Professional Documents
Culture Documents
Vasodilator Effect of 1-Trifluoromethoxyphenyl-3-1
Uploaded by
Syed Danish AliOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Vasodilator Effect of 1-Trifluoromethoxyphenyl-3-1
Uploaded by
Syed Danish AliCopyright:
Available Formats
See discussions, stats, and author profiles for this publication at: https://www.researchgate.
net/publication/326394224
Vasodilator effect of 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-
yl) urea is predominantly mediated through activation of voltage-dependent
K+ channels
Article in Tropical Journal of Pharmaceutical Research · June 2018
DOI: 10.4314/tjpr.v17i6.6
CITATION READS
1 39
5 authors, including:
Shafiq Ali Shah Malik Hassan Mehmood
Superior University Government College University Faisalabad
6 PUBLICATIONS 5 CITATIONS 84 PUBLICATIONS 1,312 CITATIONS
SEE PROFILE SEE PROFILE
Ishfaq A Bukhari Anwar-ul Hassan Gilani
King Saud University University of Haripur
65 PUBLICATIONS 932 CITATIONS 488 PUBLICATIONS 19,960 CITATIONS
SEE PROFILE SEE PROFILE
Some of the authors of this publication are also working on these related projects:
Almond's effectiveness in cardiovascular system View project
Natural products from medicinal plants View project
All content following this page was uploaded by Shafiq Ali Shah on 25 June 2021.
The user has requested enhancement of the downloaded file.
Shah et al
Tropical Journal of Pharmaceutical Research June 2018; 17 (6): 1019-1024
ISSN: 1596-5996 (print); 1596-9827 (electronic)
© Pharmacotherapy Group, Faculty of Pharmacy, University of Benin, Benin City, 300001 Nigeria.
Available online at http://www.tjpr.org
http://dx.doi.org/10.4314/tjpr.v17i6.6
Original Research Article
Vasodilator effect of 1-trifluoromethoxyphenyl-3-(1-
propionylpiperidin-4-yl) urea is predominantly mediated
through activation of voltage-dependent K+ channels
Shafiq Ali Shah1,2, Malik Hassan Mehmood1,3*, Munasib Khan2, Ishfaq Ali
4
Bukhari , Anwarul Hassan Gilani1
1
Natural Product Research Division, Department of Biological and Biomedical Sciences, Aga Khan University, Karachi,
2 3
Department of Pharmacy, University of Malakand, KPK, Department of Pharmaceutical Sciences, Government College
4
University, Faisalabad, Pakistan, Department of Pharmacology College of Medicine, King Saud University, Riyadh, Saudi
Arabia
*For correspondence: Email: malikhassan.mehmood@gmail.com; Tel: (+92 21) 3493 0051 ext 4465; Fax: (+92 21) 3493 4294
Sent for review: 25 October 2017 Revised accepted: 13 May 2018
Abstract
Purpose: To determine the mechanism of vasorelaxant effect of 1-trifluoromethoxyphenyl – 3 -(1-
propionylpiperidin–4-yl) urea (TPPU) in cardiovascular diseases, including hypertension.
Methods: Isolated rat thoracic aortic tissue preparations were mounted in an organ bath set up
integrated with isometric transducer and a Power Lab assembly. TPPU (0.3 - 100 µM) was tested for
+ +
vasorelaxant effect against low K (25 mM) and high K (80 mM)-induced contractions and its
mechanism was determined in the presence of different antagonists (glibenclamide, 4- aminopyridine
and tetraethyl ammonium).
Results: In rat aortic preparations, TPPU showed a concentration-dependent (0.3 – 100 µM) and
+
significant (p < 0.001) inhibition of low K induced contractions with complete inhibition obtained at 100
+
µM. TPPU produced significant (p < 0.05) inhibition of high K induced contractions with maximum
relaxation of 15.36 ± 1.95 % and 15.85 ± 3.35 % at 30 and 100 µM, respectively. Glibenclamide
+
(Gb,10 µM) pretreatment partially inhibited the vasorelaxant effect of TPPU against low K in a
concentration range of 1 - 30 µM. 4-Aminopyridine (4-AP, 1 mM) and tetraethyl ammonium (TEA, 10
+
mM), markedly inhibited the vasorelexant effect of TPPU against low K induced contractions with
maximum relaxation of 20.09 ± 2.40 and 21.67 ± 0.88 %, respectively, at 100 µM.
Conclusion: TPPU possesses marked vasorelaxant properties which provides sound pharmacological
evidence for its use as a potential drug candidate in the management of hypertension.
+
Keywords: 1-Trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea, Hypertension, vasodilator, K -
+
channel activation, Ca - channel antagonist
This is an Open Access article that uses a funding model which does not charge readers or their institutions
for access and distributed under the terms of the Creative Commons Attribution License
(http://creativecommons.org/licenses/by/4.0) and the Budapest Open Access Initiative
(http://www.budapestopenaccessinitiative.org/read), which permit unrestricted use, distribution, and
reproduction in any medium, provided the original work is properly credited.
