo ratings yet
- Off The Shelf Software Use in Medical Devices-Guidance For Industry and Food and Drug Administration StaffDocument29 pagesOff The Shelf Software Use in Medical Devices-Guidance For Industry and Food and Drug Administration Staffjohn DevinsNo ratings yet
- Topic 9 Heritage and Global Issues-1Document18 pagesTopic 9 Heritage and Global Issues-1AllenNo ratings yet
- Humastar 100/200: - Service ManualDocument194 pagesHumastar 100/200: - Service ManualAniket DubeyNo ratings yet
- National. Disasyer $K Redijc11On and Managemen? CouncilDocument6 pagesNational. Disasyer $K Redijc11On and Managemen? CouncilCeslhee AngelesNo ratings yet
- RMTH Chapter OneDocument42 pagesRMTH Chapter Onesosina eseyewNo ratings yet
- Loading Dari Pelabuhan Ke Kapal TongkanhgDocument10 pagesLoading Dari Pelabuhan Ke Kapal Tongkanhgfadhil AbdullahNo ratings yet
- DD Iso TS 25740-1-2011Document54 pagesDD Iso TS 25740-1-2011Олег СоловьевNo ratings yet
- Risk Assessment Process For Occupational Dive Work ExampleDocument12 pagesRisk Assessment Process For Occupational Dive Work ExampleravindraNo ratings yet
- ES5001 Course OutlineDocument4 pagesES5001 Course OutlineAriebima AbigaraNo ratings yet
- Alinity CDocument200 pagesAlinity Cjaviergamarquez50% (2)
- BS-240Pro&240E New Service Manual V3.0 enDocument419 pagesBS-240Pro&240E New Service Manual V3.0 enXavieltNo ratings yet
- ISO 9001, 14001 & 18001 INTEGRATED MANAGEMENT SYSTEM GUIDEDocument16 pagesISO 9001, 14001 & 18001 INTEGRATED MANAGEMENT SYSTEM GUIDEMeng Hwi KorNo ratings yet
- DPVNHS Contingency Plan Volcanice EruptionDocument9 pagesDPVNHS Contingency Plan Volcanice EruptionMark Neil BendañaNo ratings yet
- Nature of DisasterDocument45 pagesNature of DisasterElla PerezNo ratings yet