Professional Documents
Culture Documents
Starting Physiology: Bioelectrogenesis: How We Teach: Classroom and Laboratory Research Projects
Uploaded by
Rafael LunelliOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Starting Physiology: Bioelectrogenesis: How We Teach: Classroom and Laboratory Research Projects
Uploaded by
Rafael LunelliCopyright:
Available Formats
Adv Physiol Educ 39: 397–404, 2015;
doi:10.1152/advan.00051.2015. How We Teach: Classroom And Laboratory Research Projects
Starting physiology: bioelectrogenesis
Vander Baptista
Department of Physiological Sciences, Centre of Biological Sciences, Federal University of Santa Catarina, Florianópolis,
Santa Catarina, Brazil
Submitted 3 April 2015; accepted in final form 4 August 2015
Baptista V. Starting physiology: bioelectrogenesis. Adv Physiol The Didactic Model
Educ 39: 397– 404, 2015; doi:10.1152/advan.00051.2015.—From a
Cartesian perspective of rational analysis, the electric potential dif- As shown in Fig. 1, the didactic model is based on a single
ference across the cell membrane is one of the fundamental concepts isolated cell submersed into an electrolyte solution bath and an
for the study of physiology. Unfortunately, undergraduate students appropriate measuring device for ⌬Vm is used, just as in a real
often struggle to understand the genesis of this energy gradient, which electrophysiology experiment. However, this is a fictitious
makes the teaching activity a hard task for the instructor. The topic of situation, which assumes that a lipid bilayer sheet, freely
bioelectrogenesis encompasses multidisciplinary concepts, involves floating in an electrolyte solution, has suddenly engulfed a
several mechanisms, and is a dynamic process, i.e., it never turns off portion of the surrounding fluid and spontaneously formed a
during the lifetime of the cell. Therefore, to improve the transmission cell-like spherical structure with all the relevant intracellular
and acquisition of knowledge in this field, I present an alternative machinery. Under these imaginary conditions, we have to
didactic model. The design of the model assumes that it is possible to consider that 1) the intracellular fluid (ICF) and extracellular
build, in a series of sequential steps, an assembly of proteins within
fluid (ECF) have the same ionic composition and 2) the plasma
the membrane of an isolated cell in a simulated electrophysiology
experiment. Initially, no proteins are inserted in the membrane and the
membrane initially consists of a simple phospholipid bilayer,
cell is at a baseline energy state; the extracellular and intracellular i.e., without proteins. In addition, the didactic model also
fluids are at thermodynamic equilibrium. Students are guided through assumes that it is possible to control membrane proteins, i.e.,
a sequence of four steps that add key membrane transport proteins to we can insert or remove them. This is just the starting point for
the model cell. The model is simple at the start and becomes progres- the student’s understanding of bioelectrogenesis mechanisms.
sively more complex, finally producing transmembrane chemical and
electrical gradients. I believe that this didactic approach helps instruc- Initial Considerations
tors with a more efficient tool for the teaching of the mechanisms of
resting membrane potential while helping students avoid common Before we start building up the generation of ⌬Vm, it is
difficulties that may be encountered when learning this topic. important to remember some key concepts:
bioelectrogenesis; didactic model; sequencing instruction • Electric charges (Q) of the same sign repel each other,
whereas those of opposite signs attract each other with
electric force (F). According to Coulomb’s law (F ⬵ Q1 ⫻
MOST CELLS maintain an electrical potential difference across Q2/d2), these interactions decrease with the square of the
the cell membrane (⌬Vm), which plays many of roles in cellular distance (d).
homeostasis, such as the transmembrane transport of different • The electric potential difference (⌬V) is related to the spatial
substances. In particular, ⌬Vm in excitable cells, referred to as separation of electric charges. There is no ⌬V between two
resting membrane potential, is the basis for the action potential, circumscribed areas, A and B, if both of them have the same
the main way for fast signaling over long distances in neurons. net charge, as determined by the number of positive and
Given the importance of ⌬Vm, it is imperative that a student of negative charges in each place. However, if an external
physiology has a very clear understanding of the mechanisms force, overcoming the coulomb attraction, translocates one
by which the cell generates and maintains such a transmem- charge from A to B, ⌬V is developed between them. Since
brane energy gradient. However, even though general physi- ⌬V equals the energy (U) spent by the translocated charge
ology textbooks provide good basic explanations, the multidis- (⌬V ⫽ U/Q), the higher the number of separated electric
ciplinary concepts underlying bioelectrogenesis are not usually charges, the higher the resulting ⌬V.
presented together. The knowledge gaps can make it difficult to • ECF and ICF are electrolyte solutions and therefore electri-
qualitatively and quantitatively analyze all the mechanisms cally conductive. Furthermore, both fluids are primarily
involved as well as visualize them all working together. In electroneutral, i.e., the concentration of cations ⫽ the con-
addition, from the teacher’s perspective, it can be a hard task to centration of anions. Na⫹ and Cl⫺ are the main extracellular
link all the steps of bioelectrogenesis and successfully trans- ions, whereas K⫹, Pi, and anionic proteins are the main
form the instructor’s understanding into an effective pedagog- intracellular charge carriers. Here, for simplicity, the anions
ical approach. In the present article, aiming to improve or are not represented in the figures, but we have to keep in
facilitate the teaching-learning process, an alternative didactic mind that they are present in equivalent charge to those of
strategy for bioelectrogenesis is presented. the cations.
• The thermodynamic variables (pH, temperature, osmolarity,
etc.) of the ECF are kept constant by homeostatic mecha-
Address for reprint requests and other correspondence: V. Baptista, Dept. de
nisms. For our purpose, it is important to note that 1) Na⫹
Ciências Fisiológicas, CCB, UFSC, Campus Trindade, Florianópolis, Santa concentration is kept high (⬃142 mM) relative to K⫹ con-
Catarina 88040-970, Brasil (e-mail: vander.baptista@ufsc.br). centration (⬃4 mM), as shown in Fig. 1, where the relative
1043-4046/15 Copyright © 2015 The American Physiological Society 397
Downloaded from journals.physiology.org/journal/advances (189.004.076.190) on February 9, 2022.
How We Teach: Classroom And Laboratory Research Projects
398 TEACHING AND LEARNING BIOELECTROGENESIS
these four elements are very well organized, so a ⌬Vm
A/D 0 ranging from ⫺5 to ⫺100 mV, depending on the type of
mV cell, is established. Here, we will interconnect these require-
ments to elevate ⌬Vm from 0 mV (Fig. 1) to ⫺100 mV (Fig.
5), through a series of four basic steps, as follows.
Bath solution
[Na+] [K +] Bioelectrogenesis Steps
[Glu]
PO2
Aa
[K +] [Na+] Step 1: generation of Na⫹ and K⫹ concentration gradients.
Let’s start from the “reset” or “unloaded” cell shown in Fig. 1,
where the membrane has no proteins and ICF ⫽ ECF (note,
ΣQ+ =ΣQ -
also, that ⌬Vm ⫽ 0 mV). We can say that the system is at the
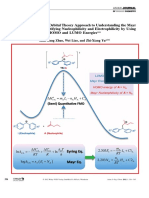
Fig. 1. The didactic model: a simplified cell at the baseline energy state as the baseline energy state. In such a state, both fluids are at
starting point of bioelectrogenesis. The extracellular fluid (ECF) and intracel- thermodynamic equilibrium so for any net change in the fluids’
lular fluid (ICF) are at thermodynamic equilibrium, and the cell membrane composition, it is necessary to supply some work to the system.
consists of a simple phospholipid bilayer. The cell forms spontaneously, and Of course, the cell membrane is not exclusively composed of
the intracellular machinery is started. The ECF is supplied with glucose
([Glu]), O2 (PO2), and amino acids (Aa). The model assumes that we can insert lipid molecules; there is a vast array of different proteins
or remove proteins on the membrane. A/D, analog to digital; Q, electron embedded in the membrane accounting for a number of dif-
charge. The brackets denote concentration. ferent functions. Na⫹-K⫹-ATPase (or pump), first reported by
J. C. Skou (21), is a transmembrane protein present in almost
all animal cells. This protein is an active transport system that
size of the letters in the ion concentration indicates the
uses metabolic energy to move Na⫹ out and K⫹ in vectorially
magnitude of the concentration, and 2) the ECF is constantly
across the membrane against their concentration gradients. The
supplied with glucose, O2, and amino acids.