Tropical Journal of Pharmaceutical Research is indexed by Science Citation Index (SciSearch), Scopus,
International Pharmaceutical Abstract, Chemical Abstracts, Embase, Index Copernicus, EBSCO, African
Index Medicus, JournalSeek, Journal Citation Reports/Science Edition, Directory of Open Access Journals
(DOAJ), African Journal Online, Bioline International, Open-J-Gate and Pharmacy Abstracts
INTRODUCTION enzyme which metabolizes epoxyeicosatrienoic
acids (EETs) to functionally less active
Soluble epoxide hydrolase (sEH) has been a produces, the dihydroxyeicosatrienoic acids
recent focus of research, is an indigenous DHETs [1]. In endothelial cells, cytochrome P450
-----------------------------------------------------------------------------------------------------------------------------------------------------
© 2018 The authors. This work is licensed under the Creative Commons TropAttribution
J Pharm 4.0 Res,International
June 2018; License
17(6): 1019
Shah et al
epoxygenase metabolizes arachidonic acid to EXPERIMENTAL
produce EET. EETs are well known for diverse
biological activities including vasodilation, anti- Chemicals
inflammatory, platelet aggregation inhibitory,
+
analgesic and cardioprotection [2,3]. Different K channel antagonists, tetraethyl
ammonium (TEA), 4-aminopyridine (4-AP) and
sEH is an indigenous enzyme which transforms glibenclamide (Gb) were acquired from Sigma
EETs to inactive dihydroxyeicosatrienoic acids Chemicals Company (St Louis, MO, USA). TPPU
DHETs [2]. The potential biological activities of was procured from Synthia laboratories Davis,
DHETs such as vasodilatation and anti- California, USA. Other chemicals used in the
inflammatory effects are less compared to EETs study such as Potassium chloride, calcium
[4]. sEH is widely distributed in intestine, liver, chloride, glucose, magnesium sulphate,
kidney, vascular smooth muscles, neuronal cells potassium dihydrogen phosphate, sodium
and astrocytes [5]. Inhibition of sEH enzyme bicarbonate and sodium chloride were obtained
from E. Merck, Darmstadt, Germany. All
activity causes elevated levels of EETs in
chemicals used were of the analytical grade.
biological fluid and tissues, thus promoting
EXPERIMENTAL ANIMALS
beneficial pharmacological actions of EETs in the
body. These findings suggest that sEH inhibition Sprague–Dawley rats of either sex and weighing
could be a potential therapeutic target for 180 – 200 g were maintained at the Animal
cardiovascular disease and pain and House facility of Aga Khan University Medical
inflammatory conditions [6]. College at 23 - 25 C. Animals had free access
to tap water, ad libitum. Animal were provided
Inhibition of sEH enzyme prevents standard diet which consists of (g/kg): flour 380,
biodegradation of EETs and enhances their fiber 380, molasses 12, NaCl 5.8, nutrivet L. 2.5,
beneficial actions. Growing body of literature powdered milk 150, vegetable oil 38, potassium
revealed that deletion of sEH or over activity of metabisulfate 1.2 and fish meal 170. Water was
CYP epoxygenase lowered blood pressure in withdrawn from rats for 12 - 14 h prior to
animal model of hypertension [7]. Large number anesthesia. The animals were euthanized
of studies have revealed anti-inflammatory, following deep anesthesia with isoflurane (2 - 5
vasodilator, antihypertensive, cardiac and renal % v/w) by inhalation in a closed chamber. After
protective effects of sEH inhibitors [6,8,9]. the achievement of deep anesthesia that was
confirmed by absence of touch and corneal
1-Trifluoromethoxyphenyl-3-(1-propionylpiperidin reflexes of the animals, thoracotomy was
-4-yl) urea (TPPU) is novel among the sEH performed followed by cardiac puncture/heart
inhibitors with relatively better pharmacokinetic excision to euthanize the animals.
and biological activity profile [10]. Human and
animals studies have also shown TPPU as a Experiments were conducted and compiled to
potent inhibiter of sEH [11]. Though TPPU has the guidelines of Institutional ethics committee
been widely studied for its diverse biological and the Institute of Laboratory Animal
activities, however, its vasorelaxant effect in Resources, Commission on Life Sciences,
intact vascular tissues is yet to be explored. The National Research Council (National Research
present study explored the possible vasodilator Council, 1996) [12]. This research work was the
activity of TPPU, mediated predominantly part of the PhD dissertation of Mr. Shafiq Ali
through activation of voltage-dependent K+ Shah, which was reviewed and approved by the
channels, which may explain the potential Board of Studies and Research at University of
therapeutic role of this compound in the Malakand, KPK, Pakistan and Aga Khan
management of hypertension. University Medical College Karachi Pakistan,
with approval no. 60-ECACU-BBS-15.
Animal studies
Rats were euthanized after they were fully
anesthetized with isoflurane by inhalation.
Isoflurane was used 2-5 % v/w, till achievement
of deep anesthesia. Once deep anesthesia was
Figure 1: Chemical structure of soluble epoxide
achieved and confirmed by absence of touch and
hydrolase inhibitor, TPPU corneal reflexes, thoracotomy was performed
followed by cardiac puncture/heart excision to
euthanize the animals. Afterwards, the thoracic
Trop J Pharm Res, June 2018; 17(6): 1020
Shah et al
aorta was isolated, cleaned of fatty tissues, cut RESULTS
into small rings and mounted individually in a
tissue bath (5 mL) filled with Kreb’s solution at 37 Vasodilating effect of TPPU in isolated
ºC and aerated with carbogen [13]. A resting rat vascular tissues
tension of 2 g was gradually applied to mounted
aortic tissue preparation. The tissues were
When tested on isolated rat aortic tissue, TPPU
initially incubated for 30 min and finally
caused significant (p < 0.001) inhibition of low
equilibrated for 1 h prior to addition of any
K+-induced contractions in a concentration
chemical agent. K+ at low (25 mM) and high (80
dependent manner with an EC50 value of 6.72
mM) concentration was used to stabilize the
µM (6.32 - 7.02, 95 % CI, n = 4), while it has
respective tissue preparations until achievement
produced complete inhibition (100% relaxation)
of constant responses usually after 2 – 3 times
at highest tested concentration of 100 µM (Figure
application followed by washing of the tissue with
2). TPPU produced mild but significant (p < 0.05)
fresh Kreb’s solution [14]. After stabilization
relaxation of high K+ (80 mM)-induced
state, low and high K+ was administered to the
contractions with resultant values of 15.36 ±
tissue to induce sustained contractions,
1.95and 15.85 ± 3.35 % at higher test
respectively. The relaxant effect of TPPU at 0.1 -
concentrations of 30 and 100 µM, respectively
100 µM was assessed against low and high K+-
(Figure 2).
induced contractions, respectively. Isometric
responses of the vessels were measured
employing isometric transducer 50-7996
(Harvard Apparatus, Holliston, MA, USA),
connected to PowerLab assembly.
