Professional Documents
Culture Documents
Reduce NOx and SOx
Uploaded by
noraiz fozanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Reduce NOx and SOx
Uploaded by
noraiz fozanCopyright:
Available Formats
Topic
“How to reduce NOX and SOX”?
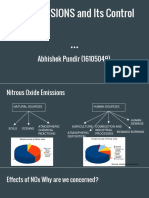
WHERE DOES NOx COME FROM?
Nitrogen oxides (NOx) are a very interesting and important family of air
polluting chemical compounds
Automobiles and other mobile sources contribute about half of the NOx that is emitted.
Electric power plant boilers produce about 40% of the NOx emissions from stationary
sources.34 Additionally, substantial emissions are also added by such anthropogenic
sources as industrial boilers, incinerators, gas turbines, reciprocating spark ignition and
Diesel engines in stationary sources, iron and steel mills, cement manufacture, glass
manufacture, petroleum refineries, and nitric acid manufacture.
NOx Control Method
Method 1. Reducing Temperature -- Reducing combustion temperature means
avoiding the stoichiometric ratio (the exact ratio of chemicals that enter into
reaction). Essentially, this technique dilutes calories with an excess of fuel, air,
flue gas, or steam. Combustion controls use different forms of this technique and
are different for fuels with high and low nitrogen content.
10
Control of NOx from combustion of high nitrogen content fuels (e.g., coal) can be
understood by the net stoichiometric ratio. Control of the NOx from combustion
of low nitrogen fuels (such as gas and oil) can be seen as lean versus rich fuel/air
ratios. Either way, this technique avoids the ideal stoichiometric ratio because
this is the ratio that produces higher temperatures that generate higher
concentrations of thermal NOx.
Combustion temperature may be reduced by: (1) using fuel rich mixtures to limit
the amount of oxygen available; (2) using fuel lean mixtures to limit temperature
by diluting energy input; (3) injecting cooled oxygen-depleted flue gas into the
combustion air to dilute energy; (4) injecting cooled flue gas with added fuel; or
(5) injecting water or steam. Low-NOx burners are based partially on this
principle.8,9,10 The basic technique is to reduce the temperature of combustion
products with an excess of fuel, air, flue gas, or steam. This method keeps the
vast majority of nitrogen from becoming ionized (i.e., getting a non-zero valence).
Method 2. Reducing Residence Time -- Reducing residence time at high
combustion temperatures can be done by ignition or injection timing with internal
combustion engines. It can also be done in boilers by restricting the flame to a
short region in which the combustion air becomes flue gas. This is immediately
followed by injection of fuel, steam, more combustion air, or recirculating flue
gas. This short residence time at peak temperature keeps the vast majority of
nitrogen from becoming ionized. This bears no relationship to total residence
time of a flue gas in a boiler.
Method 3. Chemical Reduction of NOx – This technique provides a chemically
reducing (i.e., reversal of oxidization) substance to remove oxygen from nitrogen
oxides. Examples include Selective Catalytic Reduction (SCR) which uses
ammonia, Selective Non-Catalytic Reduction (SNCR) which use ammonia or urea,
and Fuel Reburning (FR). Non-thermal plasma, an emerging technology, when
used with a reducing agent, chemically reduces NOx. All of these technologies
attempt to chemically reduce the valence level of nitrogen to zero after the
valence has become higher.11 Some low-NOx burners also are based partially on
this principle.
Method 4. Oxidation of NOx -- This technique intentionally raises the valence of
the nitrogen ion to allow water to absorb it (i.e., it is based on the greater
solubility of NOx at higher valence). This is accomplished either by using a
catalyst, injecting hydrogen peroxide, creating ozone within the air flow, or
injecting ozone into the air flow. Non-thermal plasma, when used without a
reducing agent, can be used to oxidize NOx. A scrubber must be added to the
process to absorb N2O5 emissions to the atmosphere. Any resultant nitric acid
can be either neutralized by the scrubber liquid and then sold (usually as a
calcium or ammonia salt), or collected as nitric acid to sell to customers.12, 49
Method 5. Removal of nitrogen from combustion -- This is accomplished by
removing nitrogen as a reactant either by: (1) using oxygen instead of air in the
combustion process; or (2) using ultra-low nitrogen content fuel to form less fuel
NOx.
