Professional Documents
Culture Documents
Prognostic Factors For Survival of Patients With Glioblastoma: Recursive Partitioning Analysis
Uploaded by
Sungjae AnOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Prognostic Factors For Survival of Patients With Glioblastoma: Recursive Partitioning Analysis
Uploaded by
Sungjae AnCopyright:
Available Formats
Neuro-Oncology
Prognostic factors for survival of patients with glioblastoma: Recursive partitioning analysis1
Kathleen R. Lamborn,2 Susan M. Chang, and Michael D. Prados
Department of Neurological Surgery, University of California San Francisco, San Francisco, CA 94143-0112, USA
Survival for patients with glioblastoma multiforme is short, and current treatments provide limited benet. Therefore, there is interest in conducting phase 2 trials of experimental treatments in newly diagnosed patients. However, this requires historical data with which to compare the experimental therapies. Knowledge of prognostic markers would also allow stratication into risk groups for phase 3 randomized trials. In this retrospective study of 832 glioblastoma multiforme patients enrolled into prospective clinical trials at the time of initial diagnosis, we evaluated several potential prognostic markers for survival to establish risk groups. Analyses were done using both Cox proportional hazards modeling and recursive partitioning analyses. Initially, patients from 8 clinical trials, 6 of which included adjuvant chemotherapy, were included. Subsequent analyses excluded trials with interstitial brachytherapy, and nally included
Received November 3, 2003; accepted February 9, 2004. This study was supported by grants CA13525, NS42927, CA82103, CA097257. This paper is presented on behalf of the University of California San Francisco Brain Tumor Research Center (BTRC) and is based on clinical trials that depended on leadership from a number of individuals within the BTRC.
2 1
only nonbrachytherapy trials with planned adjuvant chemotherapy. The initial analysis dened 4 risk groups. The 2 lower risk groups included patients under the age of 40, the lowest risk group being young patients with tumor in the frontal lobe only. An intermediate-risk group included patients with Karnofsky performance status (KPS) 70, subtotal or total resection, and age between 40 and 65. The highest risk group included all patients over 65 and patients between 40 and 65 with either KPS 80 or biopsy only. Subgroup analyses indicated that inclusion of adjuvant chemotherapy provides an increase in survival, although that improvement tends to be minimal for patients over age 65, for patients over age 40 with KPS less than 80, and for those treated with brachytherapy. Neuro-Oncology 6, 227235, 2004 (Posted to Neuro-Oncology [serial online], Doc. 03-062, May 13, 2004. URL http://neuro-oncology.mc.duke.edu; DOI: 10.1215/S1152851703000620)
Address correspondence to Kathleen R. Lamborn, Department of Neurological Surgery, University of California San Francisco, 400 Parnassus Avenue, San Francisco, CA 94143-0372 (lambornk@neurosurg.ucsf.edu). Abbreviations used are as follows: GBM, glioblastoma multiforme; KPS, Karnofsky performance status; RTOG, Radiation Therapy Oncology Group; RPA, recursive partitioning analysis; UCSF, University of California San Francisco.
urvival for patients with glioblastoma multiforme (GBM)3 is short. Because of the poor prognosis with existing treatments, there has been an interest in developing phase 2 protocols for experimental therapies used at the time of initial diagnosis. To do this, one needs to have a thorough understanding of the prognostic factors affecting survival and a mechanism for adjusting for these factors so that historical data can be used in the evaluation of new therapies. An understanding of these factors will help to ensure appropriate selection of treatments for phase 3 trials and will also be useful in selecting stratication variables for randomization and analysis in these phase 3 trials.
Copyright 2004 by the Society for Neuro-Oncology
Lamborn et al.: Recursive partitioning analysis: GBM and survival
Historically, the primary method of identifying prognostic factors has been through the use of the Cox proportional hazards model; however, there are a number of limitations to this approach as it is standardly implemented. First, it requires that the assumptions of the model are at least approximately met. In particular, the proportional hazards model assumes that the impact of the change in one factor on predicted survival is not dependent on the status of another factor. The standard implementation also requires that the information on all variables of interest be complete for a patients data to be included. Finally, although the proportional hazards model provides information on relative risk based on patient characteristics, it does not immediately translate into dened risk groups and, to be most useful as a tool in evaluating the efcacy of new therapies, the actual patient data from the historical database should be available for direct comparison to the data from the patients receiving the experimental treatment. While many of the limitations of the proportional hazards model can be addressed by modications of the standard methodology and additional programming, increased availability of high-speed computing has led to extensive use of an alternative approach to identifying prognostic factors the use of recursive partitioning analysis (RPA; Keles and Segal, 2002; Schmoor et al., 1993). This method makes fewer modeling assumptions and has an established procedure to adapt to missing data through use of surrogate measures. Also, because the method is designed to divide patients into groups based on length of survival, it produces natural strata for future phase 2 comparisons or for stratication in randomization for phase 3 trials. The Radiation Therapy Oncology Group (RTOG) presented results from an RPA based on all patients with high-grade gliomas (astrocytomas with anaplastic features as well as GBM) treated during their trials (Curran et al., 1993). We wished to reproduce these results, but to focus specically on pa-
tients with GBM. Since our interest was in predicting survival outcome for patients who would be likely to enroll in clinical trials, we reviewed our database for patients who were placed on clinical protocols at the time of an initial diagnosis of GBM.
Methods
This study was approved by the University of California San Francisco (UCSF) Committee on Human Research. Eight hundred thirty-two GBM patients (including 1 patient with gliosarcoma) who were enrolled in 1 of 8 clinical protocols were identied. Patients with optic, cerebellar, pineal, or brain stem tumors were not included. In more than 97% of cases, determination that the tumor was a GBM was based on central pathology review at UCSF. Recent studies have used WHO II criteria. For the earlier studies, the UCSF system differed from the standard criteria, in that UCSF pathologists did not require necrosis in order to declare the tumor to be glioblastoma. In that regard, the UCSF designation was effectively equivalent to the current WHO II criteria. Trials and key characteristics are provided in Table 1, including the number of patients and the number censored. These trials represent a combination of single-institution (UCSF) and multi-institution trials. The multi-institution trials were led by UCSF and run through the Northern California Oncology Group, a regional clinical-trials consortium sponsored by the National Cancer Institute. All protocols included provision for external-beam radiotherapy. In addition, some of these studies planned to include a temporary radioactive seed boost and/or adjuvant chemotherapy. Following the concept of intent-totreat, treatment allocation (e.g., brachytherapy or no brachytherapy, chemotherapy or no chemotherapy) was based on the protocol in which the patient was enrolled and not on the treatment that the patient actually re-
Table 1. Characteristics of the clinical trials used in analysis Protocol 6G901 BTRC 9901 6G61* 6G91* 6G902 6G82-2 8822 6G82-1* Number Number of patients censored 223 67 59 70 111 61 141 100 2 21 3 5 6 4 12 6 Radiation dose and schedule 60 Gy at 1.8- to 2.0-Gy fractions vs. 70.4 Gy at 1.6-Gy BID fractions diuoromethylornithine 60 Gy at 2.0-Gy fractions 60 Gy hydroxyurea 60 Gy hydroxyurea misonidazole 59.4 Gy at 1.8-Gy fractions hydroxyurea followed by interstitial boost hyperthermia 60 Gy at 1.8- to 2.0-Gy fractions hydroxyurea interstitial boost 60 Gy at 1.8- to 2.0-Gy fractions HU 60 Gy at 1.8- to 2.0-Gy fractions bromodeoxyuridine Adjuvant chemotherapy none temozolomide thalidomide BCNU vs. PCV procarbazine, vincristine, BCNU, 5FU none PCV 6-thioguanine, BCNU PCV Enrollment period 19911996 2000 2001 1975 1981 1979 1983 1990 1995 1982 1990 1988 1991 1982 1988
Abbreviations: 5FU, 5-uorouracil; BCNU, 1,3-bis(2-chloroethyl)-1-nitrosourea (carmustine); BID, twice daily; HU, hydroxyurea; PCV, procarbazine, CCNU (lomustine), and vincristine. *Multi-institutional study.