stoichiometry of the pump is 3Na⫹:2K⫹:1ATP, and its stron-
• According to kinetic theory, ions and molecules in the ECF,
gest activator is a high intracellular Na⫹ concentration
driven by thermal agitation [3/2 ⫻ k ⫻ T, where k is a
([Na⫹]in), so it will be quite active under the condition shown
constant and T is the temperature (in Kelvin)], randomly
in Fig. 1.
crash against the outer surface of the membrane. Hence, it is
Regarding our didactic model, certain membrane proteins
easy to figure out that at a given T, the higher the concen-
can be inserted to serve specific functional roles. In this way,
tration of a particular ionic species i ([i]), the greater the
the major factors that underlie the genesis of ⌬Vm can be
frequency of collisions of this species with the membrane.
clearly identified. Therefore, to start the process of bioelectrogen-
The relationship among T, [i], and the probability of such
collisions can be referred to as the chemical potential of i esis, we can now insert the Na⫹-K⫹ pump into the membrane (the
(i) and is expressed as i ⫽ R ⫻ T ⫻ ln[i], where R is the small circle in Fig. 2A, which represents the combined effect of
gas constant (8.31 J/K·mol). Note that if T is kept constant, thousands of pumps scattered over the membrane). The initially
i depends only on [i]; the higher [i], the higher the proba- high [Na⫹]in induces a high turnover rate of the pump. Pro-
bility of collisions. In this sense, we can therefore think that gressively, Na⫹ is extruded and K⫹ is taken up, resulting in a
i of the ECF is the tendency of i to cross the membrane decrease in [Na⫹]in and an increase in intracellular K⫹ con-
from the ECF to the ICF driven by thermal agitation. centration ([K⫹]in), as shown in Fig. 2A, where the size of the
• However, since the core of the cell membrane is hydropho- letters is correlated with concentration. As pointed out, the
bic, this structure is an insulating material. This means that activity of the pump is a function of [Na⫹]in, such that as
when an ion hits the membrane, it does not penetrate the [Na⫹]in decreases, the turnover rate of the pump slows down.
membrane. But, note also in Fig. 2A that a small ⌬Vm (equal to ⫺1 mV)
• Due to 1) the insulating properties and well-defined planar is forming. This happens because the number of ions trans-
geometry of the membrane, 2) the thinness of the membrane, ported by the pump is not equal in each direction (3Na⫹
and 3) the electrical conductivity of the biological fluids, the out:2K⫹ in); the uneven translocation of charges generates a
ECF-membrane-ICF triad is a capacitor. This means that if net outward Na⫹ current across the membrane. One net posi-
the electric potential energy of attraction overcomes the tive charge flows outwards per pump cycle, and, consequently,
kinetic energy, opposite unpaired electric charges in both a negative unpaired charge is left trapped inside the cell by
fluids will attract each other across the membrane, generat- the lipid membrane. Since the system operates as a capac-
ing a transmembrane electric field. itor, the opposite unpaired charges do not zigzag randomly
• Because the electric force is conservative, an electric poten- through the fluids; instead, they attract each other on either
tial energy (U) is stored in the electric field of the membrane, side of the membrane, forming a thin cloud of negative
which is given by U ⫽ z ⫻ F ⫻ ⌬V (where z is the valence charges scattered along the inner surface of the membrane
of the charge and F ⫽ 9.6 ⫻ 104 C/mol). and a positively charged cloud on the outer surface. The end
result of the pump’s work, in addition to the generation of
Considering the above, we can infer that for a nonzero ⌬Vm to Na⫹ and K⫹ gradients, is a slight separation of charges
exist the following requirements are necessary: 1) the presence across the membrane that gives rise to a small ⌬Vm. In a
of mobile and opposite charges, 2) a force to separate them, 3) typical mammalian neuron, the pump activity maintains an ⬃14-
a conducting pathway so that they flow separately from each fold gradient for Na⫹ and ⬃35-fold gradient for K⫹. For the
other, and 4) a device like a capacitor to keep them apart. In model cell, the working pump establishes [K⫹]in ⫽ 140 mM,
a system made up by a living cell and its surrounding fluid, [Na⫹]in ⫽ 10 mM, and ⌬Vm ⬇ ⫺2.5 mV (Fig. 2B). Note that,
Advances in Physiology Education • doi:10.1152/advan.00051.2015 • http://advan.physiology.org
Downloaded from journals.physiology.org/journal/advances (189.004.076.190) on February 9, 2022.
How We Teach: Classroom And Laboratory Research Projects
TEACHING AND LEARNING BIOELECTROGENESIS 399
A A/D ≈-1.0
separately. We assume that these channels, referred as resting
K⫹ channels, are always open, so K⫹ can diffuse freely
mV through them at all times from the ICF to the ECF and vice
versa. The K⫹ channels that we have inserted represent, of
course, the combination of a large number of them scattered
+ over the membrane. In this new configuration, the chemical
[Na+] -
[K +]
potential of intracellular K⫹ [(K⫹)in] drives K⫹ to the extra-
cellular space through K⫹ channels with a “force” equal to
3Na+
2K+
- + R ⫻ T ⫻ ln[K⫹]in. Similarly, the chemical potential of
[Na+] extracellular K⫹ [(K⫹)out] causes K⫹ influx (R ⫻ T ⫻
[K +] ln[K⫹]out). Because [K⫹]in ⬎ [K⫹]out, it follows that (K⫹)in ⬎
-
+
A ≈-2.5
B A/D
t A/D ≈-2.5 mV
mV
+
+ - +
[Na+] +
-
-
-
+
[Na+] [Na+] -
ATP
[K ]
+
- +
+ -
[K +] - +
[K +] - -
[K +] - [Na+] - + -
+
+ -
+
Fig. 2. Bioelectrogenesis step 1: generation of Na⫹ and K⫹ chemical gradi-
ents. A: the insertion of the Na⫹-K⫹ pump (small circle) establishes the active
B t A/D ≈-50
transport of Na⫹ and K⫹, which progressively increases intracellular [K⫹] mV
([K⫹]in) and decreases intracellular [Na⫹] ([Na⫹]in), as indicated by the
vertical arrows and by the size of the letters for the [ion]. The unpaired
electrical work of the pump carries positive charges to the ECF, leaving behind
+ +
in the ICF the same amount of unpaired negative charges. Due to electrical
- [Na ] - +
attraction, the unpaired opposite charges accumulate close to the surfaces of
the membrane, giving rise to a small electrical potential difference across the [Na+] +
-
+ -
- +
[K +] -
cell membrane (⌬Vm), as registered by the voltmeter. B: as the pump’s work
continues, the ICF becomes different to the ECF; at the pump’s maximal
transport capacity, the [ion] ratios are [K⫹]in/extracellular [K⫹] ([K⫹]out) ⫽ +
- +
[K +]
35, extracellular [Na⫹] ([Na⫹]out)/[Na⫹]in ⫽ 14, and ⌬Vm of ⫺2.5 mV. - -+
+ -
⫹
in this process, neither extracellular Na concentration
([Na⫹]out) nor extracellular K⫹ concentration ([K⫹]out) of the C
bulk solution are significantly altered; any tendency in this t A/D -95.4
mV
direction is canceled by the homeostatic systems of the body.
(In the model, we can assume the extracellular space to be
infinitely larger than the intracellular compartment; thus, Na⫹ + + +
- -[Na-+] - - +
and K⫹ concentrations of the surrounding fluid are not altered + +
by the pump.) Furthermore, we have to consider that only a
[Na+] +
-
- +
-
[K ]
+ - +
small imbalance in ion electroneutrality close to the membrane
surface is required to generate physiological values of ⌬Vm due + -
to the function of the membrane as an excellent capacitive [K +] + --
+ - - - - -+ +
device that allows charge separation.
Step 2: K⫹ conductance. Unlike a pure lipid bilayer, the + - - +
typical cell membrane has ionic conductance pathways, so that Fig. 3. Bioelectrogenesis step 2: the role of resting K⫹ conductance. A: upon
it acts as a leaky capacitor. Ion channels, a type of transmem- the insertion of a K⫹ channel (white ring), the K⫹ chemical potential differ-
ence (⌬K⫹ ⬵ 9,158.9 J/mol) drives K⫹ efflux (solid arrow) and the electrical
brane protein, consist of hydrophilic microdomains that allow potential energy (U ⫽ 240 J/mol) drives an inward flux (dotted arrow). As
ions to diffuse across the membrane. Furthermore, ion channels ⌬K⫹ ⬎ U, the resultant K⫹ movement is outward, as indicated by the length
are selective for different ionic species. of the arrows. B: the ongoing resultant outward K⫹ flux carries positive
Applying this to the didactic model and to mimic a real cell, charges to the ECF. This is a process of charge separation that increases ⌬Vm,
which has a membrane highly permeable to K⫹, let us insert which, in turn, increases K⫹ influx driven by U, as indicated by the increasing
length of the dotted arrow. C: as the net K⫹ efflux progresses, a steadily
selective K⫹ channels within the membrane of the model cell increasing number of electric charges is separated, increasing ⌬Vm up to U ⫽
(the white ring in Fig. 3A). At the same time, we will remove ⌬K⫹. At this point, the influx of positive electric charges is equal to their
the Na⫹-K⫹ pump so we can analyze the role of K⫹ channels efflux and ⌬Vm does not change; the cell has reached stationary equilibrium.