To explore the participation of K+ channel
opening and/or Ca++ channel antagonist
properties [15], the vasorelexant effect of TPPU
was further studied against low K+ (25 mM) and
high K+ (80 mM)-induced contractions,
respectively. After achieving sustained
contractions of vessels to K+, TPPU (0.1 - 100
µM) was added in a cumulative fashion to the
vessels to obtain its concentration-dependent
inhibitory responses. The relaxation of the tissue
preparation was expressed as percentage of the
control contraction mediated by K+.
+
To characterize and confirm the type of K -
channels involved in the vasodilating effect, the +
Figure 2. Inhibitory effect of TPPU on “□” low K (25
inhibitory effect of TPPU was reproduced in the +
mM) and “■”high K (80 mM)-induced contractions in
aortic preparation pretreated with glibenclamide isolated rat aortic rings. Values shown are mean
(Gb, 10 µM), an ATP-dependent K+ channel @
±S.E.M, n = 4-6. “ns” represents non-significant, */ p <
antagonist [16], tetraethyl ammonium (TEA, 10 0.05, **p < 0.0, ***p < 0.001, * shows comparison of the
+ +
mM), a nonselective antagonist of the K relaxant effect of TPPU on low K (25 mM) -induced
channels [17] and 4 - aminopyridine (4-AP, 1 contractions vs. 100 % control contractile response in
@
that tissue. shows comparison of the relaxant effect
mM), a voltage-dependent K+ channel blocker +
of TPPU on high K (80 mM)-induced contractions vs.
add [14]. 100 % control contractile response in that tissue and
ns = non-significant
Statistical analysis
Insight into mechanisms mediating
Data is presented as mean standard error of vasodilating effect of TPPU
mean (s.e.m, n = 4 - 6) and the median effective
concentrations (EC50) with 95 % confidence To explore the subtype of K+ channels involved
intervals (CI). Data was considered statistically in the observed vasodilator effect of TPPU, the
significant at p < 0.05. The inhibitory effects of +
tissues were pre-incubated with different K
various treatments were statistically analyzed by channel blockers. As shown in Figure 3 pre-
non-linear regression employing GraphPad treatment of tissue with 4-aminopyridine (4 - AP,
program (GraphPad, San Diego, CA, USA). 1 mM), suppressed the relaxant effect of TPPU
against low K+-induced contractions (p < 0.001)
with remaining resultant relaxation of 12.9±2.46
Trop J Pharm Res, June 2018; 17(6): 1021
Shah et al
% (n = 5) at 100 µM compared to control vessels response of vessel is usually mediated through
showing 100 % relaxation in the absence of 4 - K+ channel opening or Ca++ channel blockade
AP observed at the same concentration. Similarly [15]. In order to characterize, whether the vaso-
pretreatment of blood vessels with TEA (10 mM) relaxant effect of TPPU was mediated via similar
caused significant (p < 0.001) attenuation of the pathways, its relaxant efficacy was tested against
relaxant effect of TPPU against low K+ induced low K+(25 mM) and high K+ (80 mM)-induced
contractions with maximum relaxation of 13.5 ± contractions [19]. Interestingly, TPPU caused
3.50 % versus 100 % in control vessels in the statistically significant (p < 0.001) and dose
absence of TEA (Figure 3). Pre-incubation of the dependent inhibition of low K+-induced
blood vessels with glibenclamide (10 µM) caused contractions with partial but significant (p < 0.05)
+
partial (p < 0.01) antagonism of the inhibitory inhibition against high K -induced contractions
effect of TPPU (Figure 3). only at 30 and 100 µM. The efficacy of TPPU
against low K+ (25 mM)-induced contractions
ns ns $/+ $$$
suggesting K+ channel opening activity of this
$$$ $$$
100 compound [20]. It has been shown that
+ $$$ +
* +++ compounds that selectively inhibit the low K ( <
* +++ vs
30 mM) induced contractions are considered K+
75 +++
+++ channel opener, while Ca++channel blockers are
% of Control
vs efficacious against both low and high K+-induced
** vs contractions [13, 21].
50 +
To elucidate the type of K channels implicated in
* the vasorelaxant activity of TPPU, its effect was
assessed against low K+-induced contraction in
25 tissue pretreated with glibenclamide, a ATP-
dependent K+(KATP) channels antagonist [16],
TEA, a non-specific K+ channel blocker [17] and
0 4-Aminopyridine, a voltage dependent K+
0.1 1 10 100 channels blocker [22]. Glibenclamide had partial
inhibitory influence, while 4-AP and TEA caused
TPPU [M] marked inhibition of low K+-induced contractions
suggesting that the vasorelaxant effect of TPPU
+
Figure 3. Inhibitory effect of TPPU on “□” low K - is most likely mediated via activation voltage-
induced contractions in the absence and presence of dependent K+ channels (Kv channels) and non-
“▲” glibenclamide (Gb, 10 µM), “●”tetraethyl +
specific K channels. Moreover, these findings
ammonium (TEA, 10 mM) and “×” 4-aminopyridine (4 -
indicate the additional role of KATP channel
AP, 1 mM) in isolated rat aortic preparations. Values
shown are mean ± S.E.M, n = 4 - 6. “ns” represents activation and a weak Ca++ antagonist-like effect
+ $ ++ $$
non-significant, */ / p < 0.05, **/ / p < 0.01,
+++ $$$
/ p< [23] as possible vasorelaxant mechanism(s) of
0.001, “*”shows comparison of the relaxant effect of TPPU in part, though additional mechanism(s)
+
TPPU on low K (25 mM)-induced in the presence vs. cannot be ruled out.
