Professional Documents
Culture Documents
10 Mechanism Table 2018
10 Mechanism Table 2018
Uploaded by
Criselda Oliva CarinoCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
10 Mechanism Table 2018
10 Mechanism Table 2018
Uploaded by
Criselda Oliva CarinoCopyright:
Available Formats
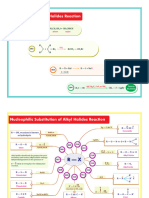
from "Master Organic Chemistry"
Identifying the Patterns in Carbonyl Reaction Mechanisms masterorganicchemistry.com
2019 Version, Copyright 2019 James A. Ashenhurst
james@masterorganicchemistry.com
O Reactions of neutral NR 2 NR 2
HO R nucleophiles with
Grignard Reaction A P R X SN2 D
R–MgX alkyl halides R R R
R R R R
Reactions of HO RO
O anionic nucleophiles R X R OR R OH R CN SN2
HO CN
Cyanohydrin NaCN A P with alkyl halides CN
formation R R R R
O O
O Enolate alkylation R X base
Ketone or aldehyde HO H D SN2
NaBH 4 (ketones or esters) R CH2R R R
reduction A P
or LiAlH4 R R R R R
O O O OH O
base HO H
Base-catalyzed aldol P
D A P Reduction of esters LiAlH4 A E A
reaction R CH2R H R R R R OR R H
R
Addition of neutral O O
nucleophiles to acid RNH 2 Grignard Reaction O HO R
A E D + acid chloride, A E A P
halides or anhydrides RMgX
R Cl R NHR ester, or anhydride R Cl R R
(e.g.amines, alcohols, water)
O O O O R
O acid N
Addition of anionic nucleophiles HO RO
A E Imine formation RNH 2 P A PT E D
to acid halides or anhydrides R Cl R OH R OR R NH 2 R R R R
H 2N
(e.g.RO , H 2N , HO )
O O O O O acid O
base PT D
Claisen condensation D A E Fischer esterification ROH P A E
*note 1 R OH R OR
R CH2R R OR R R
R
O O
O O acid
1,4 addition of Gilman R 2CuLi Amide hydrolysis H 2O P A PT E D
14A P R NR 2 R OH
reagent R R
R
O O O O O acid RO OR
base E D
Formation of acetals ROH P A PT A
Michael reaction D 14A P R R R R
R CH2R R R R
R
O O RO OR acid O
Hydrolysis of acetals H 2O P E A PT E D
RNH 2 14A PT R R R R
1,4 addition of R R
neutral nucleophiles
RHN
O O acid O
Acid catalyzed aldol P T A PT T 14E D
R CH2R H R R R
R
*note 1 - There is actually a fourth step; base removes a proton from the
acidic alpha-carbon, rendering the reaction irreversible. Acidic workup gives
the final product. The Nine Mechanistic Components (with examples)
Addition to carbonyl [1,2-addition] Elimination [1,2-elimination] SN2 Deprotonation Proton Transfer
O O O O O
O Nu O Intramolecular acid-base reaction
A O Nu E SN2 R H R D base PT
R H 3C R H 2C R example:
X R X R X R Nu R
Cα X H
Nu Removal of a proton from substrate H
HO OR H 2O OR
[1,4] addition Protonation R R R R
[1,4] elimination Keto-Enol Tautomerization
O O O O This can either be drawn as
OH H acid
O O OH P an intramolecular reaction (one step)
14A Nu 14E T or as a deprotonation (D)followed by
R R H H 2C R H 3C R
R R H 3C R R a protonation (P)
Nu H Addition of a proton to substrate
Nu X β
You might also like
- Identifying The Patterns in Carbonyl Reaction Mechanisms: R R NR NRDocument1 pageIdentifying The Patterns in Carbonyl Reaction Mechanisms: R R NR NRdjdjdhNo ratings yet
- R. K. Malik'S Newton Classes RanchiDocument2 pagesR. K. Malik'S Newton Classes RanchiVikas NagarNo ratings yet
- 9-Carbonyl 2019 PDFDocument1 page9-Carbonyl 2019 PDFNoel SibyNo ratings yet
- FunctionalGroups AnswersDocument2 pagesFunctionalGroups AnswerstaylorNo ratings yet