Advances in Physiology Education • doi:10.1152/advan.00051.2015 • http://advan.physiology.org
Downloaded from journals.physiology.org/journal/advances (189.004.076.190) on February 9, 2022.
How We Teach: Classroom And Laboratory Research Projects
400 TEACHING AND LEARNING BIOELECTROGENESIS
(K⫹)out, and, therefore, the net K⫹ movement is outwardly By applying the Nernst equation to our experimental condi-
directed. Quantitatively, the net K⫹ movement across the tions,
membrane is a function of the difference of K⫹ between the
ICF and ECF, which is (K⫹)in ⫺ (K⫹)out ⫽ ⌬K⫹ ⫽ R ⫻ 8.31 ⫻ 310 [140 ⫻ 10⫺3]
⌬Vm ⫽ ⫺ ⫻ ln ⬵ ⫺95.4 mV
T ⫻ ln[K⫹]in/[K⫹]out. Then, the resultant K⫹ efflux driven by 1 ⫻ 9.6 ⫻ 104 [4 ⫻ 10⫺3]
⌬K⫹ is represented here by a single solid arrow directed
outward, as shown in Fig. 3. At this value, ⌬Vm ⬵ ⫺95.4 mV (Fig. 3C), U (⬵9,158.9 J/mol)
Note that the resultant efflux of K⫹ is a spontaneous phenom- exactly equals ⌬K⫹, keeping the cell at a stationary state. That
enon that drives the system toward ⌬K⫹ ⫽ 0, i.e., [K⫹]in ⫽ is to say, if T and [K⫹]in/[K⫹]out are kept constant, ⌬Vm is no
[K⫹]out. However, this tendency is counteracted by U stored in longer time dependent.
the electric field of the membrane, which also drives K⫹ The ⌬Vm predicted by Nernst equation is referred to as the
through the selective channels (recall that U is directly propor- equilibrium potential of a given ionic species i (Ei). We can
tional to ⌬Vm ⬇ ⫺2.5 mV generated by the Na⫹-K⫹ pump). apply the Nernst equation to any ionic species that is chemi-
Because the inside of the cell is negative relative to the outside, cally unbalanced across the membrane. If the cell membrane is
K⫹ driven by U migrates unidirectionally, making an inward permeable to only one ionic species, the membrane potential
K⫹ current (dotted arrow in Fig. 3A), which tends to counteract will be equal to the equilibrium potential of that species. In our
the outwardly directed K⫹ current generated by ⌬K⫹. experiment, ⌬Vm ⫽ EK⫹ ⬵ ⫺95.4 mV. Note that Ei can be
Therefore, there are two types of potential energy able to considered the electrical representation of ⌬i.
move ions across the membrane: chemical and electrical. [The It is important to note that the amount of K⫹ that flows out
algebraic sum of these two parameters is called the electrochem- of the cell required to build ⌬Vm ⬵ ⫺95.4 mV is minimal
ical potential difference of an ionic species i (⌬ i).] Considering compared with the total K⫹ in the ICF. The low dielectric
[K⫹]in/[K⫹]out ⫽ 35, ⌬Vm ⬇ ⫺2.5 mV, and T ⫽ 37°C ⫽ 310 K, constant of the membrane allows the development of large
⌬K⫹ stored in the K⫹ gradient is ⬇9,158.9 J/mol, whereas U ⌬Vm for a given density of separated opposite charges. This
stored in the electric field of the membrane is ⫺240 J/mol. The means that [K⫹]in is not significantly altered during the ⌬Vm
opposite signs indicate the opposite direction of the gradients, and, generation (then, in the figures, the solid arrows that indicate
as expected, the magnitude of the gradients is directly proportional flow generated by ⌬ never change in length).
to the intensity of the K⫹ movements, as indicated by the direc- Step 3: Na⫹ conductance. Most cells have membranes not
tions and lengths of the arrows in Fig. 3A. Therefore, the ampli- exclusively permeable to K⫹; instead, they present multiple
tude of the outward K⫹ current is larger than that of the inward conductances, which can also contribute to the generation of
current. In these conditions, the inward current driven by U only ⌬Vm. Resting Na⫹ channels, the second most important con-
partially counteracts the outward current driven by ⌬K⫹, so the tributor to ⌬Vm, are highly selective for Na⫹ and are always
rate of transference of positive charges out of the cell exceeds the open, allowing Na⫹ to move across the membrane in both
inward translocation. This means that, since the membrane per- directions.
meability is selective to K⫹, the net outward K⫹ current leaves To understand the role of Na⫹ movements, we will switch
behind in the ICF an equal number of negative unpaired charges. on Na⫹ conductance (gNa⫹) by inserting a Na⫹ channel into the
Hence, because of the capacitive properties of the mem- membrane (the rectangle in Fig. 4). Using the same reasoning
brane, the unpaired negative and positive charges are pulled for K⫹ flux, we realize that both the difference in chemical
toward the membrane surfaces by electrostatic attraction. In potential for Na⫹ (⌬Na⫹) and U drive Na⫹ in the same
other words, the resultant outward K⫹ current progressively direction, from the ECF to the ICF, as shown by the arrows
increases the density of unpaired charges on the membrane inside the Na⫹ channel (Fig. 4A).
surfaces, leading to a proportional enhancement of ⌬Vm Here, a quantitative comparison is required between Na⫹
beyond of the ⫺2.5 mV generated by the Na⫹-K⫹ pump. and K⫹ movements. Since these ions are both monovalent, U
However, note that the net outward K⫹ flux is a self-limiting drives them across the membrane with equal energy. However,
process. The gradual increase in ⌬Vm (which reaches ⫺50 mV the dotted arrow in Fig. 4A, indicating K⫹ influx driven by U,
in Fig. 3B) comprises an increase in the electric driving force is longer than the dotted arrow corresponding to Na⫹ influx,
that pulls K⫹ back into the cell. The result is a gradual increase also driven by U. The reason is simple: K⫹ conductance (gK⫹)
in the inward K⫹ current as ⌬Vm increases, as shown by the is much higher than gNa⫹. In other words, the resistance of the
increased length of the dotted arrow in Fig. 3B. Then, it is clear membrane to Na⫹ flux is higher than that for K⫹, with the
that, as the hyperpolarization progresses, the increasing inward reason being that the K⫹ channels are more conductive and
K⫹ current will eventually cancel out the stable outward more numerous than Na⫹ channels. For ionic fluxes driven by
current (Fig. 3C); at that moment, U equals ⌬K⫹. At this ⌬ the same reasoning applies: although in our experimental
point, the cell is at stationary equilibrium, i.e., there is a K⫹ conditions ⌬Na⫹ (⬇6,835 J/mol) is close to ⌬K⫹ (⬇9,158.9
current in both directions across the membrane but, because J/mol), K⫹ flux is much larger than Na⫹ flux due to gK⫹ ⬎⬎
they are of identical amplitude, there is no net current and, gNa⫹.
therefore, ⌬Vm does not change. This dynamic equilibrium can Considering the above, it is easy to see that upon the insertion
be quantified by considering that the two gradients cancel each of the Na⫹ channel, the steady-state condition shown in Fig. 3C is
other out, so ⌬K⫹ ⫹ U ⫽ 0. In extended form, and solving for broken. Now, as shown in Fig. 4A, there is a net inward current
⌬Vm, we write ⌬Vm ⫽ ⫺(R ⫻ T/z ⫻ F) ⫻ ln[K⫹]in/[K⫹]out. driven by ⌬ Na⫹, which changes ⌬Vm accordingly. Recall that
This is the Nernst equation. It predicts the amplitude of the because Na⫹ channels are selective, the net influx of positive
electric potential that must exist across the membrane to charges leaves behind in the ECF the same amount of unpaired
exactly counteract the K⫹ chemical potential. negative charges. Then, the positive charges flowing into the cell
Advances in Physiology Education • doi:10.1152/advan.00051.2015 • http://advan.physiology.org
Downloaded from journals.physiology.org/journal/advances (189.004.076.190) on February 9, 2022.