absence of Gb. “+” shows comparison of the relaxant
+
effect of TPPU on low K (25 mM) -induced in the Among varied types of K+ channels, Kv
presence vs. absence of TEA. “$”shows comparison of neutralize the depolarization of the membrane
+
the relaxant effect of TPPU on low K (25 mM)-induced +
potential via K efflux [17]. Studies have also
in the presence vs. absence of 4-AP
expressed Kv channels in aortic smooth muscles
[24]. K+ channel openers are potential new class
DISCUSSION of drugs with diverse therapeutic potential in
hypertension, asthma, and gastrointestinal
Recently TPPU has got much research attention problems [25]. These compounds cause
for its efficacy as a potential therapeutic agent in membrane hyperpolarization by opening K
+
cardiovascular disorders [18]. It has been shown +
channels and increase in K efflux, decreasing
to have antihypertensive effects by enhancing intracellular free Ca++ leading to smooth muscle
EETs level in the body via sEH inhibition. EETs relaxation [17].
are well recognized for their potent vasodilator
effect. In conclusion, this study demonstrates that TPPU
possesses profound vasorelaxant properties.
The objective of this investigation was to explore These findings provide sound evidence for TPPU
the vasodilatory effect of TPPU and its possible as a potential antihypertensive agent. Future
mechanism in isolated rat aortic preparations. studies are warranted to explore the mechanism
Previous studies have shown that vasodilator
Trop J Pharm Res, June 2018; 17(6): 1022
Shah et al
of TPPU as potential vasodilator agent in the pathologic conditions. Curr Med Chem 2011; 18(4): 587-
management of cardiovascular disorders. 603.
7. Capdevila J, Falck J, Imig J, Roles of the cytochrome
DECLARATIONS P450 arachidonic acid monooxygenases in the control
of systemic blood pressure and experimental
Acknowledgement hypertension. Kidney Int 2007; 72(6): 683-689.
8. Imig JD, Walsh KA, Khan MAH, Nagasawa T, Shaw MC,
This research was supported by Deanship of Shaw SM, Hammock BD. Soluble epoxide hydrolase
Scientific Research, King Saud University, inhibition and peroxisome proliferator activated receptor
Riyadh, Kingdom of Saudi Arabia. The authors γ agonist improve vascular function and decrease renal
acknowledge the support and resources provided injury in hypertensive obese rats. Exp Biol Med 2012;
to Dr. Malik Hassan Mehmood from the 237(12): 1402-1412.
Department of Biological and Biomedical 9. Bukhari IA, Shah AJ, Gauthier KM, Walsh KA, Koduru
Sciences, The Aga Khan University. SR, Imig JD, Falck JR, Campbell WB. 11, 12, 20-
. Trihydroxy-eicosa-8 (Z)-enoic acid: a selective inhibitor
Conflict of interest of 11, 12-EET-induced relaxations of bovine coronary
and rat mesenteric arteries. Am J Physiol Heart Circ
The authors declare that they have no competing Physiol 2012; 302(8): 1574-1583.
interests with regard to this work. 10. Liu JY, Lin YP, Qiu H, Morisseau C, Rose TE, Hwang
SH, Chiamvimonvat N, Hammock BD. Substituted
Authors’ contributions phenyl groups improve the pharmacokinetic profile and
anti-inflammatory effect of urea-based soluble epoxide
We declare that this work was done by the hydrolase inhibitors in murine models. Eur J Pharm Sci
authors named in this article and all liabilities 2013; 48(4): 619-627.
pertaining to claims relating to the content of this 11. Sasso O, Wagner K, Morisseau C, Inceoglu B, Hammock
article will be borne by the authors. Malik Hassan BD, Piomelli D. Peripheral FAAH and soluble epoxide
Mehmood and Ishfaq Ali Bukhari Ishfaq designed hydrolase inhibitors are synergistically antinociceptive.
the project, Malik Hassan Mehmood and Anwarul Pharmacol Res 2015; 97: 7-15.
Hassan Gilani supervised the study and drafted 12. Council NR. Guide for the care and use of laboratory
final manuscript. Shafiq Ali Shah carried out animals. Institute of Laboratory Animal Resources,
literature search, experimental work and data Commission on Life Sciences. National Academy of
analysis. Anwar-ul-Hassan Gilani and Munasib Sciences: Washington, DC; 1996.31-36.
Khan supported in data analysis and review of 13. Khan M, Khan AU, Rehman NU, Gilani AH. Blood
the manuscript. All authors reviewed and pressure lowering, vasodilator and cardiac-modulatory
approved the final manuscript for publication. potential of Carum roxburghianum seed extract. Clin
Exp Hypertens 2015; 37(2): 102-107.