- Functional Group Reactions: C Synthesis Strategies, Chem 315/316 / Beauchamp 1Document19 pagesFunctional Group Reactions: C Synthesis Strategies, Chem 315/316 / Beauchamp 1Zia urRehman100% (1)
- Hydrocarbons Derivatives - Alkyl Halide - Aryl Halide PDFDocument15 pagesHydrocarbons Derivatives - Alkyl Halide - Aryl Halide PDFAhmed HammadNo ratings yet
- Organic Chem NotesDocument49 pagesOrganic Chem NotesPriyaNo ratings yet
- Synthesis and Evaluation of Antioxidant Activity of Semicarbazone DerivativesDocument5 pagesSynthesis and Evaluation of Antioxidant Activity of Semicarbazone DerivativesWalid EbaiedNo ratings yet
- Grupos Funcionais Organicos (Ingles)Document1 pageGrupos Funcionais Organicos (Ingles)Jefferson RibeiroNo ratings yet
- Essential Organic Chemistry Paula Yurkanis Bruice Full ChapterDocument67 pagesEssential Organic Chemistry Paula Yurkanis Bruice Full Chaptermargaret.jones429100% (7)
- Iit Reductions PDFDocument71 pagesIit Reductions PDFAshish SinghNo ratings yet
- Hydrocarbon: GMP GRDocument30 pagesHydrocarbon: GMP GRVinod AgrawalNo ratings yet
- Ebook Always Learning Paula Yurkanis Bruice Essential Organic Chemistry Pearson 2016 8Th Edition Paula Yurkanis Bruice Online PDF All ChapterDocument69 pagesEbook Always Learning Paula Yurkanis Bruice Essential Organic Chemistry Pearson 2016 8Th Edition Paula Yurkanis Bruice Online PDF All Chaptersonya.martinez866100% (9)
- Chem-353-Lecture 2Document10 pagesChem-353-Lecture 2Caleb AsharleyNo ratings yet
- C C Bond Formation: Aldol Condensation - Aldol Condensation Initially GiveDocument12 pagesC C Bond Formation: Aldol Condensation - Aldol Condensation Initially Giveaggelisgeorge8546No ratings yet
- Sach Giai Bai Tap 1 - 11Document22 pagesSach Giai Bai Tap 1 - 11Son Nguyen ThiNo ratings yet
- Synthesis of ImidazolesDocument5 pagesSynthesis of ImidazolesMuhammad SalehNo ratings yet
- Heterocyclic Chemistry: Chapter 10:pyrroles, Reactions and SynthesisDocument30 pagesHeterocyclic Chemistry: Chapter 10:pyrroles, Reactions and SynthesisTaciturnoait NihilistaNo ratings yet
- REDUCTIONS FinalDocument11 pagesREDUCTIONS Finalgamer boomerNo ratings yet
- Enantioselective (3+3) Atroposelective Annulation Catalyzed by N-Heterocyclic CarbenesDocument10 pagesEnantioselective (3+3) Atroposelective Annulation Catalyzed by N-Heterocyclic CarbenesKatrin MarchenkoNo ratings yet
- EtherDocument1 pageEtherBao TranNo ratings yet
- Reactions of Carboxylic Acids - SibiDocument4 pagesReactions of Carboxylic Acids - SibiAmir HussainNo ratings yet
- Kap 12,13,17, IIDocument12 pagesKap 12,13,17, IImuraliNo ratings yet
- Pauson Khand ReaktionDocument2 pagesPauson Khand ReaktionOrigamist KryaNo ratings yet
- Enzymatic Synthesis of Amides 2015Document44 pagesEnzymatic Synthesis of Amides 2015bluedolphin7No ratings yet
- IOC Class-9 NotesDocument22 pagesIOC Class-9 Notesmardarchod 123No ratings yet
- Northrup AldolDocument22 pagesNorthrup AldolSubham NandiNo ratings yet
- Textbook Essential Organic Chemistry Paula Yurkanis Bruice Ebook All Chapter PDFDocument53 pagesTextbook Essential Organic Chemistry Paula Yurkanis Bruice Ebook All Chapter PDFphillip.kimrey957100% (11)
- 2 OxidationDocument28 pages2 Oxidationaggelisgeorge8546No ratings yet