How We Teach: Classroom And Laboratory Research Projects
TEACHING AND LEARNING BIOELECTROGENESIS 401
⌬Vm does not change. This dynamic equilibrium can be
A quantified by applying Ohm’s law: i ⫽ g ⫻ ⌬V. However,
A/D -95.4
mV we have to consider that ⌬Vm is not the only driving force;
⌬ also drives ions across the membrane. As pointed out
and following the Nernst equation, the ⌬ of a given ionic
+ + +
- -[Na-+] - - +
+ + species may be considered, in electric terms, as the E of that
[Na+] +
-
- +
-
species. Then, taking into account that the ionic flux driven
by ⌬ (i.e., E) always generates a ⌬Vm driving an opposite
+ -
+ -
[K ]
+ - +
- +
flux, the total driving force across the membrane is given by
the algebraic difference of these two sources as ⌬Vm ⫺ E. In
[K +]
- - - - --+ other terms, if at the equilibrium potential there is no
+ + current, it means that ⌬Vm ⫽ E, and, consequently, the
driving force for an ion is measured by ⌬Vm ⫺ E. In
accordance with Ohm’s law, the coefficient that relates the
B t A/D -87.4 driving force to the ionic current is ionic conductance, so
mV that at stationary equilibrium gK⫹ ⫻ (⌬Vm ⫺ EK⫹) ⫹ gNa⫹ ⫻
(⌬Vm ⫺ ENa⫹) ⫽ 0. Solving for ⌬Vm, we write ⌬Vm ⫽ (gK⫹
⫻ EK⫹ ⫹ gNa⫹ ⫻ ENa⫹)/(gK⫹ ⫹ gNa⫹). This is a linearized
+ + version of the Goldman-Hodgkin-Katz (GHK) equation,
+ -+ -
- +
[Na+] + -
[Na ] -
-+
first derived in its standard form by Hodgkin and Katz (10).
This equation predicts the amplitude of the electric potential
[K +]
+ - [K +] -+
-+
that must exist across the membrane to exactly counteract
the K⫹ and Na⫹ chemical potentials (here expressed in
electric terms as EK⫹ and ENa⫹, respectively) and that ⌬Vm
+
- - -+ is weighted by the relative conductance to each ion. In other
words, ⌬Vm is closer to the equilibrium potential of a given
Fig. 4. Bioelectrogenesis step 3: the role of resting Na⫹ conductance. A: upon
the insertion of the Na⫹ channel (rectangle), the Na⫹ chemical potential ionic species the greater its conductance is to this ionic
difference (⌬Na⫹) and U drive Na⫹ influx (both arrows inside the Na⫹ species compared with other membrane conductances.
channel point inward). B: the net positive electric charges carried by the inward Considering that in a typical neuronal cell, under steady-
Na⫹ movement discharge the membrane capacitor, which decreases ⌬Vm and, state conditions, gK⫹:gNa⫹ is 1:0.05 (or gK⫹ ⫽ 20 ⫻ gNa⫹), we can
consequently, the electrical driving force, i.e., U (note the decrease in the
length of the dotted arrows). The discharging of the membrane continues until
rewrite the GHK equation as follows: ⌬Vm ⫽ (20 ⫻ EK⫹ ⫹
the influx of positive charges equals once again their efflux (the total lengths ENa⫹)/21. By applying this to the model cell (Fig. 4B), ⌬Vm ⫽ [20
of all inward arrows would equal the length of the outward arrow). At this ⫻ (⫺95.4 ⫻ 10⫺3) ⫹ 71.2 ⫻ 10⫺3]/21 ⬵ ⫺87.4 mV.
point, ⌬Vm ⫽ ⫺87.4 mV, the cell is at a “pseudo”-stationary equilibrium: there Exactly at this voltage value, the net positive outward
is, in fact, a net Na⫹ influx and a net K⫹ efflux, which tend to decrease the current (driven by ⌬K⫹) equals the net positive inward cur-
chemical gradients.
rents (driven by ⌬Na⫹ and U) and, therefore, ⌬Vm does not
change. This would then be the resting membrane potential of
cancel out the same amount of negative charges on the membrane the model cell. However, the cell in Fig. 4B is at risk since it
capacitance. At the same time, the unpaired negative charges left is drifting away from dynamic equilibrium. Note that although
in the ECF cancel out the same amount of positive charges on the the outward current is equal to the inward current at ⌬Vm ⫽
external surface of the membrane. This process progressively ⫺87.4 mV, there is, in fact, a net efflux of K⫹ and a net influx
discharges the membrane capacitor, decreasing ⌬Vm with a of Na⫹. Eventually, these spontaneous leakages tend to vanish
tendency toward ENa⫹ ⬵ ⫹71.2 mV (as predicted by the as ⌬K⫹ and ⌬Na⫹ decrease and disappear, leading the cell to
Nernst equation in our experimental conditions). the baseline energy state, which means cell death.
However, this tendency toward ENa⫹ is quickly counteracted Step 4: active transport. To counteract Na⫹ and K⫹ spon-
by a steadily increasing K⫹ efflux, which arises when ⌬Vm taneous flows, the cell relies on active transport: the Na⫹-K⫹
deviates from EK⫹. Note that as the membrane depolarizes (due pump. Then, we need to reinsert the pump into the membrane
to Na⫹ influx), U decreases proportionally, so inward K⫹ and of the model cell (Fig. 5A). In this configuration, while ⌬ Na⫹
Na⫹ currents driven by U also decrease, as shown by the drives a net inward Na⫹ current and ⌬ K⫹ drives a net outward
smaller length of the dotted arrows in Fig. 4B. As a conse- K⫹ current, the pump generates opposing fluxes, i.e., outward
quence, the inward Na⫹ current progressively decreases, Na⫹ and inward K⫹ currents. We can consider that the spon-
whereas the outward K⫹ current increases. Then, we can infer taneous Na⫹ influx, which tends to increase [Na⫹]in, constantly
that these opposing fluxes are driving the system to a ⌬Vm activates the pump turnover rate. In addition, as the pump
where once again the inward current (⫺) will equal the activity is electrically unpaired (3Na⫹:2K⫹), there is a resul-
outward current (⫹), i.e., ⫺i N a ⫹ (⌬ N a ⫹ ) ⫺i N a ⫹ tant positive outward current carried by Na⫹ that redistributes
(U) ⫺iK⫹(U) ⫽ ⫹iK⫹(⌬K⫹) (within the parentheses is in- the charges on membrane capacitance toward hyperpolariza-
dicated the driving force of the respective ionic current iion). tion. This displaces the voltage to a new value (⌬Vm ⬵ ⫺90.0
In other words, the net inward Na⫹ current equals the net mV; Fig. 5B), where active and spontaneous Na⫹ and K⫹
outward K⫹ current: ⫺iNa⫹(⌬ Na⫹) ⫽ ⫹iK⫹(⌬ K⫹). Exactly currents cancel each other such that there is neither a net Na⫹
at this point the cell is at stationary equilibrium, i.e., there is current nor a K⫹ current. Note that before the pump starts
no net current, iK⫹(⌬ K⫹) ⫹iNa⫹(⌬ Na⫹) ⫽ 0, and, therefore, working (Fig. 4B), the spontaneous currents are balanced, i.e.,
Advances in Physiology Education • doi:10.1152/advan.00051.2015 • http://advan.physiology.org
Downloaded from journals.physiology.org/journal/advances (189.004.076.190) on February 9, 2022.
How We Teach: Classroom And Laboratory Research Projects
402 TEACHING AND LEARNING BIOELECTROGENESIS
A A/D -87.4
stant because the pump exactly counteracts the leakage of
these ions down their ⌬ , making the cell, at the stationary
mV state, effectively impermeable to those ions at the expense
of metabolic energy.
+ + Final Considerations
+ -+ -
- +
[Na+] + -
[Na ] -
-+
The four steps to bioelectrogenesis presented above describe
[K +]
2K+ the general modus operandi of the ECF-membrane-ICF sys-
3Na+
+ - -+ tem, i.e., the primary role of membrane capacitance, electro-
[K +] -+ chemical potentials, and relative conductances of the mem-
+
- - -+ brane. However, as already mentioned, ⌬Vm ranges from ⫺5 to
⫺100 mV depending on the type of cell, demonstrating a large
functional diversity of the cell membranes. To address this
B variability, we have to keep in mind the modus operandi of the
t A/D -90.0 system and look at the determinant factors of ⌬Vm, i.e., the
mV
variables of the GHK equation: ion equilbrium potential (Eion)
and ion conductance (gion). Let us consider the following:
+ + • First, according to the Nernst equation, Eion is a function of
+ - + - -+ +
- [Na
[Na+] +-
] -
-+
intracellular ion concentration ([ion]in)/extracellular ion con-
centration ([ion]out). Note, however, that while the homeo-
[K +] - -+
2K+ static systems of the body maintain the same [ion]out for
3Na+
+- -+ most cells, [ion]in depends on each cell’s individual work.