Reduce
Traditional flue gas desulfurizer (FGD) systems are built for the express purpose of
removing SOx from the exhaust flue gases of fossil-fuel power plants and sometimes
from the emissions of other SOx emitting industrial processes. These systems generally
employ five removal methods:
Wet scrubbing that uses alkaline sorbent or seawater to scrub the flue gas;
The spray-dry scrubbing that uses similar sorbent slurries;
Wet sulfuric acid process that recovers sulfur in the form of sulfuric acid;
SNOX Flue gas desulfurization that removes sulfur dioxide, nitrogen oxides and
particulates from flue gases;
And dry sorbent injection system
Control of Sox Emissions
Following method to control Sox
Natural dispersion by dilution
Using alternative fules
Removal of sulphur by desulphurization
Control of Sox in combustion process
Treatment of fuel gases(dry&wet process)
You might also like
- The Institute of Energy's Second International Conference on COMBUSTION & EMISSIONS CONTROL: Proceedings of The Institute of Energy Conference Held in London, UK, on 4-5 December 1995From EverandThe Institute of Energy's Second International Conference on COMBUSTION & EMISSIONS CONTROL: Proceedings of The Institute of Energy Conference Held in London, UK, on 4-5 December 1995Rating: 5 out of 5 stars5/5 (1)
- COMPLEX PROBEM IcDocument2 pagesCOMPLEX PROBEM Ichafizarslanmushtaq0No ratings yet
- Complex ProblemDocument1 pageComplex Problemhafizarslanmushtaq0No ratings yet
- VOC, NOx, SOxDocument47 pagesVOC, NOx, SOxDIANA CALLISTA ARTANTINo ratings yet
- Topic 4-Control of Nitrogen OxidesDocument36 pagesTopic 4-Control of Nitrogen OxidesalyaNo ratings yet
- NOx Control in Power Plants R1Document10 pagesNOx Control in Power Plants R1Vishal JaishankarNo ratings yet
- Handbook Nitrogen Oxides Pollution Prevention and ControlDocument5 pagesHandbook Nitrogen Oxides Pollution Prevention and ControlrupigapigaNo ratings yet
- Techniques Without Using Emission Control Devices: Plant ShutdownDocument54 pagesTechniques Without Using Emission Control Devices: Plant ShutdownPower PowerNo ratings yet
- BurnersDocument4 pagesBurnersDhanny Miharja100% (1)
- 8-Clean Combustion TechnologiesDocument58 pages8-Clean Combustion TechnologiesNomaan AsimNo ratings yet
- Gas Turbine Emission Control and NOx Reduction TechnologiesDocument11 pagesGas Turbine Emission Control and NOx Reduction TechnologiesKARTHIKEYANNo ratings yet
- Boiler Combustion & EmissionDocument15 pagesBoiler Combustion & EmissionMustafa HusainNo ratings yet
- Sources and Control Methods: CE/AE 524B Air Pollution J. (Hans) Van LeeuwenDocument28 pagesSources and Control Methods: CE/AE 524B Air Pollution J. (Hans) Van LeeuwenSteve Johnson100% (1)
- Boiler Pollution Control TutorialDocument16 pagesBoiler Pollution Control TutorialsunitbhaumikNo ratings yet
- Air Pollution FinalDocument131 pagesAir Pollution FinalfaheemabbasNo ratings yet
- Technology For Air Pollution ControlDocument91 pagesTechnology For Air Pollution Controlsishu21No ratings yet
- GE Gas Turbine NOx Emissions Paper Analyzes Costs and Benefits of Achieving Very Low Emission LevelsDocument12 pagesGE Gas Turbine NOx Emissions Paper Analyzes Costs and Benefits of Achieving Very Low Emission Levelshermans57No ratings yet