REFERENCES 14. Gilani AH, Rehman NU, Khan A, Alkharfy KM. Studies on
Bronchodilator Activity of Salvia officinalis (Sage):
1. Zeldin DC. Epoxygenase pathways of arachidonic acid Possible Involvement of K+ Channel Activation and
metabolism. J Biol Chem 2001; 276(39): 36059-36062. Phosphodiesterase Inhibition. Phytother Res 2015;
2. Bukhari IA, Gauthier KM, Jagadeesh SG, Sangras B, 29(9): 1323-1329.
Falck J, Campbell WB. 14, 15-Dihydroxy-eicosa-5 (Z)- 15. Gilani AH, Khan AU, Ghayur MN, Ali SF, Herzig JW.
enoic acid selectively inhibits 14, 15-epoxyeicosatrienoic Antispasmodic Effects of Rooibos Tea (Aspalathus
acid-induced relaxations in bovine coronary arteries. J linearis) is Mediated Predominantly through K+‐Channel
Pharmacol Exp Ther 2011; 336(1): 47-55. Activation. Basic Clin Pharmacol Toxicol 2006; 99(5):
3. Morisseau C, Hammock BD. Impact of soluble epoxide 365-373.
hydrolase and epoxyeicosanoids on human health. 16. Franck H, Puschmann A, Schusdziarra V, Allescher HD.
Annual review of pharmacology and toxicology 2013; Functional evidence for a glibenclamide-sensitive K+
53: 37-58. channel in rat ileal smooth muscle. Eur J Pharmacol
4. Imig JD, Hammock BD. Soluble epoxide hydrolase as a 1994; 271(2-3): 379-386.
therapeutic target for cardiovascular diseases. Nat Rev 17. Cook NS. The pharmacology of potassium channels and
Drug Discov 2009; 8(10): 794-805. their therapeutic potential. Trends Pharmacol Sci 1988;
5. Newman JW, Morisseau C, Hammock BD. Epoxide 9(1): 21-28.
hydrolases: their roles and interactions with lipid 18. Guo Y, Luo F, Zhang X, Chen J, Shen L, Zhu Y, Xu D.
metabolism. Prog Lipid Res 2005; 44(1): 1-51. TPPU enhanced exercise-induced epoxyeicosatrienoic
6. Ingraham R, Gless R, Lo H. Soluble epoxide hydrolase acid concentrations to exert cardioprotection in mice
inhibitors and their potential for treatment of multiple after myocardial infarction. J Cell Mol Med 2018; 22(3):
1489-1500.
Trop J Pharm Res, June 2018; 17(6): 1023
Shah et al
19. Khan AU, Gilani AH. Selective bronchodilatory effect of 23. Imran I, Hussain L, Zia-Ul-Haq M, Janbaz KH, Gilani
Rooibos tea (Aspalathus linearis) and its flavonoid, AH, De Feo V. Gastrointestinal and respiratory activities
chrysoeriol. European J Nutrition 2006; 45(8): 463-473. of Acacia leucophloea. J Ethnopharmacol 2011; 138(3):
20. Khan A, AlKharfy KM, Gilani AH. Antidiarrheal and 676-682.
antispasmodic activities of Salvia officinalis are 24. Li H, Kim HW, Shin SE, Seo MS, An JR, Ha KS, Han ET,
mediated through activation of K+ channels. Bangladesh Hong SH, Firth AL, Choi IW et al. The vasorelaxant
J Pharmacol 2011; 6(2): 111-116. effect of mitiglinide via activation of voltage-dependent K
21. Hamilton T, Weir SW, Weston A. Comparison of the (+) channels and SERCA pump in aortic smooth muscle.
effects of BRL 34915 and verapamil on electrical and Life sciences 2017; 188: 1-9.
mechanical activity in rat portal vein. Br J Pharmacol 25. Lenz T, Wagner G. Potential role of potassium channel
1986; 88(1): 103-111. openers for the treatment of cardiovascular disease.
22. Okabe K, Kitamura K, Kuriyama H. Features of 4- Hypertension: Pathophysiology, diagnosis and
aminopyridine sensitive outward current observed in management. Laragh JH, Brenner BM (eds). New York,
single smooth muscle cells from the rabbit pulmonary Raven Press 1995: 2953-2968.
artery. Pflugers Arch 1987; 409(6): 561-568.