[K +] For Na⫹ and K⫹, the relatively low [Na⫹]in and high [K⫹]in
+
- - -+ + are a function of Na⫹-K⫹-ATPase activity, which does not
have the same molecular structure in every cell. Na⫹-K⫹-
Fig. 5. Bioelectrogenesis step 4: the role of Na⫹-K⫹ pump conductance. A: upon
its reinsertion, the pump establishes a net outward positive current (carried by Na⫹;
ATPase is an equimolar ␣- dimer assembly from distinct
recall that 3Na⫹ out:2K⫹ in). B: the membrane is hyperpolarized (⌬Vm ⫽ ⫺90.0 isoforms (four ␣ and three ) (5). The various combinations
mV) according to the resultant efflux of positive charges. In addition to this small of ␣- complexes are tissue specific and present different
contribution to ⌬Vm, the pump generates (Fig. 2) and maintains chemical gradi- sensitivity to regulating factors such as [Na⫹]in, [K⫹]out, pH,
ents: the Na⫹ efflux driven by the pump counteracts the Na⫹ influx driven by intracellular ATP concentration, ⌬Vm, and several circulat-
⌬ Na⫹; the K⫹ influx driven by the pump counteracts the K⫹ efflux driven by
⌬ K⫹, keeping the cell out of thermodynamic equilibrium but at stationary ing hormones (3, 5, 18), conferring wide functional variabil-
equilibrium. ity to the pump. The number of pumps, which is a determi-
nant factor for [Na⫹]in and [K⫹]in, also varies largely in
iK⫹ ⫽ ⫺iNa⫹. However, to equilibrate pump currents, the different tissues (3). Furthermore, [Na⫹]in and [K⫹]in also
spontaneous Na⫹ flux must be, at some point, 50% higher than depend on the number of Na⫹ and K⫹ leakage channels and
the spontaneous K⫹ flux. This readjustment is achieved by the on the several secondary carrier proteins that use Na⫹ and
hyperpolarization created by the pump that 1) increases iK⫹(U), K⫹ gradients, as the ion fluxes through them tend to cancel
which, in turn, decreases ⌬ K⫹, and 2) increases iNa⫹, which, in both gradients. Particularly, we can ask ourselves the fol-
turn, increases ⌬ Na⫹. The result, as shown by the longer lowing question: if the pump translocates more Na⫹ than
lengths of the dotted arrows in Fig. 5B, is a slight decrease in K⫹, why is the gradient of Na⫹ smaller than the K⫹
the outward K⫹ leakage currents and an increase in the inward gradient? This is because the distribution of Na⫹ and K⫹ is
Na⫹ leakage currents, reaching the ratio of 3/2 ⫻ iK⫹(⌬ K⫹) ⫽ not an exclusive task of the pump; instead, several secondary
⫺iNa⫹(⌬ Na⫹). This ratio exactly matches the stoichiometry of transport systems use Na⫹ and K⫹ gradients as a driving
the pump (3Na⫹:2K⫹), bringing the cell back to stationary force. Here, we can consider the Na⫹/H⫹ exchanger, a
equilibrium. This dynamic balance can be quantified by con- secondary cotransporter ubiquitously expressed, that cou-
sidering the ratio of 3/2 ⫻ iK⫹(⌬ K⫹) ⫽ ⫺iNa⫹(⌬ Na⫹). In ples the inward flux of Na⫹ driven by ⌬ Na⫹ to the
extended form, we have 3/2 ⫻ [gK⫹ ⫻ (⌬Vm ⫺ EK⫹)] ⫽ extrusion of H⫹. The stoichiometry is 1Na⫹:1H⫹; there-
⫺gNa⫹ ⫻ (⌬Vm ⫺ ENa⫹). By solving for ⌬Vm and assuming fore, it is electroneutral (15). Note that the Na⫹ influx
gK⫹ ⫽ 20 ⫻ gNa⫹, we have ⌬Vm ⫽ (30 ⫻ EK⫹ ⫹ ENa⫹)/31. decreases the Na⫹ gradient but does not interfere with the
This equation quantitatively predicts the ⌬Vm for mem- electrogenic capacity of the pump.
branes permeable only to Na⫹ and K⫹. By calculating for • Second, a given gion is not always due to a single
the model cell, ⌬Vm ⫽ [30 ⫻ (⫺95.4 ⫻ 10⫺3) ⫹ 71.2 ⫻ molecular entity; instead, it is established by multiple
10⫺3]/31 ⬵ ⫺90.0 mV (Fig. 5). The end result is that the channel types, each with distinct biophysical properties.
experiment has led the model cell to the resting membrane Each type of cell expresses a particular set of channels,
potential (⌬Vm ⬵ ⫺90.0 mV) and places it at stationary which work in concert to establish a specific value of
equilibrium. At this point, ⌬Vm does not change any further, ⌬Vm. Moreover, the ion channels are not rigid structures;
since the sum of all ionic current across the membrane is the open probability and number of the channels can be
zero. In other words, the outward currents equal the inward influenced by multiple factors like pH, temperature, volt-
currents: iK⫹(⌬ K⫹) ⫹ iNa⫹(pump) ⫽ ⫺[iNa⫹(⌬ Na⫹) ⫹ age, and chemical mediators, introducing a high degree of
iK⫹(pump)]. Na⫹ and K⫹ chemical potentials are also con- complexity in the regulation of ⌬Vm. Furthermore, in
Advances in Physiology Education • doi:10.1152/advan.00051.2015 • http://advan.physiology.org
Downloaded from journals.physiology.org/journal/advances (189.004.076.190) on February 9, 2022.
How We Teach: Classroom And Laboratory Research Projects
TEACHING AND LEARNING BIOELECTROGENESIS 403
many cells, gion is not only represented by gK⫹ and gNa⫹;
I (pA)
other conductances, which should be included in the GHK
equation, may also contribute to ⌬Vm. Particularly, many
neurons extrude Cl⫺ through K⫹-Cl⫺ cotransporter-2.
This secondary active symporter establishes an electro-
chemical gradient for Cl⫺, i.e., ECl⫺ away from ⌬Vm (23),
which makes Cl⫺ conductance a potential contributor to
⌬Vm. However, the influence of Cl⫺ can also be quite
complex due to the wide variation in the expression of +
different Cl⫺ transporters and channels.
EK+
• Third, resting gK⫹, first postulated by Hodgkin and Huxley
(9), is represented by the most diverse group of ion channels.
However, it is thought that the primary contributors to ⌬Vm fall -90 ΔVm (mV)
into two main structural classes of channels: the one-pore
domain, which encodes inward rectifier K⫹ (Kir) channels (1, -
11, 20), and two pore domains, which encode two-pore K⫹
(K2P) channels (1, 20). Each class of K⫹ channel encompasses
⬃15 different members with distinct biophysical properties.
• Fourth, resting gNa⫹, which was also described by Hodgkin and
Huxley (8), is also underlined by multiples types of channels.