- Exhaust Gas Recirculation in Four Stroke EngineDocument29 pagesExhaust Gas Recirculation in Four Stroke Enginejatin guptaNo ratings yet
- PctDocument3 pagesPctAayan ShahNo ratings yet
- Air Pollution ControlDocument24 pagesAir Pollution ControlMahmoud TarekNo ratings yet
- Burner TechnologyDocument9 pagesBurner TechnologyTint TigerNo ratings yet
- Chapter - 22 Boiler Pollution Control 1.: DLP/BOE-II/ 1-01092001Document16 pagesChapter - 22 Boiler Pollution Control 1.: DLP/BOE-II/ 1-01092001Jagdeep ArryNo ratings yet
- Unit - 4 Part - A: I. HydrocarbonsDocument20 pagesUnit - 4 Part - A: I. HydrocarbonsJVCNo ratings yet
- Air Pollution: CE1400 Environment and Safety Engineering Lecture-21Document36 pagesAir Pollution: CE1400 Environment and Safety Engineering Lecture-21Dinesh Kumar SahuNo ratings yet
- TP PC 11 02Document16 pagesTP PC 11 02Khoirul Walad100% (1)
- 1.4 Natural Gas CombustionDocument11 pages1.4 Natural Gas Combustionnoorul786No ratings yet
- Iesel Exhaust GAS AftertreatmentDocument16 pagesIesel Exhaust GAS AftertreatmentHarsh ShuklaNo ratings yet
- 4-Emission ControlDocument27 pages4-Emission ControlRASHEED MUHAMMADNo ratings yet
- 5B2. Nox Reduction Technology: Environmental Protection Technologies (Flue Gas Treatment and Gas Cleaning Technologies)Document2 pages5B2. Nox Reduction Technology: Environmental Protection Technologies (Flue Gas Treatment and Gas Cleaning Technologies)Hussein AlyahriNo ratings yet
- Nox Impacts On Environment and Human HealthDocument5 pagesNox Impacts On Environment and Human HealthnaikNo ratings yet
- What You Require To Know About NOx Reduction.20130104.123704Document2 pagesWhat You Require To Know About NOx Reduction.20130104.123704anon_931869105No ratings yet
- Industrial Oxygen: Its Generation and UseDocument12 pagesIndustrial Oxygen: Its Generation and UseJafar JilaniNo ratings yet
- Cement manufacturing emissions guidelinesDocument4 pagesCement manufacturing emissions guidelinesSambhu YadavNo ratings yet
- Air Pollution Control Methods for NOx EmissionsDocument30 pagesAir Pollution Control Methods for NOx EmissionsskywalkerNo ratings yet
- Flotation: Types of Flotation SeparationsDocument6 pagesFlotation: Types of Flotation SeparationsWaseem YousafNo ratings yet
- Nitric Acid: Chemical Process IndustriesDocument13 pagesNitric Acid: Chemical Process Industries78623No ratings yet
- NOx Reduction Techniques for ComplianceDocument2 pagesNOx Reduction Techniques for ComplianceKunal ChandNo ratings yet
- Nox Reduction Using RDF (Refuse Derived Fuel) in Cement IndustryDocument2 pagesNox Reduction Using RDF (Refuse Derived Fuel) in Cement IndustryIzaz Ulhaq YousafziNo ratings yet
- Ref7 PDFDocument7 pagesRef7 PDFJulian BermudezNo ratings yet
- Low-Emission NOx Reduction RefineriesDocument5 pagesLow-Emission NOx Reduction Refinerieslaiping_lumNo ratings yet
- Selective Catalytic ReductionDocument15 pagesSelective Catalytic ReductionJuan Esteban EnriquezNo ratings yet
- Environmental Protection in The Field of Thermal Power PlantDocument21 pagesEnvironmental Protection in The Field of Thermal Power PlantGoverdhan ShresthaNo ratings yet