228
Neuro-Oncology
J U LY 2 0 0 4
Lamborn et al.: Recursive partitioning analysis: GBM and survival
ceived. Survival was measured from the time of surgical diagnosis. All patients were enrolled prior to externalbeam radiotherapy. Variables selected for consideration were those that were consistently acquired during these trials: specically, age at diagnosis, race (Caucasian vs. other), Karnofsky performance status (KPS), gender, and anatomical site. Anatomical site was dened as frontal, temporal, parietal, or other. If tumor was present that extended beyond a single site, anatomical site was included in the other category. Also included in other were cases where the tumor was in the corpus callosum or thalamus and tumors that were occipital. Treatment variables were extent of resection, whether or not the protocol included brachytherapy, and whether or not it included adjuvant chemotherapy. Extent of resection was scored as 1, 2, or 3, respectively, according to whether the surgery was a biopsy (less than 10% resected), subtotal (10% to 90% resected), or total resection (greater than 90% resected) on the basis of the surgeons intraoperative impression in conjunction with examination of postoperative images. The denition was standard for all protocols included in this report. For patients who underwent surgery at UCSF, the standard was to obtain the postoperative magnetic resonance images within 72 h of surgery. For all patients seen at UCSF, the UCSF neuro-oncology group dened the extent of resection, whether or not the surgery was done at UCSF. Cases classied by the surgeon as gross total in which the postoperative scan was felt to show less than 90% resection were reclassied as subtotal.
meet the criterion of P 0.01 using the log-rank test were combined. Kaplan-Meier graphs are presented for the nal set of prognostic groups.
Results
The baseline characteristics of the patient groups are provided in Table 2. P-values are provided to indicate where patient characteristics may differ between treatment groups. It was anticipated that there would be some differences between the patients enrolled in brachytherapy protocols and patients enrolled in nonbrachytherapy protocols, and this was conrmed. KPS tended to be higher, the extent of resection tended to be greater, and fewer patients had a frontal tumor site in the brachytherapy studies. Among those in nonbrachytherapy trials, patients in chemotherapy trials tended to be younger and to have more extensive resections, and fewer had frontal lobe only tumors than for the protocols with no chemotherapy. In interpreting the results, it must be kept in mind that because the number of patients in the brachytherapy trials was smaller, the P-values would be higher than for the nonbrachytherapy comparisons, even if the difference between chemotherapy and nonchemotherapy studies was the same. While the differences between chemotherapy and nonchemotherapy trials in terms of age and tumor site were statistically signicant for the nonbrachytherapy studies and not signicant for the brachytherapy studies, the pattern of the differences seems to be the same. For example, in both cases, nonchemotherapy trials tended to include proportionately more patients over the age of 65. On the other hand, the patients in the nonchemotherapy trials that included brachytherapy were more likely to have had extensive resections, the opposite from the nonbrachytherapy trials. All variables presented in Table 2 were considered potential predictors of survival. Age at diagnosis was initially considered as a continuous variable and then categorized for the purposes of the RPAs as described below. Predictors of Survival (Overall Analysis) Cox proportional hazards results are presented in Table 3. Only variables statistically signicant at P 0.01 are presented. Younger age at diagnosis, higher KPS, adjuvant chemotherapy, use of brachytherapy, and greater extent of resection all predicted for improved survival. Because of missing data on one or more of the predictors, only 776 patients were included in this analysis. The results of the RPA are presented in Fig. 1. Eight hundred thirty-two patients were included in this analysis. Initially, when we used age at diagnosis as a continuous variable, the patients were split by a diagnosis age of 41.6 and then divided on the basis of the ages of 65.9 and 29.6. Based on these splits, we created an ordered age category that was age 30, age 30 to 40 inclusive,
Statistical Methods
Comparison of patient characteristics among treatment groups was done by using the Wilcoxon test for variables that were either continuous or ordered categorical (e.g., extent of resection). Age was analyzed as a continuous variable. Variables that did not have any inherent ordering were tested by using a general test for association. Initially, we evaluated the potential prognostic factors following the standard method of Cox proportional hazards modeling using backwards stepwise selection. Because of the number of variables considered, we chose to include variables in the nal model only if the results were statistically signicant at P 0.01. Our second analysis approach used RPA as described by Breiman et al. (1984). The program was constrained to have a minimum nal node size of 30 patients. Tenfold cross-validation was used. The minimum-size model within 0.1 standard error of the overall minimum cost tree was selected for review. To allow for censoring we used the method of martingale residuals described by Therneau et al. (1990). Once RPA selected the tree, we conrmed that the log-rank test met our criterion of P 0.01 for each of the splits identied. Any split that did not meet this criterion was deleted. The nal nodes were then compared. Final nodes that did not
Neuro-Oncology
J U LY 2 0 0 4
229
Lamborn et al.: Recursive partitioning analysis: GBM and survival
Table 2. Baseline characteristics of patient groups No brachytherapy Characteristic Age at diagnosis Age 30 30 age 40 age 50 age 60 age Age 65 60 70 80 90 100 Female Male White Other B STR GTR Frontal Parietal Temporal Other 40 50 60 65 Total no. (%) 26 ( 4) 85 (13) 136 (21) 181 (27) 99 (15) 133 (20) 28 (5) 78 (13) 137 (22) 280 (45) 99 (16) 249 (38) 411 (62) 557 (92) 51 (8) 101 (15) 469 (71) 86 (13) 193 147 153 167 (29) (22) (23) (25) Chemo no. (%) 21 (4.8) 59 (13.5) 96 (22.0) 126 (28.8) 65 (14.9) 70 (16.0) 15 (3.8) 51 (12.8) 88 (22.1) 194 (48.6) 51 (12.8) 161 (36.8) 276 (63.2) 393 (93) 29 (6.9) 46 (10.6) 322 (74.4) 65 (15.0) 135 (30.9) 106 (24.3) 98 (22.4) 98 (22.4) No chemo no. (%) 5 (2.2) 26 (11.7) 40 (17.9) 55 (24.7) 34 (15.3) 63 (28.2) 13 27 49 86 48 (5.8) (12.1) (22.0) (38.6) (21.5) Total no. (%) 10 (6) 13 (8) 43 (25) 54 (31) 27 (16) 25 (15) 0 11 21 79 47 Brachytherapy Chemo no. (%) 4 (6.6) 6 (9.8) 15 (24.6) 19 (31.2) 12 (19.7) 5 (8.2) 0 5 (10.6) 8 (17.0) 22 (46.8) 12 (25.5) 25 (41.0) 36 (59.0) 48 (94.1) 3 (5.9) 5 (8.2) 46 (75.4) 10 (16.4) 16 21 12 12 (26.2) (34.4) (19.7) (19.7) No chemo no. (%) 6 (5.4) 7 (6.3) 28 (25.2) 35 (31.5) 15 (13.5) 20 (18.0) 0 6 (5.4) 13 (11.7) 57 (51.4) 35 (31.5) 37 (33.3) 74 (66.7) 78 (88.6) 10 (11.4) 7 (6.3) 64 (57.7 40 (36.0) 21 34 28 28 (18.9) (30.6) (25.2) (25.2) (a) 0.001 (b) 0.43 (c) 0.18 P-value (a, b, c)* (a) 0.35 (b) 0.001 (c) 0.28