However, it seems that the Na⫹ leak channel nonselective Fig. 6. The interplay of three resting conductances on ⌬Vm. The current
(NALCN) channel is the main contributor that counterbalances (I)-⌬Vm relationships show that 1) the current carried by two-pore domain K⫹
(K2P) channels is the largest at 0 mV and drives the voltage toward K⫹
K⫹ leakage (19). Surprisingly and as its name suggests, the equilibrium potential (EK⫹); 2) as the membrane hyperpolarizes, inward
NALCN channel is not selective for Na⫹; instead, it is a rectifier K⫹ (Kir) channels lose Mg2⫹ blockage and outward currents through
cation-selective channel (19). Kir and K2P channels are added; 3) ⌬Vm does not reach EK⫹ because the current
• Finally, we also have to consider the direct contribution of the carried by Na⫹ leak channel nonselective (NALCN) channels counterbalances
the K⫹ current; and 4) at resting membrane potential, ⌬Vm ⫽ ⫺90.0 mV, the
Na⫹-K⫹ pump to ⌬Vm. Following Ohm’s law, this contribution inward Na⫹ current through NALCN channels (solid circle) is 50% larger than
depends on the amplitude of the current established by the the total outward K⫹ current through Kir and K2P channels (solid triangle).
pump activity and the resistance of the membrane. Accordingly,
the resulting ⌬Vm varies in different types of cells. The small
contribution of the Na⫹-K⫹ pump to resting membrane poten- rium (chemical potential of intracellular cations ⫽ chemical
tial, as ⫺2.5 mV in the model cell, is revealed with the potential of extracellular cations), if ⌬Vm ⫽ 0, there will be
inhibition of the pump by specific drugs, which typically results no U and, therefore, no current through NALCN channels].
in a small depolarization ranging from ⬃0.45 mV (13) up to Finally, the kinetics of the channels are quite varied. Par-
⬃10 mV (7). ticularly, at voltages that are depolarized compared with the
resting membrane potential, Kir channels are blocked by
In summary, the value of ⌬Vm is established by a particular intracellular Mg2⫹ (and by other polyvalent cations), giving
set of ion channels and transporters, of molecular compositions rise to the region of negative slope of the Kir current-voltage
and concentrations specific to the different types of cells, that relationship (20).
can also show large variability at different physiological and Taking these points into consideration, we can see on the
pathophysiological conditions. To help the students have a first graph that at ⌬Vm ⫽ 0 (at the beginning of our experiment),
look at the interactions between different conductances con- and more so at ⌬Vm ⫽ ⫺2.5 mV, when the pump has already
trolling ⌬Vm, we will only consider Na⫹-K⫹-ATPase and three built the gradients, the outward iK⫹ carried by K2P channels is
resting conductance (Kir, K2P, and NALCN). Figure 6 shows very large and drives the voltage toward EK⫹. At increasingly
the interplay between these channels, showing the amplitude of negative membrane potentials, iK⫹ through K2P channels de-
the current carried by each of them and the voltage across the creases as a function of (⌬Vm ⫺ EK⫹), Kir channels lose Mg2⫹
membrane. To analyze these curves, it is important to consider blockage, and inward current through NALCN channels in-
that the following. First, a positive current means efflux of creases progressively as a function of (⌬Vm ⫺ NALCN equi-
positive charges, i.e., Na⫹ or K⫹ leaving the cell, whereas a librium potential). Note that at resting membrane potential, i.e.,
negative current means influx of Na⫹ or K⫹. Second, at all ⌬Vm ⫽ ⫺90.0 mV, the K⫹ currents carried by K2P and Kir
voltages, ⌬K⫹ drives the efflux of K⫹, whereas ⌬Na⫹ drives channels are added and are counterbalanced by the current
the influx of Na⫹. Third, at positive voltages, U drives the through NALCN channels. In the context of this article, we
efflux of Na⫹ and K⫹, whereas at negative voltages, U will consider NALCN channels permeable to Na⫹ and K⫹, so
drives the influx of Na⫹ and K⫹. Fourth, at EK⫹ (⫺95.4 as iK⫹ and iNa⫹ are of opposite directions at ⌬Vm ⫽ ⫺90.0 mV,
mV), ⌬ K⫹ ⫽ 0, i.e., ⌬K⫹ ⫽ U, and, therefore, K⫹ the resultant inward current through NALCN channles ob-
transmembrane current is zero. Fifth, at depolarizing volt- served at the resting membrane potential is carried by Na⫹.
ages in relation to EK⫹, ⌬K⫹ is larger than U; therefore, Note then that the inward iNa⫹ through NALCN channels is
there is a resultant efflux of K⫹, i.e., a positive current. 50% larger than the outward iK⫹ (Kir ⫹ K2P channels); both
Sixth, as the NALCN channel is permeable to the main these currents are canceled by Na⫹-K⫹-ATPase, which gener-
cations, NALCN equilibrium potential ⫽ 0 (19) [we can ates outward iNa⫹ 50% larger than the inward iK⫹ keeping the
assume that since the ICF and ECF are at osmotic equilib- cell at stationary equilibrium.
Advances in Physiology Education • doi:10.1152/advan.00051.2015 • http://advan.physiology.org
Downloaded from journals.physiology.org/journal/advances (189.004.076.190) on February 9, 2022.
How We Teach: Classroom And Laboratory Research Projects
404 TEACHING AND LEARNING BIOELECTROGENESIS
Discussion and Conclusions ACKNOWLEDGMENTS
The author thanks Dr. Mariana G. Terenzi for the English revision and for
The separation of charges across the cell membrane is a valuable comments on a draft of this article. The author also thanks Clar-
process of great elegance that sustains life and runs against iswaldo Baptista and M. Marly R. Baptista for all support, guidance, and
entropy. It is very expensive for the cell, accounting for a large encouragement.
portion of the metabolic cost. To help approach this important
issue, teachers and students have the support of several books DISCLOSURES
on physiology and of some didactic models described in the No conflicts of interest, financial or otherwise, are declared by the author(s).
literature (2, 17), which provide excellent teaching tools. How-
ever, to overcome my own difficulties in explaining it clearly
AUTHOR CONTRIBUTIONS
and yielding positive outcomes, I have implemented an alter-
native model for teaching bioelectrogenesis based on a theo- Author contributions: V.B. conception and design of research; V.B. pre-
retical approach in the classroom. I believe that the strategy pared figures; V.B. drafted manuscript; V.B. edited and revised manuscript;
V.B. approved final version of manuscript.
described here gives a broader view and clearly identifies and
quantifies the various mechanisms involved in bioeletrogen-
esis. I hope that it may help others in the process of teaching REFERENCES
and learning the basis for the generation of resting membrane 1. Bayliss DA, Barrett PQ. Emerging roles for two-pore-domain potassium
potential. channels and their potential therapeutic impact. Trends Pharmacol Sci 29:
566 –575, 2008.
To develop the model, I considered the concept of cumula-
2. Cardozo DL. A model for understanding membrane potential using
tive learning, in which previously acquired knowledge func- springs. Adv Physiol Educ 29: 204 –207, 2005.
tions as building blocks for new learning (6, 12, 16). In this 3. Clausen T, Everts ME. Regulation of the Na,K-pump in skeletal muscle.
sense, I first point out in Initial Considerations some review Kidney Int 35: 1–13, 1989.
concepts upon which the bioelectrogenesis is built. In the 4. Clegg BA, DiGirolamo GJ, Keele SW. Sequence learning. Trends Cogn
Sci 2: 275–281, 1998.
classroom, I first present those basic concepts while the stu- 5. Féraille E, Doucet A. Sodium-potassium-adenosinetriphosphatase-depen-
dents are encouraged to a multidisciplinary approach to living dent sodium transport in the kidney: hormonal control. Physiol Rev 81:
systems. In fact, students have already acquired these concepts 345– 418, 2001.
in high school; we only review and emphasize that biological 6. Gagné RM. Learning hierarchies. Educ Psychol 6: 1–9, 1968.
structure is governed by the same physical and chemical laws 7. Goldman AL, Marmor MF. Steady-state contribution of the sodium
pump to the resting potential of a molluscan neurone. J Physiol 242:
that govern the universe as a whole. In addition, a number of 35– 48, 1974.
authors (4, 14, 22) have highlighted the importance of instruc- 8. Hodgkin AL, Huxley AF. A quantitative description of membrane current
tion sequencing in learning activities, so that the order and and its application to conduction and excitation in nerve. J Physiol 117:
organization of the content influence the processing and reten- 500 –544, 1952.
tion of information. Here, the model starts from a “reset cell” 9. Hodgkin AL, Huxley AF. Potassium leakage from an active nerve fibre.
J Physiol 106: 341–367, 1947.
at baseline energy state and goes through all the basic pro- 10. Hodgkin AL, Katz B. The effect of sodium ions on the electrical activity
cesses toward an energized cell at stationary equilibrium, of the giant axon of the squid. J Physiol 108: 37–77, 1949.
providing a simple-to-complex sequence of instruction in a 11. Inanobe A, Fujita A, Ito M, Tomoike H, Inageda K, Kurachi Y. Inward
constructivistic strategy. rectifier K⫹ channel Kir2.3 is localized at the postsynaptic membrane of
The key point of the model is the successive insertion of excitatory synapses. Am J Physiol Cell Physiol 282: C1396 –C1403, 2002.
12. Lee J. Cumulative learning. In: Encyclopedia of the Sciences of Learning,
different proteins into the cell membrane, which brings into edited by Seel N. New York: Springer, 2011, vol. 2, p. 887– 893.
evidence the capacitive and resistive properties of the mem- 13. Levi AJ. The electrogenic sodium/potassium pump and passive sodium
brane and highlights that the cell and its surroundings are a influx of isolated guinea pig ventricular myocytes. J Cardiovasc Electro-
system pushed out of thermodynamic equilibrium by a constant physiol 3: 225–238, 1992.
