Professional Documents
Culture Documents
Chapter 3 Electrochemistry (Notes)
Uploaded by
DivyanshuOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chapter 3 Electrochemistry (Notes)
Uploaded by
DivyanshuCopyright:
Available Formats
ABC Academy 1
Electrochemistry
Electrochemistry: is the branch of chemistry that deals with study of production of electricity from
energy released during spontaneous chemical reactions and the use of electrical energy to bring
about non-spontaneous chemical transformations.
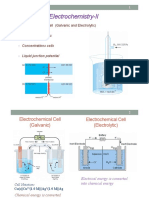
Electrochemical cell or Galvanic cell or voltaic cell : is a device that converts the chemical
energy of a spontaneous redox reaction into electrical energy.
Electrode: is a metal rod partially dipped in solution containing its own ions.
Anode: the electrode at which oxidation (loss of electrons) takes place is called anode. It has
negative potential w.r.t. the solution (electrolyte).
e.g. Zn → Zn2+ + 2e¯
Cathode: the electrode at which reduction (gain of electrons) takes place is called cathode. It has
positive potential w.r.t. the solution (electrolyte).
e.g. Cu2+ + 2e¯ → Cu
Electrode potential: is the potential difference that develops between the electrode and electrolyte.
It is the tendency of an electrode to lose or gain electrons.
Standard electrode potential: when the concentration of all the species involved in a half-cell is
unity then the electrode potential is known as standard electrode potential.
When two electrodes with different electrode potentials are connected to each other through a
conducting wire, the electrons start flowing from negative electrode to positive electrode i.e. anode
to cathode and current from cathode to anode i.e. opposite to the direction of flow of electrons.
Contact: S L Badhotra. Ph: 8826513667, 80108-64649
ABC Academy 2
Salt bridge: is U – shaped tube containing paste of an inert electrolyte like KCl or KNO3 etc. in agar
– agar.
Functions: (i) maintains electrical neutrality in two half cells.
(ii). completes internal circuit.
(iii). A salt bridge minimizes the liquid junction potential at the two solutions by preventing their
mixing.
Liquid junction potential: a potential difference sets up across the junctions of the two solutions of
the electrolytes when these are in direct contact with each other. This is known as liquid junction
potential (L.J.P.). It is due to accumulation of charges at the junction of the two solutions as a result
of different speed of ions. The charge accumulation results in electrical double layer leading to
decrease in emf of the cell.
Transport number or transfer number of the ion: is defined fraction of current carried by an ion.
𝑐𝑢𝑟𝑟𝑒𝑛𝑡 𝑐𝑎𝑟𝑟𝑖𝑒𝑑 𝑏𝑦 𝑎𝑛 𝑖𝑜𝑛
Transport number = 𝑡𝑜𝑡𝑎𝑙 𝑐𝑢𝑟𝑟𝑒𝑛𝑡 𝑐𝑎𝑟𝑟𝑖𝑒𝑑
nc + na = 1
with increase in concentration transport number decreases. For 1:1 electrolyte, if transport number
is greater than 0.5, it decreases with increase in temperature, but if it is less than 0.5, it increases
with increase in temperature and approaches 0.5 value.
Cell potential: is the potential difference between the two electrodes of a galvanic cell i.e. the
difference between the electrode potentials of the cathode and anode when circuit is closed (cell is
in use)
Cell electromotive force (emf): is the cell potential when no current is drawn through the cell i.e.
cell is not in use (circuit is open).
𝑬°𝒄𝒆𝒍𝒍 = 𝑬°𝒄𝒂𝒕𝒉𝒐𝒅𝒆 – 𝑬°𝒂𝒏𝒐𝒅𝒆
Representation of a galvanic cell: Anode is written on the left hand side and cathode on the right
hand side. Electrode (metal) and electrolyte) are separated by a vertical line and two electrolytes
are separated by double vertical line.
Cell reaction: Cu(s) + 2 Ag+(aq) → Cu2+(aq) + 2 Ag(s)
Cell representation: Cu(s) I Cu2+(aq) II Ag+(aq) I Ag(s)
Cell reaction: Zn(s) + Cu2+(𝑐1 ) → Zn2+(𝑐2 ) + Cu(s)
Cell representation: Zn(s) I Zn2+ (𝑐2 ) II Cu2+(𝑐1 ) I Cu(s)
Measurement of Electrode Potential: The potential of individual half-cell cannot be measured
because
Contact: S L Badhotra. Ph: 8826513667, 80108-64649
ABC Academy 3
i. Oxidation or reduction at an electrode takes place only when it is connected to another half-cell.
ii. Voltmeter cannot be directly connected between electrode and electrolyte
To overcome these difficulties a reference electrode known as Standard Hydrogen Electrode (SHE)
is chosen whose electrode potential is taken as zero. Then SHE is connected (coupled) with given
electrode through voltmeter to find E(cell). Hence electrode potential is calculated using
𝑬°𝒄𝒆𝒍𝒍 = 𝑬°𝒄𝒂𝒕𝒉𝒐𝒅𝒆 – 𝑬°𝒂𝒏𝒐𝒅𝒆
Standard Hydrogen Electrode (SHE): The standard hydrogen electrode consists of a platinum
electrode coated with platinum black sealed in a glass tube. The electrode is dipped in one molar
acidic solution (HCl) and pure hydrogen gas at one bar pressure is bubbled through it.
It can act as anode as well as cathode
As anode: H2→ 2H+(aq) + 2e−
As cathode: 2H+(aq) + 2e− → H2
Representation of SHE: Pt(s) | H2(g) | H+(aq)
𝑬°𝑯+/𝑯𝟐 = 0.0V
Limitations:
i. It is not easy to maintain a pressure of 1 Atm for hydrogen gas throughout.
ii. It is quite difficult to maintain the [H+] as 1M.
iii. Platinum electrode gets easily poisoned even by traces of impurities present.
It is therefore replaced by other reference electrode known as calomel electrode. It is
represented by
Hg ; Hg2Cl2(s) KCl (solution)
Different concentration of KCl solutions used give different reduction potentials e.g.