KPS
(7) (13) (50) (30)
Gender
88 (39.5) 135 (60.5) 164 (88.1) 22 (11.9) 55 (24.6) 147 (65.9) 21 (9.4) 58 41 55 69 (26.0) (18.4) (24.7) (30.9)
62 (36) 110 (64) 126 (91) 13 (9) 12 (7) 110 (64) 50 (29) 37 55 40 40 (22) (32) (23) (23)
(a) 0.68 (b) 0.53 (c) 0.32 (a) 0.71 (b) 0.04 (c) 0.29 (a, b) 0.001 (c) 0.01
Race
Extent of resection
Tumor site
(a) 0.04 (b) 0.05 (c) 0.53
Abbreviations: B, biopsy only; brachy, brachytherapy; chemo, chemotherapy; GTR, gross total resection; KPS, Karnofsky performance status; STR, subtotal resection. P-values based on Wilcoxon test except for tumor site, race, and gender, which were compared using a general test for association. *(a) Brachy vs. no brachy, (b) chemo vs. no chemo (no brachy), (c) chemo vs. no chemo (brachy).
age 40 to 50, age 50 to 60, age 60 to 65, and age 65, adding extra categories to more evenly balance the number of patients per category. With these categories, both the 40 and 65 age categories were retained. The under 30 category was no longer selected. One reason may have been that there were only 31 patients in this category. Since it was felt that the use of the categories was more logical, and the results were similar, all RPAs presented are based on this age categorization.
Table 3. Multivariate Cox proportional hazards results. All protocols (776 patients) Variable Hazard ratio 95% CI P value Chemotherapy (Y) Extent of resection KPS Age at diagnosis (years) Brachytherapy (Y) 0.60 0.75 0.97 1.03 0.73 0.52 0.71 0.65 0.86 0.96 0.98 1.02 1.04 0.60 0.89 0.001 0.001 0.001 0.001 0.002
Abbreviations: KPS, Karnofsky performance status; Y, yes
As would have been predicted from the proportional hazards model, age at diagnosis, KPS, and extent of surgery were among the factors selected to divide the patient population. The group of youngest patients (age 40) had the best outcome. Interestingly, within this relatively small group, a further split occurred into those who had frontal-only tumors versus all others. Five hundred forty patients were between the ages of 40 and 65. For the patients in this group, KPS of 70 indicated a poorer prognosis, and among those with KPS 70, biopsy-only indicated a poorer prognosis. The outcomes for these 2 groups were similar to those for the group of patients who were 65 years old. Thus 4 groups were ultimately dened, and the Kaplan-Meier curves for these 4 groups are provided in Fig. 2. The median survival and 95% CI were 132 weeks (110 226), 71 weeks (60 97), 63 weeks (58 69), and 37 weeks (32 42) for the low-risk, lowmoderate-risk, moderate-high-risk, and high-risk groups, respectively. The estimated 2-year survival rates were
230
Neuro-Oncology
J U LY 2 0 0 4
Lamborn et al.: Recursive partitioning analysis: GBM and survival
survival
weeks
Fig. 1. Recursive partitioning analysis for all patients (N 832). For terminal nodes ( ), median survival and the 95% condence interval for the median are given. N number of patients. Estimates with an asterisk (*) exclude 40 patients missing KPS and 2 patients missing extent of resection.
Fig. 2. Survival for all patients, by risk group. Fourteen patients in group 1, 12 patients in group 2, and 9 patients in group 3 lived beyond 5 years (260 weeks). Individual survival times (including those for patients who are censored) are indicated by a solid circle (). Fifty-three patients were censored (13 in group 1, 14 in group 2, 21 in group 3, and 5 in group 4). Additional detail is given in Table 4.
65%, 35%, 17%, and 4%, respectively. These results are summarized in Table 4. Impact of Postoperative Therapy It is of note that the nature of the planned postoperative treatment did not appear in the RPA, even though both chemotherapy and brachytherapy were highly statistically signicant in the Cox proportional hazards model where adjustments were made for patient characteristics. We therefore divided the patients on the basis of the protocols to see if we could more fully understand the impact of the planned treatment regimens. Nonbrachytherapy Trials Initially we considered the 660 patients who were on nonbrachytherapy trials. The proportional hazards results are presented in Table 5. Age at diagnosis, chemotherapy, extent of resection, and KPS continued to be highly statistically signicant. The hazard ratio estimates were similar to those for the overall analysis, which is not
surprising given that this group constitutes the majority of the cases. The RPA analysis was also similar (Fig. 3). Comparison of Fig. 1 and Fig. 3 reveals that the only difference is the split criterion for good-KPS patients between ages 40 and 65. Whereas previously the split for this group was based on extent of resection, in this analysis it was based on whether or not the protocol included chemotherapy. On review of the overall RPA analysis, we found that if one more split had been included, it would have been for this group (middle age, good KPS), excluding the biopsy-only patients, and would have been based on whether or not the patients received chemotherapy. Therefore, the results of the 2 analyses are not inconsistent. It seemed unlikely that the inclusion of adjuvant chemotherapy would impact the outcome for patients between the ages of 40 and 65 and not be of benet for those younger than 40. On further exploration of the regression tree algorithm, it was found that chemotherapy was a very close competitor for anatomic site as a split for the younger patients within the group of patients
Table 4. Risk-group splits, results of RPA analysis including all protocols Number of patients/number of events 50/37 84/70 134/107 370/349 286/281 Median survival in weeks (95% CI) 132 (110 226) 71 (60 97) 97 (77121) 63 (58 69) 37 (32 42) 2-Year survival estimate 65% 35% 46% 17% 4%
Category Group 1: Low risk (age 40, frontal tumor) Group 2: Low-moderate risk (age 40, other tumor sites) 2 lower-risk groups combined Group 3: Moderate-high risk (40 age 65, KPS 70, and STR or GTR) Group 4: High risk (age 65 or 40 age 65 and KPS 80 or 40 age 65, KPS 70, and biopsy only)
Abbreviations: GTR, gross total resection; KPS, Karnofsky performance status; STR, subtotal resection.