14. Lorch RF Jr, Lorch EP. Topic structure representation and text recall. J
input of metabolic energy. In my experience, the use of the
Educ Psychol 77: 137–148, 1985.
didactic model and the sequence of the topics described here 15. Malo ME, Fliegel L. Physiological role and regulation of the Na⫹/H⫹
provide an effective way of explaining and learning resting exchanger. Can J Physiol Pharmacol 84: 1081–1095, 2006.
membrane potential. Students are easily engaged in logical 16. Maton K. Cumulative and segmented learning: exploring the role of curric-
reasoning and are taken through bioelectrogenesis in a coherent ulum structures in knowledge-building. Br J Sociol Educ 30: 43–57, 2009.
17. Procopio J. Hydraulic analogs as teaching tools for bioelectric potentials.
way, from the start (reset cell) to the end (energized cell). My Adv Physiol Educ 12: 65–76, 1994.
perception is that the didactic model has a significant educa- 18. Rakowski RF, Gadsby DC, De Weer P. Voltage dependence of the Na/K
tional appeal; its design helps to match teaching with learning pump. J Membrane Biol 155: 105–112, 1997.
and allows students to qualitatively and quantitatively identify 19. Ren D. Sodium leak channels in neuronal excitability and rhythmic
the role of each element involved in ⌬Vm generation. Further- behaviors. Neuron 72: 899 –911, 2011.
20. Sepúlveda FV, Cid LP, Teulon J, Niemeyer MI. Molecular aspects of
more, this didactic model serves as a template for the teaching structure, gating, and physiology of pH-sensitive background K2P and Kir
of the action potential as well as other common mechanisms K⫹-transport channels. Physiol Rev 95: 179 –217, 2015.
such as electrotonic potentials and synaptic transmission. I 21. Skou JC. The influence of some cations on an adenosine triphosphate
must consider, however, that although the model can incorpo- from peripheral nerves. Biochem Biophys Acta 23: 394 – 401, 1957.
rate other elements such as Cl⫺ and Ca2⫹ channels as well as 22. Van Patten J, Chao CI, Reigeluth CM. A review of strategies for sequenc-
ing and synthesizing instruction. Rev Educ Res 56: 437– 471, 1986.
Donnan and border potentials, here I only analyzed the basic 23. Williams JR, Sharp JW, Kumari VG, Wilson M, Payne JA. The
mechanisms as a first approach to bioelectrogenesis; it is an neuron-specific K-Cl cotransporter, KCC2. J Biol Chem 274: 12656 –
invitation, or an introductory session, for neurophysiology. 12664, 1999.
Advances in Physiology Education • doi:10.1152/advan.00051.2015 • http://advan.physiology.org
Downloaded from journals.physiology.org/journal/advances (189.004.076.190) on February 9, 2022.
You might also like
- Membrane PotentialsDocument7 pagesMembrane PotentialsJessica Leika MatibagNo ratings yet
- Wrihtg, 2000 Generation - of - Resting - Membrane - PotentialDocument5 pagesWrihtg, 2000 Generation - of - Resting - Membrane - PotentialNathalia ReyesNo ratings yet
- ElectrophoresisDocument7 pagesElectrophoresisLalit AmbasthaNo ratings yet
- Phase Transitions in High Energy Heavy-Ion CollisionsDocument16 pagesPhase Transitions in High Energy Heavy-Ion CollisionsmeNo ratings yet
- Resting PotentialDocument16 pagesResting PotentialLuciana R LarregainNo ratings yet
- (Methods in Molecular Biology) Jac A. Nickoloff - Animal Cell Electroporation and Electrofusion Protocols-Humana Press (1995)Document358 pages(Methods in Molecular Biology) Jac A. Nickoloff - Animal Cell Electroporation and Electrofusion Protocols-Humana Press (1995)Strange LoveNo ratings yet
- Official: Á1053Ñ Capillary ElectrophoresisDocument8 pagesOfficial: Á1053Ñ Capillary ElectrophoresisDilawar BakhtNo ratings yet
- Electropermeabilization, A Physical Method For The Delivery of Therapeutic Molecules Into CellsDocument6 pagesElectropermeabilization, A Physical Method For The Delivery of Therapeutic Molecules Into CellsEspacio UniversitarioNo ratings yet
- Energy & Environmental Science: CommunicationDocument6 pagesEnergy & Environmental Science: CommunicationBhabani Sankar SwainNo ratings yet
- The Electrofusion of Cells POHLDocument19 pagesThe Electrofusion of Cells POHLAntonis TzambazakisNo ratings yet
- Bioelectronics: A Study in Cellular Regulations, Defense, and CancerFrom EverandBioelectronics: A Study in Cellular Regulations, Defense, and CancerNo ratings yet
- A Molecular Insight Into The Electro Transfer of - 2016 - Biochimica Et BiophysDocument12 pagesA Molecular Insight Into The Electro Transfer of - 2016 - Biochimica Et BiophysEduardoNo ratings yet
- Hall 1979Document6 pagesHall 1979sepot24093No ratings yet
- Computational Methods For Cardiac ElectrophysiologyDocument59 pagesComputational Methods For Cardiac ElectrophysiologyFlorinNo ratings yet
- Bioenergetics Nicholls 4th Ed. Intro To CHPT 1Document2 pagesBioenergetics Nicholls 4th Ed. Intro To CHPT 1MellyNo ratings yet
- Electrical Properties of BiomembranesDocument52 pagesElectrical Properties of Biomembranesc3rberussNo ratings yet
- Bmotor BioenergyDocument12 pagesBmotor BioenergydsecondoNo ratings yet
- 11 Objectives 16 - SilvaDocument1 page11 Objectives 16 - SilvaBryan ZhengNo ratings yet
- Law, R. & Levin, M. Bioelectric Memory. Modeling Resting Potential Bistability in Amphibian Embryos and Mammalian CellsDocument20 pagesLaw, R. & Levin, M. Bioelectric Memory. Modeling Resting Potential Bistability in Amphibian Embryos and Mammalian CellsRodolfo van GoodmanNo ratings yet
- Cellular StructureDocument12 pagesCellular StructureFekadu DagnawNo ratings yet
- Keener 1999Document17 pagesKeener 1999nmmMJKJNo ratings yet
- Beebe-Diverse Effects Wideband Non Ionizing Radiation Cells Tissues-04216264Document4 pagesBeebe-Diverse Effects Wideband Non Ionizing Radiation Cells Tissues-04216264searchtheNo ratings yet
- Bioelectrochemistry: Janja Dermol, Damijan Miklav ČičDocument10 pagesBioelectrochemistry: Janja Dermol, Damijan Miklav ČičKrishnaveni Subramani SNo ratings yet
- Membrane Potentials in Living Systems, Tools To Measure: Biological BackgroundDocument10 pagesMembrane Potentials in Living Systems, Tools To Measure: Biological BackgroundazzaassNo ratings yet
- Solar Energy Materials & Solar Cells: Buyoung Jung, Kangmin Kim, Jungwon Kim, Sehwan Kim, Eunkyoung Kim, Woochul KimDocument10 pagesSolar Energy Materials & Solar Cells: Buyoung Jung, Kangmin Kim, Jungwon Kim, Sehwan Kim, Eunkyoung Kim, Woochul KimsamiNo ratings yet
- ECE5710 Notes03 PDFDocument79 pagesECE5710 Notes03 PDFFREDERICKNo ratings yet
- 3 The Electrochemical Basis of Nerve FunctionDocument33 pages3 The Electrochemical Basis of Nerve FunctionEvets JarusNo ratings yet
- ElectrophoresisDocument12 pagesElectrophoresisbensiworkprofileNo ratings yet
- 〈1053〉 CAPILLARY ELECTROPHORESISDocument8 pages〈1053〉 CAPILLARY ELECTROPHORESISgrace_febiantyNo ratings yet
- Mass Spectrometry (MS) and Nuclear Magnetic Resonance (NMR) Applied To Biological MacromoleculesDocument13 pagesMass Spectrometry (MS) and Nuclear Magnetic Resonance (NMR) Applied To Biological Macromoleculesgigel_negoescuNo ratings yet
- 7action PotentialDocument39 pages7action PotentialsanggetharNo ratings yet