Trop J Pharm Res, June 2018; 17(6): 1024
View publication stats
You might also like
- CDM Smith Study - Eastern Pender Water System ImprovementsDocument138 pagesCDM Smith Study - Eastern Pender Water System ImprovementsMark DarroughNo ratings yet
- Article Wjpps 1427890881Document14 pagesArticle Wjpps 1427890881Pramod bhalseNo ratings yet
- Herbal Extract Loaded Chitosan-Based Nanofibers As A Potential Wound-DressingDocument11 pagesHerbal Extract Loaded Chitosan-Based Nanofibers As A Potential Wound-DressingSani IsmailNo ratings yet
- Published ArticleDocument15 pagesPublished ArticleOktavia Rahayu ANo ratings yet
- Kusirisin MC1Document10 pagesKusirisin MC1Maryem SafdarNo ratings yet
- Manfaat XanthorizolDocument16 pagesManfaat XanthorizolFauzan aprionoNo ratings yet
- Antiplatelet Activityand Quantificationof Polyphenols Contentof MethanolDocument10 pagesAntiplatelet Activityand Quantificationof Polyphenols Contentof MethanolSilviyanitaNo ratings yet
- مقاله مورفینDocument7 pagesمقاله مورفینAngham OuadahNo ratings yet
- NPR HS B.glabraDocument7 pagesNPR HS B.glabraKhy Tolero AmigableNo ratings yet
- Pharmaco-Analytical Study and Phytochemical Profile of Hydroethanolic Extract of Algerian Prickly Pear (Opuntia Ficus-Indica.l)Document12 pagesPharmaco-Analytical Study and Phytochemical Profile of Hydroethanolic Extract of Algerian Prickly Pear (Opuntia Ficus-Indica.l)lotfi9No ratings yet
- Wound Healing and Cytotoxicity Effects of Hilleria Latifolia and Laportea OvalifoliaDocument13 pagesWound Healing and Cytotoxicity Effects of Hilleria Latifolia and Laportea OvalifoliaFatamorgana AbdullahNo ratings yet
- Design and Charaterzation of Ethosome Drug Delivery System Containing Isradipine For Topical ApplicationDocument12 pagesDesign and Charaterzation of Ethosome Drug Delivery System Containing Isradipine For Topical ApplicationBaru Chandrasekhar Rao100% (1)
- Vernonia Cinerea Leaves As The Source of Phenolic Compounds, Antioxidants, and Anti-Diabetic Activity Using Microwave-Assisted Extraction TechniqueDocument13 pagesVernonia Cinerea Leaves As The Source of Phenolic Compounds, Antioxidants, and Anti-Diabetic Activity Using Microwave-Assisted Extraction TechniqueFelicia JesslynNo ratings yet
- 2020 Article 1654Document13 pages2020 Article 1654alyaNo ratings yet
- FinalpaperDocument16 pagesFinalpaperyahyaNo ratings yet
- B - G07 - English JournalDocument7 pagesB - G07 - English JournalMariani SiagianNo ratings yet
- Arctium 20 Lappa 20 FinalDocument27 pagesArctium 20 Lappa 20 Finalthiên vũ hoàngNo ratings yet
- Antioxidant Activity of Medicinal Plants ThesisDocument8 pagesAntioxidant Activity of Medicinal Plants Thesiskimcookatlanta100% (2)
- Huang Et Al-2018-Journal of Separation Science PDFDocument34 pagesHuang Et Al-2018-Journal of Separation Science PDFGeanina SorocanNo ratings yet
- Huang Et Al-2018-Journal of Separation Science PDFDocument34 pagesHuang Et Al-2018-Journal of Separation Science PDFGeanina SorocanNo ratings yet
- 26 Antiproliferative Activity of Trigona Propolis On Human Cancer CellDocument9 pages26 Antiproliferative Activity of Trigona Propolis On Human Cancer CellZulkifli ZambriNo ratings yet
- AIJPMS - Volume 3 - Issue 1 - Pages 15-31Document17 pagesAIJPMS - Volume 3 - Issue 1 - Pages 15-31Eman MahmoudNo ratings yet
- Kim 44 5 11 2003 6Document14 pagesKim 44 5 11 2003 6Simón OsésNo ratings yet
- Abortifacient - and - Antioxidant - Activities - of - A MarinaDocument16 pagesAbortifacient - and - Antioxidant - Activities - of - A MarinaDanang RaharjoNo ratings yet
- Avicequinone C Isolated From Avicennia Marina ExhiDocument14 pagesAvicequinone C Isolated From Avicennia Marina Exhidogne123456789No ratings yet
- Amylase and Dipeptidyl Peptidase 4 DPP 4 Inhibitory Effects of Melicope Latifolia Bark Extracts and Identification of Bioactive Constituents Using inDocument11 pagesAmylase and Dipeptidyl Peptidase 4 DPP 4 Inhibitory Effects of Melicope Latifolia Bark Extracts and Identification of Bioactive Constituents Using inRohaniNo ratings yet
- Effects of Extraction Solvent System, Time and Temperature On Total PhenolicDocument8 pagesEffects of Extraction Solvent System, Time and Temperature On Total PhenolicTuấn Nguyen AnhNo ratings yet
- Comparative Conventional Extraction Methods of Ethanolic Extracts of Clinacanthus Nutans Leaves On Antioxidant Activity and ToxicityDocument12 pagesComparative Conventional Extraction Methods of Ethanolic Extracts of Clinacanthus Nutans Leaves On Antioxidant Activity and ToxicityRasyidu MashuriNo ratings yet
- A Comprehensive Working, Principles and Applications of Thin Layer ChromatographyDocument19 pagesA Comprehensive Working, Principles and Applications of Thin Layer ChromatographyRizalNo ratings yet
- 255 937 1 PBDocument11 pages255 937 1 PBAnh NguyễnNo ratings yet
- AntidiabeticDocument12 pagesAntidiabeticSamantha Vhiel VicenteNo ratings yet