Concentration.of Reduction potential
KCl
0.1N +0.3338V
1.0N +0.2800V
Saturated +0.2415V
Nernst equation: is an equation used to find electrode potential of an electrode at any
concentration of electrolyte at 298K
Mn+(aq) + ne¯ → M(s)
𝑅𝑇 [𝑀]
E(Mn+/M) = E°(Mn+/M) - 𝑛𝐹
ln[𝑀𝑛+]
Contact: S L Badhotra. Ph: 8826513667, 80108-64649
ABC Academy 4
𝑹𝑻 𝟏
E(Mn+/M) = E°(Mn+/M) - ln [ concentration of solid is unity ]
𝒏𝑭 [𝑴𝒏+ ]
Similarly, in Daniell cell, the electrode potential for any given concentration of Cu2+ and Zn2+ ions.
𝑅𝑇 1
For Cathode: E(Zn2+/Zn) = E°(Zn2+/Zn) - 2𝐹
ln[𝑍𝑛2+]
𝑅𝑇 1
For Anode: E(Cu2+/Cu) = E°(Cu2+/Cu) - 2𝐹
ln[𝐶𝑢2+]
The cell potential, = E(Cu2+/Cu) - E(Zn2+/Zn)
𝑅𝑇 1 1
i.e. E(cell) = E°(Cu2+/Cu) - E°(Zn2+/Zn) - 2𝐹
[ ln[𝐶𝑢2+] - ln [𝑍𝑛2+] ]
𝑅𝑇 [𝑍𝑛2+ ]
E(cell) = E°(Cu2+/Cu) - E°(Zn2+/Zn) - 2𝐹
ln [𝐶𝑢2+]
𝑅𝑇 [𝑍𝑛2+ ]
E(cell) = E°(Cu2+/Cu) - E°(Zn2+/Zn) - 2.303 log
2𝐹 [𝐶𝑢2+ ]
𝟎.𝟎𝟓𝟗 [𝐙𝐧2+ ]
E(cell) = E°(cell) - log [𝐂𝐮2+ ] (R =8.314JK-1mol-1 and T = 298K)
𝟐
For a general reaction aA + bB → cC + dD
𝟎.𝟎𝟓𝟗 [𝑪]𝒄 [𝑫]𝒅
E(cell) = E°(cell) - log[𝑨]𝒂[𝑩]𝒃
𝒏
𝟎.𝟎𝟓𝟗 [𝒑𝒓𝒐𝒅𝒖𝒄𝒕]
E(cell) = E°(cell) - log[𝒓𝒆𝒂𝒄𝒕𝒂𝒏𝒕]
𝒏
Equilibrium constant (KC):
[𝑝𝑟𝑜𝑑𝑢𝑐𝑡]
At equilibrium E(cell) = 0 and = KC
[𝑟𝑒𝑎𝑐𝑡𝑎𝑛𝑡]
0.059
0 = E°(cell) - 𝑛
log KC
0.059
E°(cell) = 𝑛
log KC
Electrochemical cell and Gibbs energy of the reaction:
∆G = - nFE(cell)
∆G° = - nFE°(cell)
∆G° = - RTlnK
Electrode potential of a half cell reaction from two or more half cell reactions:
∆G3 = ∆G1 + ∆G2
Contact: S L Badhotra. Ph: 8826513667, 80108-64649
ABC Academy 5
-n3E3F = -n1E1F + (-n2E2F)
n3E3 = n1E1 + n2E2
Concentration cells: are cells consisting of two solutions of the same electrolyte having
different concentrations separated by a salt bridge with the electrodes of the same material
dipped in them or with electrodes at different concentration dipped in same electrolyte.
Types of concentration cells:
i. Concentration cells in which electrode is reversible w.r.t. cation:
a. Zn(s) I Zn2+ (C1) II Zn2+(C2) I Zn(s)
Net reaction:
Zn2+(C2) → Zn2+ (C1)
𝟎.𝟎𝟓𝟗 𝐶
E(cell) = E°(cell) - log 𝐶1
𝟐 2
b. Pt ; H2(1 atm) I HCl(C1) II HCl (C2) I Pt ; H2(1 atm)
𝟎.𝟎𝟓𝟗 𝐶
E(cell) = log 𝐶2
𝟏 1
Cell reaction is spontaneous in forward direction in C2 > C1
ii. Concentration cells in which electrode is reversible w.r.t. anion:
Pt(s) ; Cl2(1 atm) I 𝐶𝑙 − (C1) II 𝐶𝑙 − (C2) I Cl2(1 atm); Pt(s)
𝟎.𝟎𝟓𝟗 𝐶
E(cell) = log 𝐶1
𝟏 2
Cell reaction is spontaneous in forward direction in C1 > C2
iii. Concentration cells having electrodes at different concentrations dipped into same
electrolyte:
Pt ; H2(at 𝑃1 ) I HCl(1M) I Pt ; H2( at P2)
𝟎.𝟎𝟓𝟗 𝑃
E(cell) = log 𝑃1
𝟐 2
Cell reaction is spontaneous in forward direction in P1 > P2
Edison storage cell or Nickel – Iron accumulator:
Fe(s) I FeO(s) II KOH(aq) I Ni2O3 I Ni
At the cathode: Ni2O3 + H2O(l) + 2𝑒 − ⇌ 2NiO(s) + 𝑂𝐻 − (aq)
At the anode: Fe(s) + 𝑂𝐻 − (aq) ⇌ FeO + H2O(l) + 2𝑒 −
Overall reaction: Ni2O3 + Fe(s) ⇌ 2NiO(s) + FeO
Absolute ionic mobilities: it is defined as the speed of the ions in cm/s at infinite dilution
under a potential gradient of 1 volt/cm (potential gradient = applied EMF/distance between the
electrodes). Units of absolute ionic mobility are cm s-1/volt s-1
Contact: S L Badhotra. Ph: 8826513667, 80108-64649
ABC Academy 6
𝑖𝑜𝑛𝑖𝑐 𝑐𝑜𝑛𝑑𝑢𝑐𝑡𝑎𝑛𝑐𝑒
Absolute ionic mobility =
9500
Resistance (R): is the opposition offered by a conductor to the flow of current through it.
l
R ∝ 𝐴
; l is the length of the conductor and A is the area of cross-section of the conductor
𝑙
R = 𝜌 𝐴 ; 𝜌 is the specific resistance or resistivity of the conductor.