- Phy 107 Course SpecificationDocument14 pagesPhy 107 Course SpecificationEric John EnriquezNo ratings yet
- Temperature Effect On Ionic Current and ssDNADocument8 pagesTemperature Effect On Ionic Current and ssDNAIsabela DragomirNo ratings yet
- Evolución Del Concepto de Potencial de Reposo NeuronalDocument12 pagesEvolución Del Concepto de Potencial de Reposo NeuronalDaniela Catalina Vasquez RossierNo ratings yet
- Capillary ElectrophoresisDocument44 pagesCapillary ElectrophoresisLestari TiaNo ratings yet
- Paper 1 Unit 3 ElectrophoresisDocument34 pagesPaper 1 Unit 3 Electrophoresissagar narkarNo ratings yet
- Revisiting The Finite Temperature String Method For The Calculation of Reaction Tubes and Free EnergiesDocument18 pagesRevisiting The Finite Temperature String Method For The Calculation of Reaction Tubes and Free Energiesakrito LeeNo ratings yet
- Electrophoresis and Capillary Electrophoresis PDFDocument21 pagesElectrophoresis and Capillary Electrophoresis PDFVinay kumarNo ratings yet
- KB864 ModelCellsDocument55 pagesKB864 ModelCellsNadim AlbirNo ratings yet
- Key Points RevisionDocument124 pagesKey Points RevisionSumeyyah KemalNo ratings yet
- Lab 3Document45 pagesLab 3Gillian KwanNo ratings yet
- Electrophoresis - Part IDocument30 pagesElectrophoresis - Part IMeghaa.DNo ratings yet
- Artigo JMMDocument11 pagesArtigo JMMMaurício FalleirosNo ratings yet
- Energies 13 04726Document10 pagesEnergies 13 04726farhaNo ratings yet
- Chemistry A European J - 2019 - Munárriz - Valence Shell Electron Pair Repulsion Theory Revisited An Explanation For CoreDocument8 pagesChemistry A European J - 2019 - Munárriz - Valence Shell Electron Pair Repulsion Theory Revisited An Explanation For Coreriya singhNo ratings yet
- CE TutorialDocument14 pagesCE TutorialDenisa JucanNo ratings yet
- Quantitative Human Physiology: An IntroductionFrom EverandQuantitative Human Physiology: An IntroductionRating: 2 out of 5 stars2/5 (1)
- On Removal of Charge Singularity in Poisson-Boltzmann EquationDocument8 pagesOn Removal of Charge Singularity in Poisson-Boltzmann EquationdaskhagoNo ratings yet
- Slee 1986Document7 pagesSlee 1986Andonis AngelovNo ratings yet
- A Fully Implicit Parallel Algorithm For Simulating The Non-Linear Electrical Activity of The HeartDocument17 pagesA Fully Implicit Parallel Algorithm For Simulating The Non-Linear Electrical Activity of The HeartIbitamuno SimeonNo ratings yet
- Full PaperDocument10 pagesFull PaperĐình Thư LêNo ratings yet
- Molecular Modeling of Electron TappingDocument9 pagesMolecular Modeling of Electron TappingJINEETH JJOSEPHNo ratings yet
- Nuclear Instruments and Methods in Physics Research BDocument5 pagesNuclear Instruments and Methods in Physics Research BFederico TurcoNo ratings yet
- 1 Phys Sept 13 READING - Membrane - Potentials - 20212022Document15 pages1 Phys Sept 13 READING - Membrane - Potentials - 20212022n-gorNo ratings yet
- Electrophoresis (2002), 23 (17), 2918-2928Document11 pagesElectrophoresis (2002), 23 (17), 2918-2928Leo G. La PlataNo ratings yet
- Presented by Guided By: Ms - Arpana Pancholi M.SC Biotechnology I Semester Mr. Obulesu M Lect. Deparment of BiotechDocument30 pagesPresented by Guided By: Ms - Arpana Pancholi M.SC Biotechnology I Semester Mr. Obulesu M Lect. Deparment of Biotecharpana4317No ratings yet
- Wolfe - Cellular ThermodynamicsDocument13 pagesWolfe - Cellular Thermodynamicsandres_old_condeNo ratings yet
- Perform Basic MaintenanceDocument60 pagesPerform Basic MaintenanceJexylon TenederoNo ratings yet
- Achinstein, Peter - Speculation Within and About Science.Document297 pagesAchinstein, Peter - Speculation Within and About Science.XEx UltRavoxNo ratings yet
- Two-Level Fractional Factorial Design : Thiết Kế Lũy Thừa Phân ĐoạnDocument40 pagesTwo-Level Fractional Factorial Design : Thiết Kế Lũy Thừa Phân ĐoạnDan ARikNo ratings yet
- Quadratic Equation Using Quadratic Formula: MathematicsDocument56 pagesQuadratic Equation Using Quadratic Formula: MathematicsMariel FerreraNo ratings yet
- PDF For Color Printout1Document6 pagesPDF For Color Printout1ankit kumarNo ratings yet
- Osmometry ManualDocument7 pagesOsmometry ManualLikhithNo ratings yet
- Design and Fabrication of Groundnut Plucking Machine: Amol Junnarkar, Pankaj Jain, Ramnewash NishadDocument5 pagesDesign and Fabrication of Groundnut Plucking Machine: Amol Junnarkar, Pankaj Jain, Ramnewash NishadFiraol GudisaNo ratings yet
- Age HardeningDocument5 pagesAge Hardeninganum_nNo ratings yet
- LET-GEN-ED-General Science 100 ItemsDocument14 pagesLET-GEN-ED-General Science 100 ItemsRdo KazutoNo ratings yet
- 7af7cf0c 5378 4452 8097 9fc9707eabcc - Lesson 2 - Measuring WavesDocument12 pages7af7cf0c 5378 4452 8097 9fc9707eabcc - Lesson 2 - Measuring WavesMarcus WrightNo ratings yet
- Reading Sentence CompletionDocument3 pagesReading Sentence CompletionFahim AhmedNo ratings yet
- Vaisala RH Sensor HMT120 (Quick Guide Multilingual - 2018) PDFDocument103 pagesVaisala RH Sensor HMT120 (Quick Guide Multilingual - 2018) PDFIvan BriscoeNo ratings yet
- PSV Sizing Tool API Based Calc SheetsDocument11 pagesPSV Sizing Tool API Based Calc Sheetsvigneshpingkoo78750% (1)
- Summative Test No.1 Grade 7Document4 pagesSummative Test No.1 Grade 7LENETTE ALAGON100% (1)
- Design and Analysis of Bushed Pin Flexible Coupling: April 2017Document9 pagesDesign and Analysis of Bushed Pin Flexible Coupling: April 2017DOLSON BUTTI204008No ratings yet
- Maths Lab SyntaxDocument12 pagesMaths Lab SyntaxFS-33-Aryan KulharNo ratings yet
- Engineering Survey 1Document27 pagesEngineering Survey 1Ralph MarronNo ratings yet
- Plotting Pressure Coefficient Distribution For Airfoils: Calculation of CDocument19 pagesPlotting Pressure Coefficient Distribution For Airfoils: Calculation of CMarisha BhattiNo ratings yet
- Veb1043 Geomatics January 2020 Lab Report: Experiment: Group: Group MembersDocument8 pagesVeb1043 Geomatics January 2020 Lab Report: Experiment: Group: Group MembersAdam Kamaruzaman50% (2)
- Maths 2aDocument6 pagesMaths 2anagaraj50% (2)
- The Forms X + 32Y AND X + 64y: Irving KaplanskyDocument2 pagesThe Forms X + 32Y AND X + 64y: Irving KaplanskyJohn ChessantNo ratings yet
- Keshar Mahal Presentation - 28-07-2019Document112 pagesKeshar Mahal Presentation - 28-07-2019Aswain Tamrakar100% (1)
- Annual Portion (Grade 11 2022-2023)Document2 pagesAnnual Portion (Grade 11 2022-2023)merlinNo ratings yet
- Symmetry Correlation Diagrams-1Document36 pagesSymmetry Correlation Diagrams-1rakibNo ratings yet
- Strain GuageDocument6 pagesStrain GuageChristian EspanolNo ratings yet
- Sidra Arshad AnalystDocument2 pagesSidra Arshad Analystalrayyan.rashid03No ratings yet
- A3 Jaguar 2020Document4 pagesA3 Jaguar 2020quannguyenhoangminhNo ratings yet
- WAVES Chapter 9Document8 pagesWAVES Chapter 9shasagailNo ratings yet
- Parcial Examen Final Quiz Validación Otro XDocument2 pagesParcial Examen Final Quiz Validación Otro XJuan De DiosNo ratings yet
- Experiment 13N (Alt) RadioactivityDocument12 pagesExperiment 13N (Alt) RadioactivityAfroz Alam Ki VinesNo ratings yet