- Organic ReactionsDocument1 pageOrganic Reactionsiceman2233No ratings yet
- Synthesis of Metal Complexes of Hydroxamic AcidDocument1 pageSynthesis of Metal Complexes of Hydroxamic AcidChannal Saif100% (1)
- A. C-C Bond Forming Reactions: 04/05/2017 - Lecture:11-12Document39 pagesA. C-C Bond Forming Reactions: 04/05/2017 - Lecture:11-12asif MehmoodNo ratings yet
- Benzodiazepines andDocument49 pagesBenzodiazepines andryrahulyadav4No ratings yet
- Synthesis of Commercial Drugs 2011-12 - M2 HanoiDocument28 pagesSynthesis of Commercial Drugs 2011-12 - M2 HanoiCy MoonNo ratings yet
- Hi Let Me Download From ScribdDocument15 pagesHi Let Me Download From Scribdforfuture reviewersNo ratings yet
- Nmat Reviewer Orgchem PDFDocument15 pagesNmat Reviewer Orgchem PDFAlice Katrina100% (1)
- JURNAL Sintesis Semi KarbazoneDocument5 pagesJURNAL Sintesis Semi Karbazoneluluatul maghfirohNo ratings yet
- REaction MechanismsDocument5 pagesREaction MechanismstaizokaiNo ratings yet
- tổng quan về hydrazoneDocument32 pagestổng quan về hydrazoneHằng ĐàmNo ratings yet
- Quinoline SynthesisDocument6 pagesQuinoline SynthesisFrancesco TutinoNo ratings yet
- Ald KetoneDocument20 pagesAld Ketonejohal2530No ratings yet
- Strecker Reaction and Its ApplicationsDocument14 pagesStrecker Reaction and Its ApplicationssairachudharyNo ratings yet
- Module3 PDFDocument44 pagesModule3 PDFveenaNo ratings yet
- Reducing AgentDocument15 pagesReducing Agentpranshul jadonNo ratings yet
- Quest Journals J of Res in Pharm Sci. Vol-3-Issue-3-2016!10!19Document10 pagesQuest Journals J of Res in Pharm Sci. Vol-3-Issue-3-2016!10!19Mayur PatelNo ratings yet
- Important Reactions of Alkyl HalidesDocument3 pagesImportant Reactions of Alkyl HalidessusankmadiNo ratings yet
- Art - 10.1007 - s12039 013 0495 6 PDFDocument7 pagesArt - 10.1007 - s12039 013 0495 6 PDFمحمد بلحوتNo ratings yet
- CHM4125SAR Biomolecules Final 2023Document26 pagesCHM4125SAR Biomolecules Final 2023alec.lafrance88No ratings yet
- Organic Chemistry Prochirality Lecture Outline: S-Configuration R-ConfigurationDocument3 pagesOrganic Chemistry Prochirality Lecture Outline: S-Configuration R-ConfigurationcfmonarquiaNo ratings yet
- Chem12 C2300 SWBTDocument15 pagesChem12 C2300 SWBTAbdulrahman MaherNo ratings yet
- Chem12 C2300 SWBTDocument15 pagesChem12 C2300 SWBTAbdulrahman MaherNo ratings yet
- Ugi ReactionDocument12 pagesUgi ReactionFiruj AhmedNo ratings yet
- Organic ConversionDocument9 pagesOrganic ConversionAnonymous lmpvRsaz90% (1)
- Aldehydes and KetonesDocument1 pageAldehydes and KetonesRaiyad RezaNo ratings yet
- Advanced Organic Reactions 2000 - WarrenDocument174 pagesAdvanced Organic Reactions 2000 - Warrenshiv57100% (3)
- SelectivityDocument4 pagesSelectivitySamik BiswasNo ratings yet
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- Phosphorus-Nitrogen Compounds: Cyclic, Linear, and High Polymeric SystemsFrom EverandPhosphorus-Nitrogen Compounds: Cyclic, Linear, and High Polymeric SystemsNo ratings yet
- Fluidization Laboratory of SaskatchewanDocument4 pagesFluidization Laboratory of SaskatchewanTae Vorachit UmnuaypornNo ratings yet
- Hydro Meteorological HazardsDocument1 pageHydro Meteorological HazardsKylie Zhane Uy0% (1)