𝐴
𝜌=𝑅𝑙
Resistivity: is equal to the resistance of conductor of unit length and unit area of cross-section.
SI unit of resistance is ohm (Ω ) and that of resistivity is ohm m (Ω m).
Conductance (G): is the ease with which current flows through an electrolyte. It is equal to the
reciprocal of resistance.
1
G= ; SI unit of G is ohm-1 (Ω−1) or mho or Siemens (S).
𝑅
Conductivity (𝜿): is equal to the conductance of unit volume of solution (electrolyte). It is equal to
the reciprocal of resistivity.
1 𝒍
𝜅 = 𝜌 i.e. 𝜿=
𝑹𝑨
SI unit of conductivity is ohm-1 m-1 (Ω−1 𝑚−1 ) or mho m-1or Siemens m-1(S m-1).
Electrical conductance through metals is called metallic or electronic conductance and is due to the
movement of electrons. The electronic conductance depends on
i. The nature and structure of the metal.
ii. The number of valence electrons per atom.
iii. Temperature (it decreases with increase of temperature).
Superconductors: are materials which have zero resistivity theoretically.
When electrolytes are dissolved in water, they furnish their own ions in the solution and these ions
conduct electricity. The conductance of electricity by ions present in the solutions is called
electrolytic or ionic conductance.
The conductivity of electrolytic solutions (ionic) solutions depends on:
i. The nature of the electrolyte added: Strong electrolytes produce large number of ions as
compared weak electrolytes. Therefore conductivity of strong electrolytes is greater than weak
electrolytes.
Contact: S L Badhotra. Ph: 8826513667, 80108-64649
ABC Academy 7
ii. Size of ions produced and their salvation: smaller and less solvated ions move faster. Hence
conduct more current.
iii. The nature of the solvent and its viscosity: in polar solvents like water due to high dielectric
constant, degree of dissociation is high. Hence conductivity is greater. In highly viscous solvents,
the speed of ions is less. Therefore conductivity is low.
iv. Concentration of the electrolyte: with increase in concentration, the number of ions increases.
Therefore conductivity increases.
v. Temperature: as the temperature increases, volume increases as well as kinetic energy of of ions
increases and viscosity decreases.. Therefore ions move more freely and faster. Hence conductivity
increases.
Measurement of the conductivity of ionic solutions:
For measuring the resistance of ionic solutions we face two problems. Firstly, passing direct current
changes the composition of the solution. Secondly, a solution to measure resistance cannot be
connected to the bridge like a metallic wire or other solid conductor.
The first difficulty is resolved by using by using an alternating current. The second is solved by
using a specially designed vessel called conductivity cell.
A conductivity cell consists of two platinum electrodes coated with platinum black (finely divided
metallic Pt is deposited on the electrodes electrochemically). Electrodes have area of cross section
equal to ‘A’ and are separated by distance ‘l’.
𝑙
Cell constant (G*): The quantity 𝐴 is called cell constant. It depends upon the distance between the
electrodes and their area of cross-section and has SI unit m-1 . The measurement of l and A is not
only inconvenient but also unreliable. It is usually determined by measuring the resistance of the
cell (using Wheatstone bridge) containing a solution of known conductivity like KCl.
Once the cell constant and the resistance of the solution in the cell is determined, the conductivity
of the solution is calculated as
𝑮∗
𝜿=
𝑹
Molar conductivity (𝝀𝒎 ): is equal to the conductance of any volume of solution containing one
mole of an electrolyte kept between two electrodes with area of cross-section A and distance of unit
length.
𝜿
𝝀𝒎 = ; c is the concentration of the solution.
𝒄
SI unit of molar conductivity is S m2 mol-1 or S cm2 mol-1.
If unit of conductivity is S m-1 and concentration is mol/L.
Contact: S L Badhotra. Ph: 8826513667, 80108-64649
ABC Academy 8
𝜿
Then 𝝀𝒎 = S m2 mol-1
𝒄 ×𝟏𝟎𝟎𝟎
If unit of conductivity is S cm-1 and concentration is mol/L.
𝜿 × 𝟏𝟎𝟎𝟎
Then 𝝀𝒎 = S cm2 mol-1
𝒄
1 S m2 mol-1 = 104 S cm2 mol-1
Equivalent conductivity: is equal to the conductance of any volume of solution containing one
gram equivalent of an electrolyte kept between two electrodes with area of cross-section A and
distance of unit length.
𝜿
𝝀𝒎 =
𝒏𝒐𝒓𝒎𝒂𝒍𝒊𝒕𝒚
𝜿
𝝀𝒎 = S m2 geq-1
𝒏𝒐𝒓𝒎𝒂𝒍𝒊𝒕𝒚 ×𝟏𝟎𝟎𝟎
𝜿 × 𝟏𝟎𝟎𝟎
𝝀𝒎 = S cm2 geq-1
𝒏𝒐𝒓𝒎𝒂𝒍𝒊𝒕𝒚
Variation of conductivity with concentration: For both weak as well as strong electrolytes
the conductivity increases with increase in concentration because with increase in
concentration the number of ions per unit volume increases.
Whereas with dilution conductivity decreases because number of ions per unit volume
decreases.
Variation of Molar conductivity with concentration/dilution:
𝜿
𝝀𝒎 =
𝒄
𝒏 𝟏
𝝀𝒎 = 𝜿v ( c = 𝒗 , c = 𝒗 for n =1 )
With dilution conductivity decreases, while volume increases. The increase in volume is much
greater than decrease in conductivity. Therefore molar conductivity for weak as well as strong
electrolytes increases with dilution.