Neuro-Oncology
J U LY 2 0 0 4
231
Lamborn et al.: Recursive partitioning analysis: GBM and survival
Table 5. Multivariate Cox proportional hazards results. Nonbrachytherapy protocols (618 patients) Variable Chemotherapy (Y) Extent of resection KPS Age at diagnosis (years) Hazard ratio 0.58 0.69 0.97 1.03 95% CI 0.48 0.69 0.59 0.81 0.96 0.98 1.02 1.04 P-value 0.001 0.001 0.001 0.001
resulting survival curves with this split are presented in Fig. 5, and the summary survival estimates are provided in Table 6. Brachytherapy Trials Initially, we analyzed the data using the Cox proportional hazards model with the variables identied in the overall Cox proportional hazards model (Table 7). Again, KPS and age at diagnosis were clearly important. The importance of chemotherapy and extent of surgery was less clear. While some increase in P-value would be expected because of the smaller sample size, the hazard ratio estimates give no indication of benet from increased resection or addition of adjuvant chemotherapy in the setting of brachytherapy. The number of patients on brachytherapy trials was too small to complete an independent RPA. However, we did assign patients from these protocols to groups based on the risk assignments from the overall RPA analysis to conrm that the results were consistent. Figure 6 provides the Kaplan-Meier curves that resulted for all patients on these trials, and Fig. 7 includes only those on chemotherapy trials. On the basis of Fig. 6, it would appear that the strata selected provide a meaningful grouping of patients in this new data set, although the number in the lowest risk group is small. The similarity in the curves comparing outcome in all patients and in the chemotherapy-only group is consistent with the assessment that chemotherapy has a smaller role to play when brachytherapy is given. Anatomic Site as a Predictor in Younger Patients The nding that age, KPS, and adjuvant chemotherapy predicted survival was expected. The nding of the
Abbreviations: KPS, Karnofsky performance status; Y, yes.
on nonbrachytherapy trials. In fact, if the 111 patients under 40 were split according to chemotherapy protocol (Y/N), median survival was 60 weeks (CI, 37 76) and 110 weeks (CI, 86 141) for nonchemotherapy protocols and chemotherapy protocols, respectively. This is in contrast to the patients over 65, where the median survival was 32 weeks for both chemotherapy protocols and nonchemotherapy protocols. One major purpose of this analysis was to identify stratication variables for planning future studies. For this purpose, it was useful to consider only patients on trials including adjuvant chemotherapy. This also provided a group of patients who could be considered to have been on equivalent postoperative therapy. Figure 4 is the result of this analysis of 437 patients on nonbrachytherapy trials who received adjuvant chemotherapy. The resulting tree cut points are similar to the overall tree, although the estimated median survival is slightly better for those groups that previously included some patients who were not on adjuvant chemotherapy protocols. With the smaller number of patients, the split on anatomical site in the younger patient group no longer met the criterion of P 0.01 based on the log-rank test. However, it did meet the other RPA criteria and is included in the gures for comparison purposes. The
Fig. 3. Recursive partitioning analysis for all patients on nonbrachytherapy protocols (n 660). For terminal nodes ( ), median survival and the 95% condence interval for the median are given. N number of patients. *Twenty-eight patients without KPS were excluded from the estimates.
Fig. 4. Recursive partitioning analysis for all patients on nonbrachytherapy protocols that included adjuvant chemotherapy (n 437). For terminal nodes ( ), median survival and the 95% condence interval for the median are given. N number of patients. *Twenty-eight patients with missing KPS were excluded from the estimates.
232
Neuro-Oncology
J U LY 2 0 0 4
Lamborn et al.: Recursive partitioning analysis: GBM and survival
Table 6. Risk-group splits, results of RPA analysis including nonbrachytherapy protocols with adjuvant chemotherapy Category Group 1: Low risk (age 40, frontal tumor) Group 2: Low-moderate risk (age 40, other tumor sites) 2 lower risk groups combined Group 3: Moderate-high risk (40 age 65, KPS 70) Group 4: High risk (age 65 or 40 age 65 and KPS 80)
Abbreviation: KPS, Karnofsky performance status.
Number of patients / number of events 34/22 46/38 80/60 212/191 117/114
Median survival in weeks (95% CI) 131 (102 334) 79 (61141) 110 (86141) 62 (56 71) 37 (26 44)
2-Year survival estimate 66% 41% 51% 21% 5%
Table 7. Multivariate Cox proportional hazards model. Brachytherapy protocols (158 patients) Variable Chemotherapy (Y) Extent of resection KPS Age at diagnosis (years) Hazard ratio 0.93 1.05 0.97 1.04 95% CI 0.65 1.3 0.78 1.42 0.95 0.99 1.03 1.06 P value 0.69 0.74 0.004 0.001
survival
Abbreviations: KPS, Karnofsky performance status; Y, yes.
anatomic site of tumor as a predictor was not. We therefore evaluated this further using Cox proportional hazards models. When analyses included all ages, there was some trend toward improved survival among those with frontal-only tumors, but the results were never statistically signicant at the 0.05 level. However, when the patient group was limited to those 40 years old at the time of diagnosis, the results were statistically signicant. The results of this analysis for all patients 40 are provided in Table 8. Initially, we hypothesized that tumor site might be a surrogate for extent of resection. However, the Cox proportional hazards model indicates that the association with location is much stronger than any association with extent of resection. On review of the data, few of these younger patients had biopsy only (11% of cases), and 73% of those with frontal tumors had a subtotal resection compared to between 68% and 73% in the remaining three groups. Thus, the lack of association with extent of resection is likely due to the high proportion of patients with subtotal resections, limiting the ability to detect impact of resection, and the association of frontal-only tumors compared with other locations remains to be conrmed and explained with further studies.
weeks
Fig. 5. Survival for patients on nonbrachytherapy protocols that included adjuvant chemotherapy. Nine patients in group 1, 6 patients in group 2, and 7 patients in group 3 survived beyond 5 years (260 weeks). Individual survival times (including those for patients that are censored) are indicated by a solid circle (). Fortyfour patients were censored (12 in group 1, 8 in group 2, 21 in group 3, and 3 in group 4). Additional detail is given in Table 6.