- Natural Gas CombustionDocument10 pagesNatural Gas CombustionLakshmi Pathi BojjaNo ratings yet
- 6 Tips For Improving Efficiency and Reducing NOX - 2018-09-13 - Process HeatingDocument15 pages6 Tips For Improving Efficiency and Reducing NOX - 2018-09-13 - Process Heatingwest kestNo ratings yet
- Combustion Principles and ControlDocument32 pagesCombustion Principles and ControlThalia de la FuenteNo ratings yet
- Gas Natural PDFDocument10 pagesGas Natural PDFCristian Andres Muñoz AguilarNo ratings yet
- Gas Turbine Emissions and Their ControlDocument23 pagesGas Turbine Emissions and Their Controlgrkgupta100% (2)
- Measurement of ExhaustDocument25 pagesMeasurement of ExhaustSreeram NandakumarNo ratings yet
- Recent Development in MarineDocument44 pagesRecent Development in MarinerajishrrrNo ratings yet
- Chapter 5 - Microturbine Fuels and EmissionsDocument4 pagesChapter 5 - Microturbine Fuels and EmissionsGarip GerçeklerNo ratings yet
- Technologies To Reduce Air PollutionDocument18 pagesTechnologies To Reduce Air PollutionSambiri PisiraiNo ratings yet
- VDZ-Onlinecourse 7 3 enDocument20 pagesVDZ-Onlinecourse 7 3 enAnonymous iI88LtNo ratings yet
- 10 Technologies/Methods For Controlling Nox & Sox Emissions From ShipsDocument2 pages10 Technologies/Methods For Controlling Nox & Sox Emissions From ShipsRaji MNNo ratings yet
- Nox Emissions and Its Control: Abhishek Pundir (16105049)Document27 pagesNox Emissions and Its Control: Abhishek Pundir (16105049)SavitarNo ratings yet
- Application of SNCR and SCR in Cement PlantsDocument8 pagesApplication of SNCR and SCR in Cement PlantsmuhammedNo ratings yet
- IncinerationDocument50 pagesIncinerationinder4180100% (1)
- The Diesel Catalytic ConverterDocument4 pagesThe Diesel Catalytic ConvertersayeemNo ratings yet
- SNCRDocument21 pagesSNCRtunganh1110No ratings yet
- Carbon Capture Technologies for Gas-Turbine-Based Power PlantsFrom EverandCarbon Capture Technologies for Gas-Turbine-Based Power PlantsNo ratings yet
- Cyclone SeparatorsDocument9 pagesCyclone Separatorsnoraiz fozanNo ratings yet
- Naeem CVDocument3 pagesNaeem CVnoraiz fozanNo ratings yet
- Computing Fundamentals: By: Hina ArshadDocument40 pagesComputing Fundamentals: By: Hina Arshadnoraiz fozanNo ratings yet
- F1 Visa Interview Questions and AnswersDocument8 pagesF1 Visa Interview Questions and AnswersSanket Shah71% (7)
- Epoxy Polymer Surface Roughness Modeling Based On Kinetic Studies of Wet Chemical TreatmentsDocument2 pagesEpoxy Polymer Surface Roughness Modeling Based On Kinetic Studies of Wet Chemical TreatmentsAzzedine AllalNo ratings yet
- Temperature and Heat 3Document18 pagesTemperature and Heat 3Samud MorrisNo ratings yet
- Environmental Management in The Oil, Gas and Related Energy Industries in GhanaDocument7 pagesEnvironmental Management in The Oil, Gas and Related Energy Industries in GhanaKwame D BoatengNo ratings yet
- Charpy Impact TestDocument4 pagesCharpy Impact TestChinmay Deo80% (5)
- JSW Casting EquipDocument23 pagesJSW Casting EquipArnab GhoshNo ratings yet
- Adhesion of Cells To Polystyrene SurfaceDocument7 pagesAdhesion of Cells To Polystyrene Surfacef20212314No ratings yet