For strong electrolytes increase in molar conductivity is represented as
𝝀𝑐𝑚 = 𝝀°𝑚 - 𝐴√𝑐 (Huckel Onsagar equation)
The value of the constant ‘A’ for a given solvent and temperature depends on the type of electrolyte
i.e., the charges on the cation and anion produced on the dissociation of the electrolyte in the
solution. Thus, NaCl, CaCl2, MgSO4 are known as 1-1, 2-1 and 2-2 electrolytes respectively. All
electrolytes of a particular type have the same value for ‘A’.
Contact: S L Badhotra. Ph: 8826513667, 80108-64649
ABC Academy 9
For weak electrolytes molar conductivity is generally less than strong electrolytes because of lower
degree of dissociation of weak electrolytes.
At infinite dilution molar conductivity of weak electrolytes increases sharply. This is because at
infinite dilution weak electrolyte dissociates completely and produces large number of ions in total
volume of solution that contains one mole of electrolyte.
Effect of pressure: the viscosity of dilute solutions decreases with increasing pressure, therefore,
equivalent/molar conductivity increases provided the pressure is not too high.
Conductivity water: it is highly purified water whose own conductance is very very small. It is
prepared by demineralization of ordinary water by passing through cation and anion exchange
resins.
Limiting molar conductivity (𝝀°𝒎 ): molar conductivity at infinite dilution i.e. when concentration
approaches zero is called limiting molar conductivity.
Degree of dissociation and dissociation constant of weak electrolyte is given as
𝜆𝑐𝑚
𝛼 =
𝜆°𝑚
cα2
Ka =
(1− α)
Kohlrausch’s law of independent migration of ions: limiting molar conductivity of an electrolyte
can be represented as the sum of individual limiting molar conductivities of ions (cations and
anions) produced upon dissociation/ionization of the electrolyte.
𝝀°𝑚(NaCl) = 𝝀°m(Na+) + 𝝀°m(Cl−)
𝝀°𝑚(MgCl2 ) = 𝝀°m(Mg2+) + 2 𝝀°m(Cl−)
Electrolytic cells and electrolysis: is a device in which external source of voltage is used to bring
about a chemical reaction. Electrolytic cells are used for electrolytic reduction metals like sodium,
magnesium and Aluminium from their respective salts (chlorides and oxides) when no suitable
reducing agents are available.
Quantitative aspects of electrolysis:
Faraday’s laws of electrolysis:
Contact: S L Badhotra. Ph: 8826513667, 80108-64649
ABC Academy 10
I. First law of electrolysis: the amount chemical reaction which occurs (amount of a substance
produced or dissolved) at any electrode is proportional to the quantity of electricity passed through
the electrolyte (solution or melt).
m 𝛼Q
m = Z I t; Z is constant of proportionality known as electrochemical equivalent, I
is current and t is time for which current is passed through the solution.
Electrochemical equivalent: is equal to the mass of a substance deposited when one ampere
current is passed through an electrolyte for one second.
𝑒𝑞𝑢𝑖𝑣𝑎𝑙𝑒𝑛𝑡 𝑚𝑎𝑠𝑠 𝑎𝑡𝑜𝑚𝑖𝑐 𝑚𝑎𝑠𝑠
Z= = ,
𝐹 𝑛𝐹
𝑎𝑡𝑜𝑚𝑖𝑐 𝑚𝑎𝑠𝑠
Equivalent mass = 𝑛𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝑒𝑙𝑒𝑐𝑡𝑟𝑜𝑛𝑠 𝑔𝑎𝑖𝑛𝑒𝑑 𝑜𝑟 𝑙𝑜𝑠𝑡
F (Faraday is the unit of charge).
One Faraday is the amount of charge carried by one mole of electrons. Therefore,
1F = 1.6 × 10-19 × 6.022 × 1023
1F = 96487C (1F = 96500C)
II. Second law: the amounts of different substances liberated by the same quantity of electricity
passing through the electrolytic solution are proportional their equivalent masses or electrochemical
equivalents. Or
The ratio of masses of different substances liberated by the same quantity of electricity passing
through the electrolytic solution is equal to the ratio of their equivalent masses or electrochemical
equivalents.
𝑚1 𝐸1 𝑚1 𝑍1
= or =
𝑚2 𝐸2 𝑚2 𝑍2
Products of electrolysis: products of electrolysis depend on
i. Nature of material being electrolysed and the type of electrodes being used.
ii. Different oxidizing and reducing species present in the electrolytic cell
iii. Concentration of electrolytes.
Some of the electrochemical processes although feasible, are so slow kinetically that at lower
voltages these do not seem to take place and extra potential (over potential) has to be applied,
which makes such process more difficult to occur.
At cathode, the reaction with higher reduction potential takes place and at anode with lower
reduction potential.
Products of electrolysis:
1) Molten NaCl
At cathode: Na+ (aq) + 1e- → Na (s)
Contact: S L Badhotra. Ph: 8826513667, 80108-64649
ABC Academy 11
At anode: Cl- (aq) → ½ Cl2 (g) + e-
2) Aq NaCl
-
At cathode: H2O (l) + 1e- → ½ H2 (g) + OH (aq)
Or H+ (aq) + 1e- → ½ H2 (g)
At anode: Cl- (aq) → ½ Cl2 (g) + e- (due to over potential).
3) Dilute H2SO4
At cathode: H2O (l) + 1e- → ½ H2 (g) + OH- (aq)
Or H+ (aq) + 1e- → ½ H2 (g)
At anode: 2H2O → O2 (g) + 4H+ (aq) + 4e-
4) Concentration H2SO4
At cathode: H2O (l) + 1e- → ½ H2 (g) + OH- (aq)
+
Or H (aq) + 1e- → ½ H2 (g)
At anode: 2SO42- (aq) → S2O82- (aq) + 2e-
5) Aq AgNO3 with silver electrodes
At cathode: Ag+ (aq) + 1e- → Ag (s)
+
At anode: Ag (s) → Ag (aq) + 1e-
6) Aq AgNO3 with platinum electrodes
+
At cathode: Ag (aq) + 1e- → Ag (s)
At anode: 2H2O → O2 (g) + 4H+ (aq) + 4e-
7) Aq CuCl2 with platinum electrodes
2+
At cathode: Cu (aq) + 2e- → Cu (s)
At anode: Cl- (aq) → ½ Cl2 (g) + 1e- (due to over potential).