Discussion
A difference in survival among patients with GBM has been consistently seen to depend on age and KPS. Although individual studies have generally not shown improved survival with the use of adjuvant chemotherapy, meta-analyses have observed improvement in survival with adjuvant chemotherapy (GMT, 2002). The roles of extent of resection and use of brachytherapy are
survival
weeks
Fig. 6. Survival for all patients on brachytherapy protocols (n 160). Risk groups are as dened in Table 4 for all patients. Three patients in group 1 and 5 patients in group 2 lived beyond 5 years (260 weeks). Individual survival times (including those for patients that are censored) are indicated by a solid circle (). Eight patients were censored (1 in group 1, 5 in group 2, 2 in group 3, and 0 in group 4).
Neuro-Oncology
J U LY 2 0 0 4
233
Lamborn et al.: Recursive partitioning analysis: GBM and survival
weeks
Fig. 7. Survival for patients on brachytherapy protocols that included adjuvant chemotherapy (n 49). Risk groups are as dened for all patients in Table 4. Two patients in group 1 and 2 patients in group 2 lived beyond 5 years (260 weeks). Individual survival times (including those for patients that are censored) are indicated by a solid circle (). Two patients were censored (both in group 2).
less certain. Two randomized trials of brachytherapy did not show an improvement in outcome (Laperriere et al., 1998; Selker et al., 2002). In the case of extent of resection, this variable is highly dependent on resectability and, therefore, difcult to assess. Prior to this study, investigators have relied on the RTOG RPA to identify risk groups when planning and evaluating treatment regimens for patients with newly diagnosed GBM in phase 2 single-arm studies. This study differs in a number of respects from that study. This study included only patients with GBM, while the RTOG study was open to patients with any high-grade glioma. Clearly, the inclusion of only GBM patients has affected some of the splits in the RPA. This study focused on protocols led by clinicians at UCSF, although some were open to patient entry at multiple institutions. The studies reported here had a smaller portion of patients with low KPS, which will also affect splits. The RTOG study did have the advantage of some additional variables that were not routinely collected on these trials and so could not be studied as possible predictors.
Table 8. Multivariate Cox proportional hazards model including anatomic site and other variables as predictors. Patient age 40 (124 patients) Variable Chemotherapy (Y) Extent of resection KPS Age at diagnosis (years) Brachytherapy Frontal-only tumor Hazard ratio 0.44 0.80 0.96 1.05 0.50 0.53 95% CI 0.28 0.69 0.54 1.20 0.94 0.98 1.011.10 0.27 0.92 0.34 0.83 P-value 0.001 0.28 0.001 0.027 0.025 0.005
Recursive partitioning is an exploratory tool that has found favor in recent years because it provides a method of categorizing patients into risk groups. However, the technique has limitations. It is an exploratory tool with the possibility of selecting apparently prognostic factors by chance, and no probability statements can be made related to the nal splits because all these analyses are post hoc tests. The selection of factors may vary because of small changes in the patient group studied. This can occur if one or more variables are highly correlated. It can also occur if 2 variables have close to the same discrimination ability, which can happen even if the 2 variables are not highly correlated. The choice between chemotherapy and tumor location as the selection criterion among younger patients seen in the analyses reported here is an example of this. It is also important to recognize that the denition of risk groups does not mean that there is no predictive ability of variables within risk groups. This can be seen in the analysis of the data in the younger patients, where several factors were still predictive even among this goodrisk patient group. What the risk groups do is dene a limited set of characteristics that are the most meaningful for grouping patients based on prediction of outcome. More and more, it is being recognized that patients with GBM do not have a homogeneous prognosis. While many of todays efforts are focused on identifying molecular markers for prognosis and targeting treatments to specic patients, the use of clinical factors to assign risk groups continues to be of importance. In randomized phase 3 trials it is possible to use the actual values in proportional hazards models for the nal analyses, but the denition of risk groups provides a practical method of stratifying patients at the time of randomization to ensure reasonable balance among the treatment groups. Most phase 2 trials use historical controls to evaluate whether the therapy is worth studying in a phase 3 trial. Use of actual historical data if available is optimal; however, it is often not available. The identication of risk groups with estimates of the expected outcomes for each provides some level of assurance that results that seem either promising or discouraging are not primarily due to a particular selection bias for that study. In summary, it may be possible to further rene groupings if more information is available, but the risk groups identied in this study have meaningful differences in estimated survival and should be readily used in a multicenter environment, either at the time of randomization or as a basis for historical comparison.
survival
Conclusions
Based on the analyses reported here, a recommendation for grouping of patients for evaluation of clinical trials involving chemotherapy and standard radiotherapy would appear to be ages 40, ages between 40 and 65, and ages
Abbreviations: KPS, Karnofsky performance status; Y, yes.
234
Neuro-Oncology
J U LY 2 0 0 4
Lamborn et al.: Recursive partitioning analysis: GBM and survival
65. Within the middle age group, patients should be further split by KPS 80 versus 80, with patients with low KPS grouped with the older patients (ages 65) for purposes of stratication. For prospective randomized trials, the 3 categories could be the basis for stratied randomization. For single-arm trials, the analysis could take into account the proportion of patients who t into each of the 3 groups. The identication of tumor site as a predictor of survival in the youngest patients is a new nding and so is subject to more questions about its usefulness. The number of these patients is likely to be a small subset of those in any trial; however, the observed difference in survival was large. In instances where the protocol is such that it tends to favor selection of younger patients with more circumscribed tumors, this report would indicate that
tumor location might need to be considered as a factor in evaluating the results. This would especially be true if our results are conrmed in future studies. This study is consistent with results seen in metaanalyses indicating that inclusion of adjuvant chemotherapy provides an increase in survival, although that improvement tends to be minimal for patients over age 65, for those over age 40 with a KPS less than 80, and for patients treated with brachytherapy.
Acknowledgment
The authors thank Sharon Reynolds, Department of Neurological Surgery, University of California San Francisco, for editorial support.
