Professional Documents
Culture Documents
Write Your Name and ID No. On The Question Paper. All Questions Are Compulsory
Uploaded by
APOORVA OJHAOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Write Your Name and ID No. On The Question Paper. All Questions Are Compulsory
Uploaded by
APOORVA OJHACopyright:
Available Formats
Birla Institute of Technology & Science, Pilani, Rajasthan - 333 031
IInd Semester 2015-2016
CHEM F342 ORGANIC CHEMISTRY IV Mid-Term Exam (Closed Book)
Max. Marks: 50 Duration: 90 minutes Date: 17th March 2016
Write your name and ID no. on the question paper. All questions are compulsory.
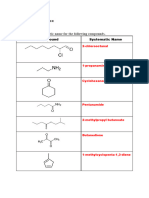
Q1. (i) Write the IUPAC or Hantzsch-Widman system names of the following compounds. [4x1=4]
(ii) Electrophilic substitution in thiophene takes place preferably at C-2 position as compared to C-3
position. Explain [3]
(iii) Arrange furan, benzene, thiophene, and pyrrole in the decreasing order of reactivity towards
electrophilic substitution and electrophilic addition reactions. [2x1=2]
Q2. (i) Identify the major product and propose a detailed mechanism for its formation, in each of the
following chemical transformations: [4+4+4+2=14]
Q3. (i) Identify the final products (A-H) in the following chemical transformations (No mechanism
required): [8x1=8]
(ii) Using appropriate reagents/solvents/reactants, convert succinic acid to diethyl furan-3,4-dicarboxylate
[4]
.............................................................................................................................................................................
P.T.O Page 1
Q4. (i) Draw the acyclic carbon chain framework of a sesquiterpenoid obtained by joining isoprene units.[1]
(ii) Citral, an acyclic monoterpenoid with molecular formula C10H16O, exists as two geometric isomers: (E)-
Citral and (Z)-Citral. When either of the isomers is first treated with Na in EtOH and then with dil. acid,
both form the corresponding cyclic alcohol: -terpineol (mol formula C10H18O). Write down the
corresponding two-step reactions. Explain, by showing the mechanism, which of the two isomers undergoes
faster cyclisation and why. [2+2]
(iii) Complete the following reaction sequence. What important structural information about camphor can be
inferred from these reactions? [2]
Q5. (a) Cholesterol is a monohydroxy steroid. In determining the position of the –OH group, cholesterol is
converted to Diel’s hydrocarbon. Show the reaction conditions and the products formed during the
conversion of cholesterol to Diel’s hydrocarbon in four steps consisting of reduction, oxidation, Grignard
reaction, and heating with Se. [3]
(b) Draw the chair conformer of a trans-trans-trans fused general steroid core. Show clearly the wedge-dash
bonds at the ring fusion. [2]
(c) The following reaction sequence represents the partial synthesis of estrone (a sex hormone) from
androstane. Complete the reaction sequence. [3]
..............................................................................................................................................................................
END Page 2
You might also like
- Transition Metal-Catalyzed Pyridine Synthesis: Transition Metal-Catalyzed Heterocycle Synthesis SeriesFrom EverandTransition Metal-Catalyzed Pyridine Synthesis: Transition Metal-Catalyzed Heterocycle Synthesis SeriesNo ratings yet
- Write Your Name and ID No On The Question Papers. All Questions Are CompulsoryDocument8 pagesWrite Your Name and ID No On The Question Papers. All Questions Are CompulsoryAPOORVA OJHANo ratings yet
- Chemistry Past Paper Ch1.7Document12 pagesChemistry Past Paper Ch1.7Raymond ChanNo ratings yet
- Chemistry Past Paper Ch1.7Document16 pagesChemistry Past Paper Ch1.7Raymond ChanNo ratings yet
- Chem F342 1562 C 2017 1Document8 pagesChem F342 1562 C 2017 1APOORVA OJHANo ratings yet
- Organic Cover Work 7th FebruaryDocument6 pagesOrganic Cover Work 7th FebruaryjcdiekcNo ratings yet
- AS Level Topic 6A TestDocument14 pagesAS Level Topic 6A TestMorvan BarnesNo ratings yet
