Professional Documents
Culture Documents
Timeline MDR
Timeline MDR
Uploaded by
gdfOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Timeline MDR
Timeline MDR
Uploaded by
gdfCopyright:
Available Formats
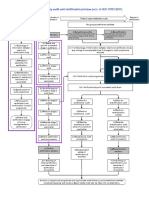
Timeline MDR certification
Step 7:
Step 4:
Conformity
SIQ performs the
assessment
technical
Step 2: Step 6: procedure
documentation Step 5:
SIQ performs the SIQ performs a (Annex X, Annex
and clinical Organization
pre-check of post-audit and XI part B, Annex
evaluation submits
technical issues an audit XI part A, Annex
Step 3: assessment and Corrective action Step 8:
documentation report - IX,…)
Organization issues an audit report Notified body
Step 1: report
submits Commission
Organization
amended takes place, the
submits the
technical Depending on chosen decision on
technical 1-2 weeks 12-14 weeks up to 6 months 10-12 weeks
documentation* assessment route granting
documentation
certificate is
* in case of e.g. accepted;
missing Step 5 and 6 may be repeated in case EU certificate is
Annex IX, Annex XI part A –
documentation nonconformities are not resolved in Quality management granted
at pre-check first post-audit (additional timing) system – certification audit
stage 1, stage 2 (audit +
report – 8 weeks/stage) +
possible post audit (8
weeks/audit)
You might also like
- Business Plan of Budget Airline CompanyDocument43 pagesBusiness Plan of Budget Airline Companynafees3981% (27)
- Internal Audit IMS ChecklistDocument11 pagesInternal Audit IMS ChecklistBobby Lawrence100% (3)
- Action Plan For ISO:TS 22163 PDFDocument1 pageAction Plan For ISO:TS 22163 PDFKarthi Thiyagarajan100% (2)
- CCTV Pipe Survey Inspection Test PlanDocument1 pageCCTV Pipe Survey Inspection Test Planmohandes06No ratings yet
- AC7140 Rev CDocument73 pagesAC7140 Rev CRanga100% (1)
- FSSC 22000 Certification ProcessDocument6 pagesFSSC 22000 Certification Processcollege food&beverageNo ratings yet
- Audit and Certification Process - 00 - PreparationDocument4 pagesAudit and Certification Process - 00 - PreparationHamza HamNo ratings yet
- WP04 L - D01e 1 Certification Procedure ISO21001Document9 pagesWP04 L - D01e 1 Certification Procedure ISO21001Abdul Ghaffar Iso WalaNo ratings yet
- Iso 14001 Certification ProcessDocument5 pagesIso 14001 Certification Processepoube marcelNo ratings yet
- NC Report Hermosillo 2Document10 pagesNC Report Hermosillo 2AdanCeballosNo ratings yet
- Procedure التصرف بالأجهزةDocument5 pagesProcedure التصرف بالأجهزةMed Hédi BANNANINo ratings yet
- Quality System Audit Report TemplateDocument5 pagesQuality System Audit Report TemplateIshara VithanaNo ratings yet
- Completing The Audit: Chapter ObjectivesDocument9 pagesCompleting The Audit: Chapter ObjectivesEmmaNo ratings yet
- ISO Auditing-2Document25 pagesISO Auditing-2suniljayaNo ratings yet
- ANSI Audit FlowDocument1 pageANSI Audit FlowhtdvulNo ratings yet
- Audit Report-4Document4 pagesAudit Report-4Ranjeet BhureNo ratings yet
- Audit Process Flowchart MC PDFDocument1 pageAudit Process Flowchart MC PDFDemi BangayanNo ratings yet
- PlanningDocument29 pagesPlanningKLONE DONNo ratings yet
- RA7920 Visitation Process MapDocument1 pageRA7920 Visitation Process MapjanmczealNo ratings yet
- Skema Audit InternalDocument4 pagesSkema Audit InternaligoeneezmNo ratings yet
- PBU PPVC MAS Flowchart New Application RenewalDocument1 pagePBU PPVC MAS Flowchart New Application RenewalNicky LimNo ratings yet
- Iso 13485 Certification ProcessDocument10 pagesIso 13485 Certification ProcessJohn ThompsonNo ratings yet
- Audit Report - Surveillance - Hayat IndustriesDocument9 pagesAudit Report - Surveillance - Hayat Industriessajid waqasNo ratings yet
- Sa 230Document12 pagesSa 230meghanaNo ratings yet
- Audit Findings Report JanuaryDocument2 pagesAudit Findings Report JanuaryBrian KamskyNo ratings yet
- 1.08.accreditation & Certification ProcessDocument14 pages1.08.accreditation & Certification ProcessSrishti TripathyNo ratings yet