- In Vitro Antioxidant Activities of Chloroform Extract of Ctenolepsis CerasiformisDocument4 pagesIn Vitro Antioxidant Activities of Chloroform Extract of Ctenolepsis CerasiformisiajpsNo ratings yet
- Cistanche Tubulosa Laminaria JaponicaDocument8 pagesCistanche Tubulosa Laminaria JaponicaJaTi NurwigatiNo ratings yet
- Application of Atractylodes Macrocephala Koidz Extract in M - 2017 - Procedia enDocument6 pagesApplication of Atractylodes Macrocephala Koidz Extract in M - 2017 - Procedia enstavrosNo ratings yet
- 2019 18 12 15 PDFDocument7 pages2019 18 12 15 PDFRachel Lalaine Marie SialanaNo ratings yet
- Zahid, 2450-Naila Masood Galley ProofDocument12 pagesZahid, 2450-Naila Masood Galley ProofNaila MasoodNo ratings yet
- British J Pharmacology - 2015 - Pastore - Transdermal Patches History Development and PharmacologyDocument31 pagesBritish J Pharmacology - 2015 - Pastore - Transdermal Patches History Development and PharmacologyEdwin VillavicencioNo ratings yet
- Black Corn KetanDocument9 pagesBlack Corn KetanJericho J JardenNo ratings yet
- Inhibition of Hormone Sensitive Lipase and PancreaDocument9 pagesInhibition of Hormone Sensitive Lipase and PancreaAbril HopeNo ratings yet
- Effect of Fagonia Arabica (Dhamasa) On in Vitro ThrombolysisDocument7 pagesEffect of Fagonia Arabica (Dhamasa) On in Vitro ThrombolysisnidaNo ratings yet
- Article54 Antimalarial Ngemenya FinDocument8 pagesArticle54 Antimalarial Ngemenya FinNjeodoNo ratings yet
- Antioxidant Activities of Phenolic Components FromDocument10 pagesAntioxidant Activities of Phenolic Components FromValerie LevaNo ratings yet
- Antioxidant, Antidiabetic, Antihyperlipidemic, Reproduction Stimulatory Properties and Safety of Essential Oil of Satureja Khuzestanica in Rat in Vivo: A Toxicopharmacological StudyDocument6 pagesAntioxidant, Antidiabetic, Antihyperlipidemic, Reproduction Stimulatory Properties and Safety of Essential Oil of Satureja Khuzestanica in Rat in Vivo: A Toxicopharmacological StudyArini Dwi NastitiNo ratings yet
- Vet - Res. 0928-4249 2004 35 1 ART0007Document11 pagesVet - Res. 0928-4249 2004 35 1 ART0007Rocio BautistaNo ratings yet
- 33-oleanolic-TLCJ Med PlantsDocument6 pages33-oleanolic-TLCJ Med PlantsShar KhanNo ratings yet
- Effect of Astaxanthin On Cutaneous Wound Healing: Clinical, Cosmetic and Investigational Dermatology DoveDocument7 pagesEffect of Astaxanthin On Cutaneous Wound Healing: Clinical, Cosmetic and Investigational Dermatology DoveRaziel Alvarez RebolloNo ratings yet
- Ejpbs E Ganistrus in VivoDocument9 pagesEjpbs E Ganistrus in VivoRahayu UtamiNo ratings yet
- The Investigation of Clone and Expression of ButyrylcholinesteraseDocument8 pagesThe Investigation of Clone and Expression of ButyrylcholinesteraseMonaNo ratings yet
- Antioxidant Effects of Astaxanthin in Various Diseases-A ReviewDocument16 pagesAntioxidant Effects of Astaxanthin in Various Diseases-A ReviewHelenaNo ratings yet
- Heli Yon PaperDocument16 pagesHeli Yon Paperbmounika 206No ratings yet
- Safety, Analgesic, and Anti-Inflammatory Effects of Aqueous and Methanolic Leaf Extracts of Hypericum Revolutum Subsp. KenienseDocument11 pagesSafety, Analgesic, and Anti-Inflammatory Effects of Aqueous and Methanolic Leaf Extracts of Hypericum Revolutum Subsp. KenienseInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Study On In-Vitro Thrombolytic Activity of Methanolic Extract of Mesua Ferrea LeavesDocument4 pagesStudy On In-Vitro Thrombolytic Activity of Methanolic Extract of Mesua Ferrea LeavesASIF AL MAHMOODNo ratings yet
- DR Dicky - Antiinflamatory Effect of ABM in High Fat Diet Induced MiceDocument7 pagesDR Dicky - Antiinflamatory Effect of ABM in High Fat Diet Induced MiceFuma AjiNo ratings yet
- 2014 Revchim MwparaspDocument4 pages2014 Revchim Mwparasp199 DIHTA MARWATINo ratings yet
- New Aspects of Therapy of Hepatocellular Carcinoma PDFDocument16 pagesNew Aspects of Therapy of Hepatocellular Carcinoma PDFKrrliveNo ratings yet
- Banana Pheolic ContentDocument11 pagesBanana Pheolic ContentLakshmipriya GopinathNo ratings yet
- Phytochemical Investigation, Antiulcer, Cyclooxygenase-2, and 15-Lipoxygenase Inhibitory Activities of Echinops Erinaceus Kit TanDocument22 pagesPhytochemical Investigation, Antiulcer, Cyclooxygenase-2, and 15-Lipoxygenase Inhibitory Activities of Echinops Erinaceus Kit Tanshrooq sweilamNo ratings yet
- Nutritional and Healthical Aspects of Yacon (Smallanthus Sonchifolius) For Human, Animals and PoultryDocument11 pagesNutritional and Healthical Aspects of Yacon (Smallanthus Sonchifolius) For Human, Animals and PoultryWiro JuangNo ratings yet
- Publications 24 31Document8 pagesPublications 24 31biodoc biodocNo ratings yet
- Antioxidant and Anti Inflammatory in VitDocument8 pagesAntioxidant and Anti Inflammatory in VitDamaris Amabel Mendoza MartínezNo ratings yet
- The HDL Handbook: Biological Functions and Clinical ImplicationsFrom EverandThe HDL Handbook: Biological Functions and Clinical ImplicationsNo ratings yet