- Orbital Motors: OMEW Standard and With Low Speed OptionDocument20 pagesOrbital Motors: OMEW Standard and With Low Speed OptionMINH Phạm Mai NhậtNo ratings yet
- Rajesh Joshi Enhanced LectureDocument9 pagesRajesh Joshi Enhanced LectureHimanshu Goel100% (3)
- AC MUZ HJ35VA MSZ HJ35VA 1713423466 25 178f45c6Document6 pagesAC MUZ HJ35VA MSZ HJ35VA 1713423466 25 178f45c6Book LebanonNo ratings yet
- LNP: April 10, 2016: News PresentationDocument80 pagesLNP: April 10, 2016: News PresentationLNP MEDIA GROUP, Inc.No ratings yet
- Quantum Mechanics Course PeriodicpotentialsDocument4 pagesQuantum Mechanics Course PeriodicpotentialsjlbalbNo ratings yet
- The Following Is The List of Registered Exporters of Fresh Frozen ChilledDocument21 pagesThe Following Is The List of Registered Exporters of Fresh Frozen Chilledtapioca leNo ratings yet
- Network of More Than 2,500 Growers (Strong Established Distribution System)Document8 pagesNetwork of More Than 2,500 Growers (Strong Established Distribution System)Anqi LiuNo ratings yet
- Rain Water Harvesting ProjectDocument19 pagesRain Water Harvesting ProjectManoj Janardan Jayashree Terekar100% (1)
- Nemo Outdoor 6.41 ManualDocument380 pagesNemo Outdoor 6.41 ManualPunky HeroNo ratings yet
- Grammar Test 1Document5 pagesGrammar Test 1Vy NguyễnNo ratings yet
- SyllabusDocument26 pagesSyllabusmasoodmuhidNo ratings yet
- How To Prevent, Find, and Treat Head LiceDocument12 pagesHow To Prevent, Find, and Treat Head LiceOmer ErgeanNo ratings yet
- Artificial Tears Reference Guide: LubricatingDocument5 pagesArtificial Tears Reference Guide: LubricatingPaola Ortega FrancoNo ratings yet
- Metalwork: PAPER 1 Theory, Drawing and DesignDocument12 pagesMetalwork: PAPER 1 Theory, Drawing and Designmstudy123456No ratings yet
- High Speed Milling of Hardened Steel Convex SurfaceDocument12 pagesHigh Speed Milling of Hardened Steel Convex SurfaceZerina ČustovićNo ratings yet
- Location and Design For Traffic SignalsDocument7 pagesLocation and Design For Traffic SignalsGauri JagtapNo ratings yet
- Restricted Earth Fault Technical ReportDocument5 pagesRestricted Earth Fault Technical ReportVijai PrasathNo ratings yet
- Intracellular Compartments and Vesicular Traffic - 2016Document32 pagesIntracellular Compartments and Vesicular Traffic - 2016Muhammad Akip PoapaNo ratings yet
- A Message From The Past: 2 Exercise 1Document12 pagesA Message From The Past: 2 Exercise 1Theklia GeorghiadouNo ratings yet
- TableDocument10 pagesTableRome GentaNo ratings yet
- Epilepsy in ChildrenDocument34 pagesEpilepsy in ChildrenAdam MochtarNo ratings yet
- Top 25 Google Maps Interview Questions and AnswersDocument12 pagesTop 25 Google Maps Interview Questions and AnswersTutorialsMateNo ratings yet
- MagnetometerDocument16 pagesMagnetometerButnariu CodrutNo ratings yet
- The Sloop's Log Fall 2012Document24 pagesThe Sloop's Log Fall 2012Chebeague Island Historical SocietyNo ratings yet
- PWRSLMDocument3 pagesPWRSLMGANESH MURUGANNo ratings yet
- Boettcher ThermodynamicsDocument78 pagesBoettcher ThermodynamicsCHE.ENG1734No ratings yet
- Activity 4Document4 pagesActivity 4Mark Galerio100% (1)
- 03 Cutterbit PDFDocument6 pages03 Cutterbit PDFsanty222No ratings yet