- OPMAN-Part-2 - Joel Factoriza Misa - ID 21226220 - ETEEAP BSBADocument20 pagesOPMAN-Part-2 - Joel Factoriza Misa - ID 21226220 - ETEEAP BSBAOnice BallelosNo ratings yet
- INS-4 2-01 Instruction For Management Systems Certification Bodies - InglésDocument11 pagesINS-4 2-01 Instruction For Management Systems Certification Bodies - InglésDiego TobrNo ratings yet
- Process Validation and Revalidation in Medical Device ProductionDocument7 pagesProcess Validation and Revalidation in Medical Device ProductionBREWSKINo ratings yet
- Audit ManagementDocument21 pagesAudit ManagementeldhoisaacNo ratings yet
- ISO 20000 Implementation Diagram - ENDocument1 pageISO 20000 Implementation Diagram - ENNeymar Moura CarvalhoNo ratings yet
- Internal Audit MapDocument1 pageInternal Audit Maptauqeer25No ratings yet
- PED20Document5 pagesPED20Kubilay Fatih ŞimşekNo ratings yet
- 1.QP01 QCDocument6 pages1.QP01 QCpankaj bhargaveNo ratings yet
- Mileston e Description of Milestone Target Time Date Range: TH STDocument1 pageMileston e Description of Milestone Target Time Date Range: TH STNikeshNo ratings yet
- CB Communique 2016-009 - IATF ADP Entry PDFDocument5 pagesCB Communique 2016-009 - IATF ADP Entry PDFSelvaraj SNo ratings yet
- MaPl 6000236 RevDocument10 pagesMaPl 6000236 RevAditya Sandi nugrahaNo ratings yet
- SYS Procedure - Internal Quality Audit P1Document1 pageSYS Procedure - Internal Quality Audit P1sumanNo ratings yet
- R20.55IATF IATF 16949 Documentation ReviewDocument7 pagesR20.55IATF IATF 16949 Documentation ReviewPrakash kumarTripathiNo ratings yet
- Action Plan For ISO:TS 22163Document1 pageAction Plan For ISO:TS 22163Karthi ThiyagarajanNo ratings yet
- Guiding Principles For Plant Quality - 20140708Document4 pagesGuiding Principles For Plant Quality - 20140708didik dadtNo ratings yet
- 10 Steps To Asset CareDocument13 pages10 Steps To Asset CareDamianNo ratings yet
- Checklist For Evaluation of Audit DocumentsDocument7 pagesChecklist For Evaluation of Audit DocumentsTrương CườngNo ratings yet
- Audit Guide Full DayDocument17 pagesAudit Guide Full DayDan DumbravescuNo ratings yet
- Quality Execution Plan CREH ApprovedDocument19 pagesQuality Execution Plan CREH Approvedkarthikeyan.dNo ratings yet
- 1.09.Auccreditation & Certification ProcessDocument14 pages1.09.Auccreditation & Certification Processcaxoro1799No ratings yet
- ISO 9001 2015 Comprehensive Audit Checklist NlngogDocument13 pagesISO 9001 2015 Comprehensive Audit Checklist NlngogAlmerindo DOS SANTOS0% (1)
- Audit Cycle Stage 2Document4 pagesAudit Cycle Stage 2Mohamed FouadNo ratings yet
- 01 Internal Auditing Technique Rev. 05 12 09 2018Document40 pages01 Internal Auditing Technique Rev. 05 12 09 2018Syed Maroof AliNo ratings yet
- ISO 9001 Process Procedure QPP-092-1 Internal AuditDocument4 pagesISO 9001 Process Procedure QPP-092-1 Internal Auditmahm.tahaNo ratings yet
- F050-2 AUDIT REPORT S1152.S2.9K - FinalDocument13 pagesF050-2 AUDIT REPORT S1152.S2.9K - FinalMuhammad IrfanNo ratings yet
- 282 - Knight Frank - IMS ProposalDocument6 pages282 - Knight Frank - IMS ProposalManimegalai RajendiranNo ratings yet
- Day 3 TC on ISO 19011 2018Document31 pagesDay 3 TC on ISO 19011 2018Twinkle MiguelNo ratings yet
- Reminders For QMS Documentation of DBTC MatiDocument7 pagesReminders For QMS Documentation of DBTC MatiCamelle Kate BarbasNo ratings yet
- Stage 1 Audit Report and SummaryDocument39 pagesStage 1 Audit Report and Summaryabie mumuNo ratings yet
- Structural Integrity Management: Ageing Structures Workshop April 8thDocument10 pagesStructural Integrity Management: Ageing Structures Workshop April 8thonnly1964No ratings yet
- Section 9.2 - Internal AuditDocument2 pagesSection 9.2 - Internal Auditturkih_1988No ratings yet
- UKAS RG6 Edition 2 Mar 06Document10 pagesUKAS RG6 Edition 2 Mar 06moheb botrosNo ratings yet
- The CePIETSO PCP Certification Process PDocument2 pagesThe CePIETSO PCP Certification Process PAhmad AizatNo ratings yet