Battery/cell: Any battery (actually it may have one or more than one cell connected in series) or
cell that we use as a source of electrical energy is basically galvanic cell where the chemical energy
of the redox reaction is converted into electrical energy.
Primary batteries: The batteries in which the reaction occurs only once and after use over a period
of time battery becomes dead and cannot be reused again. The most familiar example of this type
is the dry cell.
Dry cell: The cell consists of a zinc container that also acts as anode and the cathode is a carbon
(graphite) rod surrounded by powder manganese dioxide and carbon. The space between the
electrodes is filled by a moist paste of ammonium chloride (NH4Cl) and zinc chloride (ZnCl2).
Anode: Zn(s) → Zn2+ + 2e–
Cathode: MnO2 + 𝑁𝐻4+ + e– → MnO(OH) + NH3
The cell has a potential of nearly 1.5 V.
Contact: S L Badhotra. Ph: 8826513667, 80108-64649
ABC Academy 12
Mercury Cell: Suitable for low current devices like hearing aids, watches, etc. consists of zinc –
mercury amalgam as anode and a paste of HgO and carbon as the cathode. The electrolyte is a
paste of KOH and ZnO.
Anode: Zn(Hg) + 2OH– → ZnO(s) + H2O + 2e–
Cathode: HgO + H2O + 2e– → Hg(l) + 2OH–
The overall reaction is
Zn(Hg) + HgO(s) → ZnO(s) + Hg(l)
The cell potential is approximately 1.35 V and remains constant during its life as the overall reaction
does not involve any ion in solution whose concentration can change during its life time. The
disadvantage of mercury cell is its disposal as mercury is poisonous.
Secondary Batteries: A secondary cell after use can be recharged by passing current through it in
the opposite direction so that it can be used again.
The most important secondary cell is the lead storage battery commonly used in automobiles and
invertors. It consists of a lead anode and a grid of lead packed with lead oxide (PbO2) as cathode. A
38% solution of sulphuric acid is used as an electrolyte.
The cell reactions when the battery is in use are:
Anode: Pb (s) + SO4 2– (aq) → PbSO4 (s) + 2e–
Cathode: PbO2 (s) + SO4 2–-(aq) + 4H+ (aq) + 2e– → PbSO4 (s) + 2H2O (I)
The overall cell reaction is:
Pb (s) + PbO2 (s) + 2H2SO4 (aq) → 2PbSO4(s) + 2H2O (I)
On charging the battery, the reaction is reversed and PbSO4(s) on anode and cathode is converted
into Pb and PbO2 respectively.
Another important secondary cell is the Nickel – Cadmium battery which has longer life than the
lead storage battery but more expensive to manufacture.
The overall reaction during discharge is:
Cd (s) + 2Ni(OH)3 (s) → CdO (s) + 2Ni(OH)2 (s) + H2O (I)
Lithium batteries: are latest and widely used in cell phones, laptops, cam-corders, cameras etc.
lithium is the metal with highest reduction potential (about 3.0 V). only 6.94 g of Li is required to
provide 1 mole electrons. Li batteries consist of Li anode, either Li metal itself or Li atoms inserted
into graphite electrode, a metal oxide or metal sulphide cathode that can incorporate Li+ ions and an
electrolyte that contains Li salt, such LiClO4 in an organic solvent. Solid state polymer electrolytes
that can transport Li+ ions have also been used.
When cathode material is MnO2, the electrode reactions are
Anode: Li(s) → Li+ + 1e–
Cathode: MnO2(s) + Li+ + 1e–→ LiMnO2(s)
Fuel cells: are electrochemical cells in which the energy of combustion of fuels like hydrogen,
methane, methanol, etc. is directly into electrical energy.
Contact: S L Badhotra. Ph: 8826513667, 80108-64649
ABC Academy 13
Hydrogen – oxygen fuel cell: One of the most successful fuel cells uses the reaction of hydrogen
with oxygen to form water. In the cell, hydrogen and oxygen are bubbled through porous carbon
electrodes into concentrated aqueous sodium hydroxide solution. Catalysts like finely divided
platinum or palladium metal are incorporated into the electrodes for increasing the rate of electrode
reactions.
Cathode: O2 (g) + 2H2O (I) + 4e– → 4OH– (aq)
Anode: 2H2 (g) + OH– (aq) → 4H2O (I) + 4e–
Overall reaction is:
2H2 (g) + O2 (g) → 2 H2O(I)
The cell runs continuously as long as the reactants are supplied. Fuel cells produce electricity with
an efficiency of about 70% compared to thermal plants whose efficiency is about 40%. Fuel cells
are pollution free.
Corrosion
Corrosion slowly coats the surfaces of metallic objects with oxides or other oxides of the metal. The
rusting of iron, tarnishing of silver, development of green coating on copper and bronze are some of
the examples of corrosion.
Corrosion as an electrochemical process: At a particular spot of an object made of iron,
oxidation takes place and that spot behaves as an anode.
Anode: 2Fe (s) → 2Fe2+ + 4 e– E°(Fe2+/ Fe) = − 0.44 V
Electrons released at anode move through the metal and go to another spot on the metal and
reduce oxygen in presence of H+ (which is believed to be available from H2CO3 formed due to
dissolution of carbon dioxide from air into water). Hydrogen ion in water may also be available due
to dissolution of other acidic oxides from the atmosphere. This spot behaves as cathode:
Cathode: O2 (g) + 4 H+ (aq) + 4 e– → 2 H2O (I) E° H+ / O2 / H2O = 1.23 V
The overall reaction is:
2Fe(s) + O2 (g) + 4H+(aq) → 2Fe2+(aq) + 2 H2O (I) E°(cell) = 1.67 V
The ferrous ions are further oxidized by atmospheric oxygen to ferric ions which come out as rust in
the form of hydrated ferric oxide (Fe2O3.xH2O) and with further production of hydrogen gas.