- Topic 15 Set-3 QS 3 Nov 2021 - 211101 - 171324Document9 pagesTopic 15 Set-3 QS 3 Nov 2021 - 211101 - 171324Fatheena MusfiraNo ratings yet
- As Level Chemistry: Answer All Questions Max 80 MarksDocument15 pagesAs Level Chemistry: Answer All Questions Max 80 MarksemiliaNo ratings yet
- As Level Chemistry: Answer All Questions Max 80 MarksDocument14 pagesAs Level Chemistry: Answer All Questions Max 80 MarksChryssa EconomouNo ratings yet
- Mod 4 Topic Test Core Organic ChemistryDocument21 pagesMod 4 Topic Test Core Organic ChemistryrainsoothingnoiseNo ratings yet
- 9: Organic Chemistry 2 - Topic Questions: Year Series Paper NumberDocument9 pages9: Organic Chemistry 2 - Topic Questions: Year Series Paper NumberSumaira AliNo ratings yet
- Topic 15 SET - 4 QS 3 NOV 21 - 211101 - 202426Document8 pagesTopic 15 SET - 4 QS 3 NOV 21 - 211101 - 202426Fatheena MusfiraNo ratings yet
- Alkenes 2 QPDocument10 pagesAlkenes 2 QPIyad AbdallahNo ratings yet
- 4.4 4.5 TestDocument10 pages4.4 4.5 TestesfwefweNo ratings yet
- Alkenes 1 QPDocument11 pagesAlkenes 1 QPasddf asdafNo ratings yet
- Organic As QuestionsDocument184 pagesOrganic As Questionsan7li721No ratings yet
- Stereoselective Intramolecular 1 3 DipolDocument226 pagesStereoselective Intramolecular 1 3 DipolTrần Văn ĐạtNo ratings yet
- ACJC H2 Chem 2021 P3 QPDocument32 pagesACJC H2 Chem 2021 P3 QPantesipation ฅ'ω'ฅNo ratings yet
- A2 Hydrocarbons: Zahid Iqbal Warraich 0333-4200541Document7 pagesA2 Hydrocarbons: Zahid Iqbal Warraich 0333-4200541Grace KamauNo ratings yet
- Tiffins 2019 ExamDocument17 pagesTiffins 2019 ExamUNKNOWNNo ratings yet
- Edexcel - IAS - Organic Chemistry - 1Document21 pagesEdexcel - IAS - Organic Chemistry - 1mostafa barakatNo ratings yet
- Chemistry Paper 3 SLDocument14 pagesChemistry Paper 3 SLamrdeck1No ratings yet
- A-Level Paper 2 pp2Document16 pagesA-Level Paper 2 pp2Charlie MarstonNo ratings yet
- Carboxylic Acids & Esters 3 QPDocument10 pagesCarboxylic Acids & Esters 3 QPjasonNo ratings yet
- Exam Questions Organic ChemistryDocument4 pagesExam Questions Organic Chemistrymalikimran28No ratings yet
- Chapter 10 Organic Chemistry HL - SLDocument88 pagesChapter 10 Organic Chemistry HL - SLrozalia.kozinskaNo ratings yet
- One-Step Synthetic Ofp-Tert-Butylcalix (4) Arene Derivative Via Direct Benzoylation: Mechanism Reaction StudiesDocument4 pagesOne-Step Synthetic Ofp-Tert-Butylcalix (4) Arene Derivative Via Direct Benzoylation: Mechanism Reaction StudiesIJAR JOURNALNo ratings yet
- Alcohols & Carboxylic Acids 3 QPDocument10 pagesAlcohols & Carboxylic Acids 3 QPAyca UgurluNo ratings yet
- Chemistry Unit 4 Goodie BagDocument29 pagesChemistry Unit 4 Goodie BagJacob Salkin100% (2)
- A2 Level Chemistry: Answer All Questions Max 64 MarksDocument16 pagesA2 Level Chemistry: Answer All Questions Max 64 Marksbejeweled1308No ratings yet
- Chapter 4 QuestionsDocument2 pagesChapter 4 Questionsdaniday1977100% (1)
- Organic Chem ExDocument15 pagesOrganic Chem Exwan zhouNo ratings yet
- 1 Which Intermolecular Force Is The Weakest?Document4 pages1 Which Intermolecular Force Is The Weakest?Loh Jun Xian100% (1)
- Spectroscopy Questions - Student VersionDocument37 pagesSpectroscopy Questions - Student Versionanon_205713503No ratings yet
- Edexcel - IAS - Chemical Analysis - 1Document12 pagesEdexcel - IAS - Chemical Analysis - 1mostafa barakatNo ratings yet
- Alcohols & Carboxylic AcidsDocument7 pagesAlcohols & Carboxylic Acidsk76xhwbsddNo ratings yet
- 2020 MI H2 Chemistry Paper 3Document30 pages2020 MI H2 Chemistry Paper 3clarissa yeoNo ratings yet
- Benzene Is An Important Starting Material in The Production of Dyes, Detergents and Medicines. (A)Document9 pagesBenzene Is An Important Starting Material in The Production of Dyes, Detergents and Medicines. (A)Rayyan BariNo ratings yet
- Amines 2 QPDocument9 pagesAmines 2 QPdanyNo ratings yet
- Q1. (A) Gas Oil (Diesel), Kerosine (Paraffin), Mineral Oil (Lubricating Oil) and PetrolDocument96 pagesQ1. (A) Gas Oil (Diesel), Kerosine (Paraffin), Mineral Oil (Lubricating Oil) and Petrolwerkape1No ratings yet