- Establishing A CGMP Laboratory Audit System: A Practical GuideFrom EverandEstablishing A CGMP Laboratory Audit System: A Practical GuideNo ratings yet
- The Sarbanes-Oxley Section 404 Implementation Toolkit: Practice Aids for Managers and AuditorsFrom EverandThe Sarbanes-Oxley Section 404 Implementation Toolkit: Practice Aids for Managers and AuditorsNo ratings yet
- Julius Caesar Persuasive EssayDocument8 pagesJulius Caesar Persuasive Essayezm8kqbt100% (2)
- Afisco Insurance Corp v. CADocument3 pagesAfisco Insurance Corp v. CAJulianNo ratings yet
- Syndicate 2 - MarvelDocument9 pagesSyndicate 2 - Marveldeeto_88No ratings yet
- Tata Consultancy Services Limited - Chapter One (Profile of The Company)Document12 pagesTata Consultancy Services Limited - Chapter One (Profile of The Company)Prajaktha PrakashNo ratings yet
- Emplifi Report State of Influencer Marketing 2023 ENDocument27 pagesEmplifi Report State of Influencer Marketing 2023 ENthaongo7126No ratings yet
- Practice Test 4: SECTION 1 Questions 1-12Document5 pagesPractice Test 4: SECTION 1 Questions 1-12Nhật Hường TrầnNo ratings yet
- MeetingsDocument1 pageMeetingsCloieRjNo ratings yet
- Checklist EU SDSDocument3 pagesChecklist EU SDSZulfadli RaniNo ratings yet
- Spiral Wound Gasket: Bharat Heavy Electricals Limited Tiruchirappalli-620 014Document54 pagesSpiral Wound Gasket: Bharat Heavy Electricals Limited Tiruchirappalli-620 014Ramalingam PrabhakaranNo ratings yet
- Offshore Outsourcing - The Complete Guide (Forrester)Document112 pagesOffshore Outsourcing - The Complete Guide (Forrester)2styleNo ratings yet
- Creating A Prototype - Software: Professor Kartik HosanagarDocument8 pagesCreating A Prototype - Software: Professor Kartik HosanagarRidhya MahajanNo ratings yet
- Financial Accounting Terms Final 29Document30 pagesFinancial Accounting Terms Final 29Mehdi MemesNo ratings yet
- MKTG 1 Complete ModuleDocument64 pagesMKTG 1 Complete ModuleAngelo Paolo AcostaNo ratings yet
- Ciia 52472 PDFDocument18 pagesCiia 52472 PDFPritz Marc Bautista MorataNo ratings yet
- Chapter 1Document7 pagesChapter 1Rhosvic VargasNo ratings yet
- IOIPG-Enterprise Risk Management Framework PDFDocument56 pagesIOIPG-Enterprise Risk Management Framework PDFpejaaNo ratings yet
- Integrated Communication Campaign Plan Regarding Covid-19 AwarenessDocument11 pagesIntegrated Communication Campaign Plan Regarding Covid-19 AwarenessAbid hasanNo ratings yet
- An Introduction To Cost Terms and Purposes Problems 1 11Document3 pagesAn Introduction To Cost Terms and Purposes Problems 1 11b894qr949tNo ratings yet
- Equal Risk Contribution Portfolios: Erik Forseth, Ed TrickerDocument6 pagesEqual Risk Contribution Portfolios: Erik Forseth, Ed TrickerGeorge ChenNo ratings yet
- Excitel - Procare A DecDocument2 pagesExcitel - Procare A Decmdumar0164No ratings yet
- Acknowledgement Receipt: Philippine Institute of Civil Engineers - Csu ChapterDocument4 pagesAcknowledgement Receipt: Philippine Institute of Civil Engineers - Csu ChapterLarry XNo ratings yet
- NP Chart, P Chart, C Chart, U Chart .Attribute DataDocument17 pagesNP Chart, P Chart, C Chart, U Chart .Attribute DataSakshi SinghNo ratings yet
- AFST Practice Set 02 Partnership (Part 2)Document3 pagesAFST Practice Set 02 Partnership (Part 2)Alain CopperNo ratings yet
- Rent A Bike For Life - UPLB CHE SCDocument5 pagesRent A Bike For Life - UPLB CHE SCAura Carla TolentinoNo ratings yet
- BBA Course in Nepal Your Ultimate Career GuideDocument8 pagesBBA Course in Nepal Your Ultimate Career Guidequeens.kpisNo ratings yet
- CHAPTER 1 Caselette - Accounting CycleDocument51 pagesCHAPTER 1 Caselette - Accounting CycleKaren MagsayoNo ratings yet
- Cse332 Industry Ethics and Legal IssuesDocument2 pagesCse332 Industry Ethics and Legal IssuesJenish ButaniNo ratings yet
- AbbreviationDocument4 pagesAbbreviationPost Your FeedbackNo ratings yet
- Sponsorship LetterDocument2 pagesSponsorship LetterJonela LazaroNo ratings yet