Atmospheric oxidation: 2Fe2+ (aq) + 2H2O(I) +1/2O2(g) → Fe2O3(s) + 4H+
Prevention of corrosion: is of prime importance. It not only saves money but also helps in
preventing accidents such as a bridge collapse or failure of a key component due to corrosion.
One of the simplest methods of preventing corrosion is to prevent the surface of the metallic object
to come in contact with atmosphere by covering the surface with paint or by some chemicals (e.g.
bisphenol).
Another simple method is to cover the surface by other metals (Sn, Zn, etc.) that are inert or react
to save the object e.g. galvanization or nickel plating.
Galvanization is the process of depositing the layer of zinc over the surface of iron objects by
dipping in molten zinc.
An electrochemical method is to provide a sacrificial electrode of another metal (like Mg, Zn, etc.)
which corrodes itself but saves the object.
Contact: S L Badhotra. Ph: 8826513667, 80108-64649
ABC Academy 14
Contact: S L Badhotra. Ph: 8826513667, 80108-64649
You might also like
- Electrochemistry FinalDocument58 pagesElectrochemistry Finalrp7workNo ratings yet
- Feynman Lectures Simplified 2C: Electromagnetism: in Relativity & in Dense MatterFrom EverandFeynman Lectures Simplified 2C: Electromagnetism: in Relativity & in Dense MatterNo ratings yet
- Electrochemistry Anil HssliveDocument10 pagesElectrochemistry Anil HssliveRanit MukherjeeNo ratings yet
- Electrochemistry: Key ConceptsDocument10 pagesElectrochemistry: Key ConceptsTr Mazhar PunjabiNo ratings yet
- Electronic Structure of Molecules: Diatomic Molecules, Small Molecules, Saturated Hydrocarbons, Conjugated Molecules, Molecules of Biochemical InterestFrom EverandElectronic Structure of Molecules: Diatomic Molecules, Small Molecules, Saturated Hydrocarbons, Conjugated Molecules, Molecules of Biochemical InterestNo ratings yet
- Hsslive Xii CH 2 Electro Chemistry AnilDocument10 pagesHsslive Xii CH 2 Electro Chemistry AnilFathima NithinshaNo ratings yet
- Chap.1 ElectrochemistryDocument93 pagesChap.1 ElectrochemistryAnushkaSinhaNo ratings yet
- ElectrochemistryDocument80 pagesElectrochemistryNitin NishantNo ratings yet
- ELECTROCHEMISTRY REFERENCESDocument80 pagesELECTROCHEMISTRY REFERENCESAshish KumarNo ratings yet
- Electrochemistry and Storage CellsDocument14 pagesElectrochemistry and Storage CellsDivithNo ratings yet
- Chemical Energy Fuel: Dept of Chemistry, ANITSDocument10 pagesChemical Energy Fuel: Dept of Chemistry, ANITS320126512165 VSAICHARANGUPTANo ratings yet
- ElectrochemistryDocument80 pagesElectrochemistrykunalwahNo ratings yet
- CHEMISTRY (XI, XII & Medical) by VIJAY KUMAR (M.Sc. B.Ed.)Document10 pagesCHEMISTRY (XI, XII & Medical) by VIJAY KUMAR (M.Sc. B.Ed.)Vijay KumarNo ratings yet
- Electrochemistry FinalDocument76 pagesElectrochemistry Finalsmudgegaming4989No ratings yet
- Basics of electrochemistryDocument41 pagesBasics of electrochemistryMehul Khimani100% (1)
- Electrode System Notes - FinalDocument13 pagesElectrode System Notes - Finalrockymounesh177No ratings yet
- Electromotiveforcefinal 200727 135128Document15 pagesElectromotiveforcefinal 200727 135128Mohit AgarwalNo ratings yet
- Electrochemistry Cell PotentialDocument19 pagesElectrochemistry Cell PotentialRoxanneNo ratings yet
- Electrochemistry: Standard Reduction PotentialsDocument45 pagesElectrochemistry: Standard Reduction PotentialsDalitso SimonNo ratings yet
- Lecture 09. 28122020 E&MEDocument41 pagesLecture 09. 28122020 E&MEMuhammad TalhaNo ratings yet
- Electrochemical CellsDocument4 pagesElectrochemical CellsPROTHSA K.No ratings yet
- 202004021930364692ranvijay ElectrochemistryDocument11 pages202004021930364692ranvijay ElectrochemistryThantzinNo ratings yet
- ElectrochemistryDocument40 pagesElectrochemistryՏɑʍ ՏɑղԵհօՏհNo ratings yet
- Electrochemistry NotesDocument19 pagesElectrochemistry NotesLinaNo ratings yet
- Electrode ChemDocument17 pagesElectrode Chemapi-372366467% (3)
- B. Electrochemistry 2 1Document37 pagesB. Electrochemistry 2 1Dank CoderNo ratings yet
- Principles of Electrochemistry: Potential & ThermodynamicsDocument13 pagesPrinciples of Electrochemistry: Potential & ThermodynamicsGonzalo AlmeidaNo ratings yet
- ElectrochemistryDocument78 pagesElectrochemistryGM VillaneaNo ratings yet
- ElectrolysisDocument24 pagesElectrolysisSMELLY CATNo ratings yet
- Electrochemical Cell HLDocument36 pagesElectrochemical Cell HLRyan BoukaaNo ratings yet
- Electrochemistry Complete NCERTDocument20 pagesElectrochemistry Complete NCERTNitesh YadavNo ratings yet
- Electrochemical CellDocument30 pagesElectrochemical CellSubhu100% (1)
- 5 - Electrochemistry PDFDocument15 pages5 - Electrochemistry PDFthinkiit100% (1)
- 3 Electro Chemistry 1Document40 pages3 Electro Chemistry 1Kalpana BidhanNo ratings yet
- Electrochemical Cells GuideDocument9 pagesElectrochemical Cells Guideyyy ntNo ratings yet
- Electrochemistry I - Measuring Voltage of Galvanic CellsDocument10 pagesElectrochemistry I - Measuring Voltage of Galvanic CellsWilliam FernandoNo ratings yet