References
Breiman, L., Friedman, J.H., Olshen, R.A., and Stone, C.J. (1984) Classication and Regression Trees. Belmont, Calif.: Wadsworth International Group. Curran, W.J., Jr., Scott, C.B., Horton, J., Nelson, J.S., Weinstein, A.S., Fischbach, A.J., Chang, C.H., Rotman, M., Asbell, S.O., Krisch, R.E., and Nelson, D.F. (1993) Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J. Natl. Cancer Inst. 85, 704 710. GMT. Glioma Meta-analysis Trialists (GMT) Group (2002) Chemotherapy in adult high-grade glioma: A systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet 359, 10111018. Keles, S., and Segal, M.R. (2002) Residual-based tree-structured survival analysis. Stat. Med. 21, 313 326. Laperriere, N.J., Leung, P.M.K., McKenzie, S., Milosevic, M., Wong, S., Glen, J., Pintilie, M., and Bernstein, M. (1998) Randomized study of brachytherapy in the initial management of patients with malignant astrocytoma. Int. J. Radiat. Oncol. Biol. Phys. 41, 1005 1011. Schmoor, C., Ulm, K., and Schumacher, M. (1993) Comparison of the Cox model and the regression tree procedure in analysing a randomized clinical trial. Stat. Med. 12, 2351 2366. Selker, R.G., Shapiro, W.R., Burger, P., Blackwood, M.S., Deutsch, M., Arena, V.C., Van Gilder, J.C., Wu, J., Malkin, M.G., Mealey, J., Jr., Neal, J.H., Olson, J., Robertson, J.T., Barnett, G.H., Bloomeld, S., Albright, R., Hochberg, F.H., Hiesiger, E., Green S., and Brain Tumor Cooperative Group (2002) The Brain Tumor Cooperative Group NIH trial 87-01: A randomized comparison of surgery, external radiotherapy, and carmustine versus surgery, interstitial radiotherapy boost, external radiation therapy, and carmustine. Neurosurgery 51, 343 357. Therneau, T.M., Grambsch, P.M., and Fleming, T.R. (1990) Martingalebased residuals for survival models. Biometrika 77, 147160.
Neuro-Oncology
J U LY 2 0 0 4
235
You might also like
- NO102 08 LambornDocument9 pagesNO102 08 LambornAbhilash VemulaNo ratings yet
- (583950958) Journal Leucopenia Treatment Effiicacy NPCDocument8 pages(583950958) Journal Leucopenia Treatment Effiicacy NPCDaniel HoseaNo ratings yet
- 10.12921 cmst.2006.12.02.143-147 MoczkoDocument5 pages10.12921 cmst.2006.12.02.143-147 Moczkoshital shermaleNo ratings yet
- Annotated BibDocument16 pagesAnnotated Bibapi-542958465No ratings yet
- Clinical Impact of External Radiotherapy in Non-Metastatic Esophageal Cancer According To Histopathological SubtypeDocument12 pagesClinical Impact of External Radiotherapy in Non-Metastatic Esophageal Cancer According To Histopathological SubtypesilviailieNo ratings yet
- MeduloblastomaDocument7 pagesMeduloblastomasilvia erfanNo ratings yet
- Prognostic Factors in Nasopharyngeal Carcinoma With Synchronous Liver Metastasis: A Retrospective Study For The Management of TreatmentDocument7 pagesPrognostic Factors in Nasopharyngeal Carcinoma With Synchronous Liver Metastasis: A Retrospective Study For The Management of TreatmentChairul Nurdin AzaliNo ratings yet
- Prediction Model For Shortterm Mortality After Palliative Therapy For Patients Having Advanced Cancer - A Cohort Study From Routine Electronic Medical DataDocument10 pagesPrediction Model For Shortterm Mortality After Palliative Therapy For Patients Having Advanced Cancer - A Cohort Study From Routine Electronic Medical DataHollis LukNo ratings yet
- Prognostic Model For Survival of Local Recurrent Nasopharyngeal Carcinoma With Intensity-Modulated RadiotherapyDocument7 pagesPrognostic Model For Survival of Local Recurrent Nasopharyngeal Carcinoma With Intensity-Modulated Radiotherapypp kabsemarangNo ratings yet
- Long-Term Survival in Patients With Non-Small Cell Lung CancerDocument7 pagesLong-Term Survival in Patients With Non-Small Cell Lung CancerMrBuu2012No ratings yet
- International Seminars in Surgical OncologyDocument8 pagesInternational Seminars in Surgical OncologyAhmad ShafiqNo ratings yet
- RRP Radical ProstatectomyDocument6 pagesRRP Radical ProstatectomybojanvuckovicNo ratings yet
- Sequential Chemoradiotherapy With Gemcitabine and Cisplatin For Locoregionally Advanced Nasopharyngeal CarcinomaDocument9 pagesSequential Chemoradiotherapy With Gemcitabine and Cisplatin For Locoregionally Advanced Nasopharyngeal CarcinomadheaonyonNo ratings yet
- Hyerbaric Oxygenation - Final DraftDocument17 pagesHyerbaric Oxygenation - Final Draftapi-542958465No ratings yet
- JCO 2003 Lin 631 7Document7 pagesJCO 2003 Lin 631 7Adhika Manggala DharmaNo ratings yet
- Comparison of Radical Cystectomy With Conservative Treatment in Geriatric ( 80) Patients With Muscle-Invasive Bladder CancerDocument9 pagesComparison of Radical Cystectomy With Conservative Treatment in Geriatric ( 80) Patients With Muscle-Invasive Bladder CancerjustforuroNo ratings yet
- Cam40002 0234Document9 pagesCam40002 0234Domenico LombardiniNo ratings yet
- A Genomic StrategyDocument11 pagesA Genomic StrategyAmirahShalehaNo ratings yet
- Strouthos 2017Document8 pagesStrouthos 2017Evelynππ θσυNo ratings yet
- Nejmoa 060467Document11 pagesNejmoa 060467Juan Camilo LandazuriNo ratings yet
- Radiotherapy and OncologyDocument8 pagesRadiotherapy and OncologyDINDA PUTRI SAVIRANo ratings yet
- Accepted Manuscript: Advances in Radiation OncologyDocument28 pagesAccepted Manuscript: Advances in Radiation OncologyRonald André Buleje HinostrozaNo ratings yet
- Neoadjuvant Paclitaxel For Operable Breast Cancer: Multicenter Phase II Trial With Clinical OutcomesDocument6 pagesNeoadjuvant Paclitaxel For Operable Breast Cancer: Multicenter Phase II Trial With Clinical OutcomesSubhash SugathanNo ratings yet
- 551 PDFDocument5 pages551 PDFfaidgustisyarifNo ratings yet
- Endometrial CancerDocument12 pagesEndometrial CancermersinonkolojiNo ratings yet
- Protocol SummaryDocument4 pagesProtocol Summaryapi-631272802No ratings yet
- COGNITION A Prospective Precision Oncology TrialDocument12 pagesCOGNITION A Prospective Precision Oncology Trialveaceslav coscodanNo ratings yet
- FDA Approval Summary - Atezolizumab and Durvalumab in Combination With Platinum-Based Chemotherapy in Extensive Stage Small Cell Lung CancerDocument6 pagesFDA Approval Summary - Atezolizumab and Durvalumab in Combination With Platinum-Based Chemotherapy in Extensive Stage Small Cell Lung CancerasdffdsaNo ratings yet