- Regulations and Rules For MRCPCH and DCH ExaminationsDocument18 pagesRegulations and Rules For MRCPCH and DCH ExaminationsSyed Danish AliNo ratings yet
- DHL Express Usa Overview - enDocument14 pagesDHL Express Usa Overview - enSyed Danish AliNo ratings yet
- SICOT SyllabusDocument33 pagesSICOT SyllabusSyed Danish AliNo ratings yet
- HBL Discounts List - May 2022Document1 pageHBL Discounts List - May 2022Syed Danish AliNo ratings yet
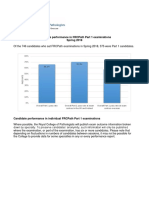
- Examination Performance Report Spring 2018 Part 1Document3 pagesExamination Performance Report Spring 2018 Part 1Syed Danish AliNo ratings yet
- September 2018: Frcpath Questions Hemato-OncologyDocument24 pagesSeptember 2018: Frcpath Questions Hemato-OncologySyed Danish Ali100% (1)
- Part 1 Examination: Candidates Must Answer All Questions. Each Question Is Worth A Total of 25 MarksDocument15 pagesPart 1 Examination: Candidates Must Answer All Questions. Each Question Is Worth A Total of 25 MarksSyed Danish AliNo ratings yet
- A) Normal Referral Letter:: Most Likely, Grave's DiseaseDocument8 pagesA) Normal Referral Letter:: Most Likely, Grave's DiseaseSyed Danish AliNo ratings yet
- Transfer Letter Oet Writing Task PracticeDocument3 pagesTransfer Letter Oet Writing Task PracticeSyed Danish Ali100% (1)
- Online Course in Science Journalism: Lesson 2 - Finding and Judging Science StoriesDocument22 pagesOnline Course in Science Journalism: Lesson 2 - Finding and Judging Science StoriesleksremeshNo ratings yet
- Peds - Research ArticleDocument2 pagesPeds - Research Articleapi-662219485No ratings yet
- Ficha Tecnica E803Document2 pagesFicha Tecnica E803Zenen AlarconNo ratings yet
- Loyola Public School, Loyola Nagar, Guntur - 522005 Promotion From Class VIII To IXDocument1 pageLoyola Public School, Loyola Nagar, Guntur - 522005 Promotion From Class VIII To IXDevi KalyanNo ratings yet
- USAA Tax Exempt Intermediate-Term Fund 2Q '22Document2 pagesUSAA Tax Exempt Intermediate-Term Fund 2Q '22ag rNo ratings yet
- The Role of The Nurse in Health Promotion PDFDocument1 pageThe Role of The Nurse in Health Promotion PDFCie Ladd0% (1)
- Rule: Pesticide Tolerance: ProthioconazoleDocument6 pagesRule: Pesticide Tolerance: ProthioconazoleJustia.comNo ratings yet
- Brockton Police Log March 18Document20 pagesBrockton Police Log March 18BBNo ratings yet
- Temporomandibular Disorders: A Term Whose Time Has Passed!: PerspectivesDocument2 pagesTemporomandibular Disorders: A Term Whose Time Has Passed!: PerspectivesUmer HussainNo ratings yet
- Forthcoming Grand Chamber Case Vavricka and Others v. Czech RepublicDocument3 pagesForthcoming Grand Chamber Case Vavricka and Others v. Czech RepublicZiarul de GardăNo ratings yet
- Abnormal PuerperiumDocument23 pagesAbnormal PuerperiumLynee OlvianaNo ratings yet
- NMC Hospital Abu Dhabi - 2011!06!08Document16 pagesNMC Hospital Abu Dhabi - 2011!06!08Manjul TakleNo ratings yet
- 1 SMDocument14 pages1 SMRosalina Pertiwi GultomNo ratings yet
- Jerusalem SyndromeDocument5 pagesJerusalem Syndromele_papillon15No ratings yet
- Articles-Personality Process and Levels of DevelopmentDocument16 pagesArticles-Personality Process and Levels of DevelopmentbenhazanNo ratings yet
- An EUA For Casirivimab and ImdevimabDocument3 pagesAn EUA For Casirivimab and ImdevimabAllan FradiqueNo ratings yet
- 1.neuro Intro-1 UMTDocument51 pages1.neuro Intro-1 UMTLOVELY WALEEDNo ratings yet
- WWW - England.nhs - Uk: Business Continuity Management NHS WorkshopDocument52 pagesWWW - England.nhs - Uk: Business Continuity Management NHS WorkshopForbetNo ratings yet
- Letter To ParentsDocument4 pagesLetter To ParentsGary DetmanNo ratings yet
- Complementary and Alternative Health Care Client Bill of RightsDocument2 pagesComplementary and Alternative Health Care Client Bill of Rightsapi-31121402No ratings yet
- Important Numbers For SMLEDocument3 pagesImportant Numbers For SMLEAkpevwe EmefeNo ratings yet
- Assign Chem in EverydayLife 16Document1 pageAssign Chem in EverydayLife 16Shubham AsthanaNo ratings yet
- 21 CFR Part 11 RegulationsDocument25 pages21 CFR Part 11 RegulationsVinita SharmaNo ratings yet
- Turkish Exhibitors: 8-11 MARCH 2013Document9 pagesTurkish Exhibitors: 8-11 MARCH 2013qday1No ratings yet
- Aseptic TechniqueDocument34 pagesAseptic TechniqueJessa Adenig100% (1)
- Acute Neurotherapeutics and Neuromonitoring FellowshipDocument7 pagesAcute Neurotherapeutics and Neuromonitoring FellowshippuspoNo ratings yet
- MSDS To 1020 60 SNXDocument8 pagesMSDS To 1020 60 SNXdeiva balanNo ratings yet
- Enteral Nutrition (Gastrojejunotomy)Document30 pagesEnteral Nutrition (Gastrojejunotomy)Hairul IzlanNo ratings yet
- The Impact of Workplace Wellness Programs On Employee Health and ProductivityDocument43 pagesThe Impact of Workplace Wellness Programs On Employee Health and ProductivityVishal VishwakarmaNo ratings yet