- M1 ElectrochemistryDocument11 pagesM1 ElectrochemistryMalvika RkNo ratings yet
- Skcet Class LectureDocument50 pagesSkcet Class Lecturedineshsilambam2305No ratings yet
- Electrochemistry StudentDocument88 pagesElectrochemistry StudentCtNabihahAmilaMarminNo ratings yet
- Department of Chemical EngineeringDocument12 pagesDepartment of Chemical EngineeringSheikh AliNo ratings yet
- ElectroChemistry Slides PDFDocument44 pagesElectroChemistry Slides PDFHenry OkoyeNo ratings yet
- Redox Systems Guide ElectrochemistryDocument10 pagesRedox Systems Guide ElectrochemistryStephany Solis GuerraNo ratings yet
- Unit 3 ElectrochemistryDocument14 pagesUnit 3 ElectrochemistrySuresh Dasaraddi100% (1)
- Chem101 Ho4Document4 pagesChem101 Ho4cyrusryan21No ratings yet
- Unit 3 ElectrochemistryDocument8 pagesUnit 3 ElectrochemistryYashvee GuptaNo ratings yet
- Electrochemistry Part 3Document13 pagesElectrochemistry Part 3Shofwa AnnisaaNo ratings yet
- Hsslive Xii Chem 3. ElectrochemistryDocument20 pagesHsslive Xii Chem 3. ElectrochemistryHakim AbbasNo ratings yet
- Notes Chem NewDocument17 pagesNotes Chem Newilias1973No ratings yet
- Electrochemistry 1Document17 pagesElectrochemistry 1dwijsaidwhatNo ratings yet
- Galvanic Cell: Cell Consists of Two Half-Cells. in Its Simplest Form, Each Half-CellDocument6 pagesGalvanic Cell: Cell Consists of Two Half-Cells. in Its Simplest Form, Each Half-CellcracasttaNo ratings yet
- Electrochemistry Notes 24-25Document10 pagesElectrochemistry Notes 24-25Mohammed FahadNo ratings yet
- Chap 2Document64 pagesChap 2Swe Zin Zaw MyintNo ratings yet
- Electrochemistry 12Document19 pagesElectrochemistry 12Manas ChhabraNo ratings yet
- Electrochemistry Syllabus: Sengunthar Engineering College Department of ChemistryDocument10 pagesElectrochemistry Syllabus: Sengunthar Engineering College Department of ChemistrydeeparamaniNo ratings yet
- Electro Chemistry SND CorrosionDocument10 pagesElectro Chemistry SND CorrosiondrgviswaNo ratings yet
- Electrochemistry - NotesDocument4 pagesElectrochemistry - Notesn611704No ratings yet
- Chapter Five Introduction To Electroanalytical ChemistryDocument16 pagesChapter Five Introduction To Electroanalytical ChemistryZekarias LibenaNo ratings yet
- SEO-Optimized Electrochemistry and Corrosion Syllabus TitleDocument10 pagesSEO-Optimized Electrochemistry and Corrosion Syllabus TitledeeparamaniNo ratings yet
- 1 Digit Numeric Displays: DimensionDocument1 page1 Digit Numeric Displays: DimensionhenriquezrsNo ratings yet
- Practice Problems For Expressing ConcentrationsDocument4 pagesPractice Problems For Expressing ConcentrationsPamela ElumbaNo ratings yet
- Chapter 1 Solutions (Global Edition) : Prob. 1.1Document10 pagesChapter 1 Solutions (Global Edition) : Prob. 1.1靑山なつきNo ratings yet
- Rbber Exxpansion JointsDocument64 pagesRbber Exxpansion Jointsramon duldulaoNo ratings yet
- Assessment of Fib Bulletin 90 Design Provisions Fo PDFDocument23 pagesAssessment of Fib Bulletin 90 Design Provisions Fo PDFangelaNo ratings yet
- CONTROLLED DRUG DELIVERY SYSTEMSDocument19 pagesCONTROLLED DRUG DELIVERY SYSTEMSPramod ChoudharyNo ratings yet
- RAC by Two Stage Mixing ApproachesDocument16 pagesRAC by Two Stage Mixing ApproachesAnupEkboteNo ratings yet
- Study On The Properties and Applications of Molten Salts: December 2010Document6 pagesStudy On The Properties and Applications of Molten Salts: December 2010Goran OsmakNo ratings yet
- Iso 9516 1 enDocument11 pagesIso 9516 1 enAc AbNo ratings yet
- Civil ItpDocument60 pagesCivil ItpBiswas100% (1)
- Balancing Equations: Practice ProblemsDocument10 pagesBalancing Equations: Practice ProblemsAdeenaNo ratings yet
- PML and RNF4 Remove Misfolded Proteins in Mammalian CellsDocument16 pagesPML and RNF4 Remove Misfolded Proteins in Mammalian CellsAndonis AngelovNo ratings yet
- Amity Institute of Applied Sciences: B Tech Engineering Chemistry CHEM136Document8 pagesAmity Institute of Applied Sciences: B Tech Engineering Chemistry CHEM136gaurav toppoNo ratings yet
- Experiment 7 - Gravimetric Determination of Aluminum As OxinateDocument2 pagesExperiment 7 - Gravimetric Determination of Aluminum As OxinateSavita ChemistryNo ratings yet
- Biotek 1Document23 pagesBiotek 1Ratna AnjarNo ratings yet
- The Thermal Decomposition of Metal: Complexes - XiiDocument7 pagesThe Thermal Decomposition of Metal: Complexes - XiiCrhiiztiian RojjazNo ratings yet
- Dhaka University Microbiology Syllabus 2015-2017Document33 pagesDhaka University Microbiology Syllabus 2015-2017Janus MalikNo ratings yet
- Development of Poly (Vinyl Chloride) /wood Composites. A Literature ReviewDocument11 pagesDevelopment of Poly (Vinyl Chloride) /wood Composites. A Literature ReviewNasir QayyumNo ratings yet
- 1 s2.0 S2090447921003506 MainDocument9 pages1 s2.0 S2090447921003506 MainRAFIQUE UR REHMANNo ratings yet
- Vianord EVO 2 Technical Manual V1.1Document37 pagesVianord EVO 2 Technical Manual V1.1Juan CaceresNo ratings yet