- Ashland, Technical Data SheetDocument5 pagesAshland, Technical Data Sheetyasi heeruNo ratings yet
- Freon 134a Si Thermodynamic PropertiesDocument52 pagesFreon 134a Si Thermodynamic PropertiesAyu LestariNo ratings yet
- Extension Module 6 of ChemistryDocument6 pagesExtension Module 6 of Chemistryangelo aquinoNo ratings yet
- Science Lab ReportDocument8 pagesScience Lab Reportapi-452660914No ratings yet
- AA IGCSE Unit 1,2Document9 pagesAA IGCSE Unit 1,2Yee MeiNo ratings yet
- Wet II Presentation Group 3 Antimicrobial FinishDocument29 pagesWet II Presentation Group 3 Antimicrobial FinishZillur Rahman SaykatNo ratings yet
- Effect of 1-Thioglycerol As Capping Agent On ZNS Nanoparticles: Structural and Optical CharacterizationDocument4 pagesEffect of 1-Thioglycerol As Capping Agent On ZNS Nanoparticles: Structural and Optical CharacterizationInternational Journal of Science and Engineering InvestigationsNo ratings yet
- CH01 Analy ChemDocument29 pagesCH01 Analy Chemαγαπημένη του ΧριστούNo ratings yet
- Physical Science SHS 4.1 Worksheet 2Document2 pagesPhysical Science SHS 4.1 Worksheet 2Maricris Jane PeranteNo ratings yet
- Kilfrost Winter Best Practice Guide - Edition 3Document44 pagesKilfrost Winter Best Practice Guide - Edition 3DimitriosMonogios100% (1)
- Intro To Methyl Chloride Plant 1Document57 pagesIntro To Methyl Chloride Plant 1Kimberly ConleyNo ratings yet
- Us3479310 PDFDocument7 pagesUs3479310 PDFKhoi Nguyen DangNo ratings yet
- 4th Opp Final ReportDocument30 pages4th Opp Final ReportLEBOGANG MASHABELANo ratings yet
- Minor07 Ans DLP NEET20 (Pmtcorner - In)Document8 pagesMinor07 Ans DLP NEET20 (Pmtcorner - In)Jennifer AkhtarNo ratings yet
- MEM 661 - Applied Welding Engineering Individual Assignment Instruction and Guidelines CASE STUDY (Document10 pagesMEM 661 - Applied Welding Engineering Individual Assignment Instruction and Guidelines CASE STUDY (Shaikhan NadzemiNo ratings yet
- Nessler Reagent PDFDocument14 pagesNessler Reagent PDFBernardoNo ratings yet
- Production process of artists' paintDocument2 pagesProduction process of artists' paintkinley dorjeeNo ratings yet
- Internship Report On Railway Coach FactoryDocument39 pagesInternship Report On Railway Coach FactoryJyotiraj ThakuriaNo ratings yet
- A Type-Curve Matching Procedure For Material Balance Analysis of Production Data From Geopressured Gas Reservoirs A.K. AmbasthaDocument6 pagesA Type-Curve Matching Procedure For Material Balance Analysis of Production Data From Geopressured Gas Reservoirs A.K. AmbasthaAnderson JimenezNo ratings yet
- Magnetic PropertiesDocument29 pagesMagnetic PropertiesSiyan ShivaNo ratings yet
- Bio-Vision - SSLC Chemistry SmileDocument19 pagesBio-Vision - SSLC Chemistry SmilemujeebNo ratings yet
- Shell Tellus S2M68 Msds - 00107669Document7 pagesShell Tellus S2M68 Msds - 00107669Jasmine TsoNo ratings yet
- Chemical Bonding Worksheet ReviewDocument2 pagesChemical Bonding Worksheet ReviewCatherine JeaneNo ratings yet
- Manufacturing of Acrylonitrile Internship ReportDocument33 pagesManufacturing of Acrylonitrile Internship ReportHirenNo ratings yet