- JonathanDocument5 pagesJonathanKissiedu YirenkyiNo ratings yet
- It Is "2-Imino-4-Thiazolidinones" and Not Thiohydantoins As The Reaction Product of 1,3-Disubstituted Thioureas and ChloroacetylchlorideDocument7 pagesIt Is "2-Imino-4-Thiazolidinones" and Not Thiohydantoins As The Reaction Product of 1,3-Disubstituted Thioureas and Chloroacetylchloridelox agencyNo ratings yet
- Isomerism 1 QPDocument10 pagesIsomerism 1 QPClemency OuroussoffNo ratings yet
- Enthalpy QuestionsDocument8 pagesEnthalpy QuestionsShiloh FrederickNo ratings yet
- Alk EnesDocument22 pagesAlk EnesgasNo ratings yet
- Anic Chemistry AK 2018-19Document22 pagesAnic Chemistry AK 2018-19XXXNo ratings yet
- SCH 112 - Cat1 2023-2024Document3 pagesSCH 112 - Cat1 2023-2024obarakevin421No ratings yet
- Mini Mock Unit 4 4 To 4 11 A2 Organic Chemistry and Structure DeterminationDocument15 pagesMini Mock Unit 4 4 To 4 11 A2 Organic Chemistry and Structure DeterminationSahanNivanthaNo ratings yet
- A B C D: Winter 1999 CH4Document8 pagesA B C D: Winter 1999 CH4HAC83No ratings yet
- PolymerDocument59 pagesPolymerFatema Khatun0% (1)
- Chemistry HL P3Document22 pagesChemistry HL P3Juan Fernando Velasco ForeroNo ratings yet
- TH Q7Document4 pagesTH Q7Hazel Dela PazNo ratings yet
- As Level Chemistry: Answer All Questions Max 50 MarksDocument10 pagesAs Level Chemistry: Answer All Questions Max 50 MarksChryssa EconomouNo ratings yet
- Chemistry Olympiad 2010 PaperDocument11 pagesChemistry Olympiad 2010 PaperAlokShuklaNo ratings yet
- A Level Chemistry: Topic 17 - Carboxylic Acids, Amines, Esters and Acylation Assessed HomeworkDocument16 pagesA Level Chemistry: Topic 17 - Carboxylic Acids, Amines, Esters and Acylation Assessed HomeworklawrenceNo ratings yet
- Halogenalkanes Elimination and OzoneDocument88 pagesHalogenalkanes Elimination and Ozone22S48 SUNDARAM RAMASUBBU RAKSHANo ratings yet
- CLASS TEST 3 (Introducing Organic, Hydrocarbons) : Academic Session: 2018-2019Document4 pagesCLASS TEST 3 (Introducing Organic, Hydrocarbons) : Academic Session: 2018-2019GM Ali KawsarNo ratings yet
- Sanitizermachine ZeichenDocument7 pagesSanitizermachine ZeichenprasannaNo ratings yet
- Mass Transfer by Migration & Diffusion (Ch. 4)Document16 pagesMass Transfer by Migration & Diffusion (Ch. 4)Shekel DeninoNo ratings yet
- Unit QuestionsDocument155 pagesUnit QuestionsSanya KhanNo ratings yet
- Created by C. Mani, Education Officer, KVS RO Silchar: ST ND RD ST ND RD ST ND RDDocument51 pagesCreated by C. Mani, Education Officer, KVS RO Silchar: ST ND RD ST ND RD ST ND RDjaindevansh100% (2)
- Pipe Fabrication Brochure CompressedDocument2 pagesPipe Fabrication Brochure CompressedLeDzungNo ratings yet
- Angle Chase As PDFDocument7 pagesAngle Chase As PDFNM HCDENo ratings yet
- Radionics PatentsDocument112 pagesRadionics Patentschad ballNo ratings yet
- Failure Modes For Flooded & VRLA BatteriesDocument46 pagesFailure Modes For Flooded & VRLA Batteriesazhagaan100% (1)
- Brochure - Digilogic Systems PVT Ltd.Document28 pagesBrochure - Digilogic Systems PVT Ltd.ManojGRamakrishnaNo ratings yet
- 10K Ductile Cast Iron Gate Valve (Flange Type) TOYO VALVE Gate ValvesDocument4 pages10K Ductile Cast Iron Gate Valve (Flange Type) TOYO VALVE Gate ValvesFredie LabradorNo ratings yet
- Day 2 - S3 S4 - Introduction To Jbase Database1Document48 pagesDay 2 - S3 S4 - Introduction To Jbase Database1alasad parvezNo ratings yet
- 2021 10 11 - Intro ML - InsermDocument41 pages2021 10 11 - Intro ML - Insermpo esperitableNo ratings yet
- Open-Air Theatre in The Centre of The City: Acoustic Design and Noise Environment ControlDocument3 pagesOpen-Air Theatre in The Centre of The City: Acoustic Design and Noise Environment ControlArchitectural Visualizations ArchitecturelandsNo ratings yet
- Ak98 Preset ListDocument21 pagesAk98 Preset ListHichem NaghmouchiNo ratings yet
- Chap. 49 Modes: 3. Modes On Regular Triangular DrumDocument9 pagesChap. 49 Modes: 3. Modes On Regular Triangular DrumfudogNo ratings yet