- Transistor Current ComponentsDocument3 pagesTransistor Current ComponentsDiptendu MitraNo ratings yet
- 1.argentometric Titration: Techniques-8665193.html - Id CMO:0002145&MSID B000901FDocument3 pages1.argentometric Titration: Techniques-8665193.html - Id CMO:0002145&MSID B000901Fyonela dorrisNo ratings yet
- Determination of Solute ConcentrationDocument6 pagesDetermination of Solute ConcentrationbhupinderNo ratings yet
- 05 V4+R VRF Service ManualDocument104 pages05 V4+R VRF Service ManualMAQNAARZH COOLTECHNo ratings yet
- Metallurgical SlagDocument27 pagesMetallurgical SlagADITYA RAHMANNo ratings yet
- Extremophilic Microorganisms: Biochemical Adaptation and Biotechnological Application (Review)Document14 pagesExtremophilic Microorganisms: Biochemical Adaptation and Biotechnological Application (Review)Arely PradoNo ratings yet
- Mole Concept Stoichiometry (SUMMARY CHEMISTRY CHAPTER)Document5 pagesMole Concept Stoichiometry (SUMMARY CHEMISTRY CHAPTER)the lillyNo ratings yet
- Series and Patterns Test QuestionsDocument96 pagesSeries and Patterns Test Questionssaroj2058No ratings yet
- FdgsfdcpaeperDocument6 pagesFdgsfdcpaeperzakkyNo ratings yet
- Dr. Ihsan Soayed - IGCSE - Cambridge - 0610 - Biology - ExperimentsDocument30 pagesDr. Ihsan Soayed - IGCSE - Cambridge - 0610 - Biology - ExperimentsAbdullah ZubairNo ratings yet
- A Brief History of Time: From the Big Bang to Black HolesFrom EverandA Brief History of Time: From the Big Bang to Black HolesRating: 4 out of 5 stars4/5 (2193)
- Knocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldFrom EverandKnocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldRating: 3.5 out of 5 stars3.5/5 (64)
- A Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceFrom EverandA Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceRating: 4 out of 5 stars4/5 (51)
- Dark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseFrom EverandDark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseRating: 3.5 out of 5 stars3.5/5 (69)
- The Physics of God: How the Deepest Theories of Science Explain Religion and How the Deepest Truths of Religion Explain ScienceFrom EverandThe Physics of God: How the Deepest Theories of Science Explain Religion and How the Deepest Truths of Religion Explain ScienceRating: 4.5 out of 5 stars4.5/5 (23)
- The Tao of Physics: An Exploration of the Parallels between Modern Physics and Eastern MysticismFrom EverandThe Tao of Physics: An Exploration of the Parallels between Modern Physics and Eastern MysticismRating: 4 out of 5 stars4/5 (500)
- Quantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessFrom EverandQuantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessRating: 4 out of 5 stars4/5 (6)
- Summary and Interpretation of Reality TransurfingFrom EverandSummary and Interpretation of Reality TransurfingRating: 5 out of 5 stars5/5 (5)
- Lost in Math: How Beauty Leads Physics AstrayFrom EverandLost in Math: How Beauty Leads Physics AstrayRating: 4.5 out of 5 stars4.5/5 (125)
- Packing for Mars: The Curious Science of Life in the VoidFrom EverandPacking for Mars: The Curious Science of Life in the VoidRating: 4 out of 5 stars4/5 (1395)
- Black Holes: The Key to Understanding the UniverseFrom EverandBlack Holes: The Key to Understanding the UniverseRating: 4.5 out of 5 stars4.5/5 (13)
- Quantum Physics for Beginners Who Flunked Math And Science: Quantum Mechanics And Physics Made Easy Guide In Plain Simple EnglishFrom EverandQuantum Physics for Beginners Who Flunked Math And Science: Quantum Mechanics And Physics Made Easy Guide In Plain Simple EnglishRating: 4.5 out of 5 stars4.5/5 (18)
- The Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeFrom EverandThe Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeNo ratings yet
- Quantum Physics: What Everyone Needs to KnowFrom EverandQuantum Physics: What Everyone Needs to KnowRating: 4.5 out of 5 stars4.5/5 (48)
- The Holographic Universe: The Revolutionary Theory of RealityFrom EverandThe Holographic Universe: The Revolutionary Theory of RealityRating: 4.5 out of 5 stars4.5/5 (76)
- The Power of Eight: Harnessing the Miraculous Energies of a Small Group to Heal Others, Your Life, and the WorldFrom EverandThe Power of Eight: Harnessing the Miraculous Energies of a Small Group to Heal Others, Your Life, and the WorldRating: 4.5 out of 5 stars4.5/5 (54)
- Bedeviled: A Shadow History of Demons in ScienceFrom EverandBedeviled: A Shadow History of Demons in ScienceRating: 5 out of 5 stars5/5 (5)
- In Search of Schrödinger’s Cat: Quantum Physics and RealityFrom EverandIn Search of Schrödinger’s Cat: Quantum Physics and RealityRating: 4 out of 5 stars4/5 (380)
- The End of Everything: (Astrophysically Speaking)From EverandThe End of Everything: (Astrophysically Speaking)Rating: 4.5 out of 5 stars4.5/5 (155)
- Midnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterFrom EverandMidnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterRating: 4.5 out of 5 stars4.5/5 (409)