- Concurrent Cisplatin, Etoposide, and Chest Radiotherapy in Pathologic Stage IIIB Non-Small-Cell Lung CancerDocument7 pagesConcurrent Cisplatin, Etoposide, and Chest Radiotherapy in Pathologic Stage IIIB Non-Small-Cell Lung Cancerdurgesh kumarNo ratings yet
- Neurosurg Focus Article PE4Document8 pagesNeurosurg Focus Article PE4emilyNo ratings yet
- Evaluation of Outcome and Prognostic Factors in Patients of Glioblastoma Multiforme A Single Institution ExperienceDocument11 pagesEvaluation of Outcome and Prognostic Factors in Patients of Glioblastoma Multiforme A Single Institution Experienceafdhal.888980No ratings yet
- EJGO2022054 Cervical CaDocument8 pagesEJGO2022054 Cervical CaRahmayantiYuliaNo ratings yet
- Vaginal Dose Is Associated With Toxicity in Image Guided Tandem Ring or Ovoid-Based BrachytherapyDocument7 pagesVaginal Dose Is Associated With Toxicity in Image Guided Tandem Ring or Ovoid-Based BrachytherapyFlor ZalazarNo ratings yet
- Lung Stereotactic Ablative Body Radiotheray SABR - Patient o - 2019 - ClinicalDocument1 pageLung Stereotactic Ablative Body Radiotheray SABR - Patient o - 2019 - ClinicalSENo ratings yet
- Esofago Carvical Valmasoni 2018Document9 pagesEsofago Carvical Valmasoni 2018Carlos N. Rojas PuyolNo ratings yet
- The Significance of Neutrophil/lymphocyte Ratio As A Possible Marker of Underlying Papillary Microcarcinomas in Thyroidal Goiters: A Pilot StudyDocument6 pagesThe Significance of Neutrophil/lymphocyte Ratio As A Possible Marker of Underlying Papillary Microcarcinomas in Thyroidal Goiters: A Pilot StudyagusNo ratings yet
- JurnalDocument11 pagesJurnalPuput Indah PratiwiNo ratings yet
- Impact of Time To Surgery After Neoadjuvant Chemotherapy in Operable Breast Cancer PatientsDocument6 pagesImpact of Time To Surgery After Neoadjuvant Chemotherapy in Operable Breast Cancer PatientsPani lookyeeNo ratings yet
- Reuter 2010Document8 pagesReuter 2010Hector Javier BurgosNo ratings yet
- Survival Rates For Patients With Resected Gastric Adenocarcinoma Finally Have Increased in The United StatesDocument7 pagesSurvival Rates For Patients With Resected Gastric Adenocarcinoma Finally Have Increased in The United StatesXavier QuinteroNo ratings yet
- Abstracts: Annals of OncologyDocument1 pageAbstracts: Annals of OncologySole GonzalezNo ratings yet
- Patient Experience in The Treatment of Metastatic Castration-Resistant Prostate Cancer: State of The ScienceDocument11 pagesPatient Experience in The Treatment of Metastatic Castration-Resistant Prostate Cancer: State of The ScienceLuis ReyNo ratings yet
- A Phase II Study Evaluating The Efficacy and Safety of AMG 102 (Rilotumumab) in Patients With Recurrent GlioblastomaDocument10 pagesA Phase II Study Evaluating The Efficacy and Safety of AMG 102 (Rilotumumab) in Patients With Recurrent GlioblastomaSubhash SugathanNo ratings yet
- Kolagocarsinoma PDFDocument7 pagesKolagocarsinoma PDFBerlyan RuslanNo ratings yet
- NPCDocument8 pagesNPCArsy Mira PertiwiNo ratings yet
- Clin Cancer Res-2010-Tabchy-Evaluation of A 30-Gene Paclitaxel, Fluorouracil, Doxorubicin, and Cyclophosphamide Chemotherapy Response Predictor in A Multicenter Randomized Trial in Breast CancerDocument11 pagesClin Cancer Res-2010-Tabchy-Evaluation of A 30-Gene Paclitaxel, Fluorouracil, Doxorubicin, and Cyclophosphamide Chemotherapy Response Predictor in A Multicenter Randomized Trial in Breast CancerOncology FatmawatiNo ratings yet
- 2014 Article 1491Document5 pages2014 Article 1491Glauce L TrevisanNo ratings yet
- Eligibility Criteria: Previous Sectionnext SectionDocument6 pagesEligibility Criteria: Previous Sectionnext Sectionbettzy21No ratings yet
- 1 s2.0 S1083879111002916 MainDocument8 pages1 s2.0 S1083879111002916 MainNunungTriwahyuniNo ratings yet
- 1677 5538 Ibju 42 04 0694Document10 pages1677 5538 Ibju 42 04 0694Lucas VibiamNo ratings yet
- Pros TataDocument8 pagesPros TataMartin ElenaNo ratings yet
- Prognostic Predictive Markers Prognostic Predictive Prognostic PredictiveDocument5 pagesPrognostic Predictive Markers Prognostic Predictive Prognostic Predictivepre_singh2136No ratings yet
- CPCNP MTXDocument9 pagesCPCNP MTXMary CogolloNo ratings yet
- ConutDocument9 pagesConutargamaviNo ratings yet
- MÁS RADIOTERAPIA SI RECAÍDA y Biopsia A Todos. 310 PersonasDocument13 pagesMÁS RADIOTERAPIA SI RECAÍDA y Biopsia A Todos. 310 Personasouf81No ratings yet
- Concurrent Radiotherapy and Weekly Paclitaxel For Locally Advanced Squmous Cell Carcinoma of Uterine Cervix-Treated Patients at Rural Centre in IndiaDocument5 pagesConcurrent Radiotherapy and Weekly Paclitaxel For Locally Advanced Squmous Cell Carcinoma of Uterine Cervix-Treated Patients at Rural Centre in IndiaIjsrnet EditorialNo ratings yet
- Review: Second Consensus On Medical Treatment of Metastatic Breast CancerDocument11 pagesReview: Second Consensus On Medical Treatment of Metastatic Breast Cancermohit.was.singhNo ratings yet
- Analysis of Compliance, Toxicity and Survival WeeklyDocument11 pagesAnalysis of Compliance, Toxicity and Survival Weeklydanu20No ratings yet
- Curran 2011Document9 pagesCurran 2011Nguyễn Hoàng PhúcNo ratings yet
- Does Testosterone Have A Role in Erectile Function?: Review AJM Theme Issue: Men's HealthDocument10 pagesDoes Testosterone Have A Role in Erectile Function?: Review AJM Theme Issue: Men's HealthNandia SeptiyoriniNo ratings yet
- Methotrexate in Rheumatoid Arthritis Efficacy and Safety 2329 6887 2 127Document4 pagesMethotrexate in Rheumatoid Arthritis Efficacy and Safety 2329 6887 2 127Saifuddin HaswareNo ratings yet
- Pengetahuan Pasangan Usia Subur (Pus) Tentang Kanker ServiksDocument6 pagesPengetahuan Pasangan Usia Subur (Pus) Tentang Kanker ServiksgraceNo ratings yet
- Clinical Factors Responsible For Gastric Outlet Obstruction in Patients From Bihar RegionDocument4 pagesClinical Factors Responsible For Gastric Outlet Obstruction in Patients From Bihar RegionzapomannNo ratings yet
- Bleeding Disorder ApproachDocument9 pagesBleeding Disorder Approachmalik003No ratings yet
- Fashion Theatre and Media Make-Up Sample Client Consultation FormDocument3 pagesFashion Theatre and Media Make-Up Sample Client Consultation Formindiecindy91100% (1)
- High-Dose Vitamin Therapy Stimulates Variant Enzymes With Decreased Coenzyme Binding AffinityDocument43 pagesHigh-Dose Vitamin Therapy Stimulates Variant Enzymes With Decreased Coenzyme Binding AffinityytreffalNo ratings yet