- Clinical Practice Guideline On Diagnosis and Treatment of Hyponatremia PDFDocument12 pagesClinical Practice Guideline On Diagnosis and Treatment of Hyponatremia PDFLuis Mochas HClNo ratings yet
- Windy Hill Middle School - Trumpet Warm Up BookDocument61 pagesWindy Hill Middle School - Trumpet Warm Up BookGleyce VieiraNo ratings yet
- EE2202 Electromagnetic Theory Lecture NotesDocument125 pagesEE2202 Electromagnetic Theory Lecture NoteskanjaiNo ratings yet
- Excel FunctionsDocument13 pagesExcel Functionsfhlim2069No ratings yet
- Spiking Into Aqueous Samples: Standard Guide ForDocument6 pagesSpiking Into Aqueous Samples: Standard Guide Formohdhafizmdali100% (1)
- Experiment 6Document11 pagesExperiment 6CarlosLorenzoSaninNo ratings yet
- MPMC Unit 2Document31 pagesMPMC Unit 2nikitaNo ratings yet
- Hypothesis Testing in Stata PDFDocument9 pagesHypothesis Testing in Stata PDFMarisela FuentesNo ratings yet
- Way4 Manager Menu Editor: Openway Group System Administrator ManualDocument47 pagesWay4 Manager Menu Editor: Openway Group System Administrator ManualEloy Escalona100% (1)
- Unit 9: Areas and PerimetersDocument22 pagesUnit 9: Areas and PerimetersSanchit GargNo ratings yet
- 4-DatAdvantage Advanced Installation For Microsoft Platforms 8.6 - M365 PatchDocument158 pages4-DatAdvantage Advanced Installation For Microsoft Platforms 8.6 - M365 PatchyaritzaNo ratings yet
- WS-Problems Solved by Quadratic EquationsDocument1 pageWS-Problems Solved by Quadratic EquationsNorul AzimNo ratings yet
- KKC Model Number System2Document3 pagesKKC Model Number System2zayerirezaNo ratings yet
- QT 5 Inferential Chi SquareDocument23 pagesQT 5 Inferential Chi SquareSaad MasoodNo ratings yet
- ICH Quality Guidelines: An Implementation GuideFrom EverandICH Quality Guidelines: An Implementation GuideAndrew TeasdaleNo ratings yet
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (14)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactFrom EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactRating: 5 out of 5 stars5/5 (5)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeRating: 4 out of 5 stars4/5 (1)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- AP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeFrom EverandAP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeRating: 5 out of 5 stars5/5 (1)
- It's Elemental: The Hidden Chemistry in EverythingFrom EverandIt's Elemental: The Hidden Chemistry in EverythingRating: 4 out of 5 stars4/5 (10)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactFrom EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactRating: 5 out of 5 stars5/5 (1)
- Taste: Surprising Stories and Science About Why Food Tastes GoodFrom EverandTaste: Surprising Stories and Science About Why Food Tastes GoodRating: 3 out of 5 stars3/5 (20)
- AP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeFrom EverandAP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeNo ratings yet
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideFrom EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNo ratings yet
- Guidelines for Defining Process Safety Competency RequirementsFrom EverandGuidelines for Defining Process Safety Competency RequirementsRating: 3 out of 5 stars3/5 (1)
- The Periodic Table: A Very Short IntroductionFrom EverandThe Periodic Table: A Very Short IntroductionRating: 4.5 out of 5 stars4.5/5 (3)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (90)
- The Production of Volatile Oils and Perfumery Plants in the United StatesFrom EverandThe Production of Volatile Oils and Perfumery Plants in the United StatesNo ratings yet
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsFrom EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsNo ratings yet
- The Billion-Dollar Molecule: The Quest for the Perfect DrugFrom EverandThe Billion-Dollar Molecule: The Quest for the Perfect DrugRating: 5 out of 5 stars5/5 (2)
- Essential Chemistry for Formulators of Semisolid and Liquid DosagesFrom EverandEssential Chemistry for Formulators of Semisolid and Liquid DosagesRating: 5 out of 5 stars5/5 (2)
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)