- Cervical Cancer Patho.2Document2 pagesCervical Cancer Patho.2Verni Dela CruzNo ratings yet
- Advance Endoscopy Assessment FormDocument2 pagesAdvance Endoscopy Assessment FormRomelia CampuzanoNo ratings yet
- History TakingDocument15 pagesHistory TakingacildevilNo ratings yet
- Antibacterial Activity of Passion Fruit (Passiflora SP.) Leaf ExtractsDocument23 pagesAntibacterial Activity of Passion Fruit (Passiflora SP.) Leaf ExtractsJaslynn PintoNo ratings yet
- Bacterial Flash Cards (Part 1 of 4)Document25 pagesBacterial Flash Cards (Part 1 of 4)Nafis Shamsid-DeenNo ratings yet
- Techniques of Bowel Resection and AnastomosisDocument6 pagesTechniques of Bowel Resection and AnastomosisFachrul SyafruddinNo ratings yet
- NPTEL Virology MCQDocument19 pagesNPTEL Virology MCQTawfeeq AuqbiNo ratings yet
- RAdiology Related Infratemporal Fossa, Pterygo Palatine Fossa, Parapharyngeal SpaceDocument11 pagesRAdiology Related Infratemporal Fossa, Pterygo Palatine Fossa, Parapharyngeal SpacehaneefmdfNo ratings yet
- IndianJRadiolImaging253288-7172209 195522 (7lmbr)Document8 pagesIndianJRadiolImaging253288-7172209 195522 (7lmbr)Ami WilliamsNo ratings yet
- HER Health Expo Manual: by Charles H. Cleveland, MPHDocument32 pagesHER Health Expo Manual: by Charles H. Cleveland, MPHRes Resilia100% (1)
- ATT Induced Liver Injury: Dr. Zubair Sarkar JR2, Deptt. of Medicine J.N.M.C.H., Aligarh Muslim UniversityDocument61 pagesATT Induced Liver Injury: Dr. Zubair Sarkar JR2, Deptt. of Medicine J.N.M.C.H., Aligarh Muslim UniversityFahadKamalNo ratings yet
- Breast and Testicular Self-ExaminationDocument4 pagesBreast and Testicular Self-ExaminationLala MumuNo ratings yet
- Types of Gene TherapyDocument8 pagesTypes of Gene TherapyAqib KhalidNo ratings yet
- Blood ConservationDocument7 pagesBlood ConservationbobbykrishNo ratings yet
- Colposcopy CourseDocument50 pagesColposcopy CourseNam Le100% (1)
- Student ProjectDocument25 pagesStudent ProjectHananya ManroeNo ratings yet
- Equine Diseases Caused by Known Genetic MutationsDocument12 pagesEquine Diseases Caused by Known Genetic Mutationsalvesguida100% (1)
- Draft Citizen Charter-DSHindiDocument34 pagesDraft Citizen Charter-DSHindibarwalaNo ratings yet
- MSDS NiFe2O4Document8 pagesMSDS NiFe2O4aku2505kamuNo ratings yet
- Thesis StatementDocument4 pagesThesis StatementBlessyJoyPunsalanNo ratings yet
- BIRAC IITK Edited by Vishal SirDocument45 pagesBIRAC IITK Edited by Vishal SirRobin hoodNo ratings yet
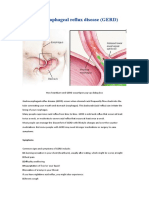
- Gastroesophagus Reflux Disease (GERD)Document5 pagesGastroesophagus Reflux Disease (GERD)to van quyenNo ratings yet
- The Age of Magical Overthinking: Notes on Modern IrrationalityFrom EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityRating: 4 out of 5 stars4/5 (23)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsFrom EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsNo ratings yet
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedFrom EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedRating: 5 out of 5 stars5/5 (80)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsFrom EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsRating: 5 out of 5 stars5/5 (1)
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeFrom EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeNo ratings yet
- Summary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisFrom EverandSummary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisRating: 4.5 out of 5 stars4.5/5 (42)
- The Obesity Code: Unlocking the Secrets of Weight LossFrom EverandThe Obesity Code: Unlocking the Secrets of Weight LossRating: 4 out of 5 stars4/5 (5)
- Why We Die: The New Science of Aging and the Quest for ImmortalityFrom EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityRating: 4 out of 5 stars4/5 (3)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaFrom EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaRating: 4.5 out of 5 stars4.5/5 (266)
- Mindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessFrom EverandMindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessRating: 4.5 out of 5 stars4.5/5 (328)
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisFrom EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisRating: 3.5 out of 5 stars3.5/5 (2)
- Gut: the new and revised Sunday Times bestsellerFrom EverandGut: the new and revised Sunday Times bestsellerRating: 4 out of 5 stars4/5 (392)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsFrom EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsRating: 3.5 out of 5 stars3.5/5 (3)
- Raising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsFrom EverandRaising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsRating: 4.5 out of 5 stars4.5/5 (169)
- The Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeFrom EverandThe Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeRating: 4.5 out of 5 stars4.5/5 (253)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.From EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Rating: 4.5 out of 5 stars4.5/5 (110)
- Cult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryFrom EverandCult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryRating: 4 out of 5 stars4/5 (44)
- 12 Rules for Life by Jordan B. Peterson - Book Summary: An Antidote to ChaosFrom Everand12 Rules for Life by Jordan B. Peterson - Book Summary: An Antidote to ChaosRating: 4.5 out of 5 stars4.5/5 (207)
- How to ADHD: The Ultimate Guide and Strategies for Productivity and Well-BeingFrom EverandHow to ADHD: The Ultimate Guide and Strategies for Productivity and Well-BeingNo ratings yet
- An Autobiography of Trauma: A Healing JourneyFrom EverandAn Autobiography of Trauma: A Healing JourneyRating: 5 out of 5 stars5/5 (2)
- Sleep Stories for Adults: Overcome Insomnia and Find a Peaceful AwakeningFrom EverandSleep Stories for Adults: Overcome Insomnia and Find a Peaceful AwakeningRating: 4 out of 5 stars4/5 (3)
- Outlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisFrom EverandOutlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisRating: 4 out of 5 stars4/5 (1)
- Troubled: A Memoir of Foster Care, Family, and Social ClassFrom EverandTroubled: A Memoir of Foster Care, Family, and Social ClassRating: 4.5 out of 5 stars4.5/5 (26)