Professional Documents
Culture Documents
Interpreting Spectra for Organic Compounds
Uploaded by
Iván SalazarOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Interpreting Spectra for Organic Compounds
Uploaded by
Iván SalazarCopyright:
Available Formats
Interpreting Infrared and Nuclear Magnetic Resonance
Spectra of Simple Organic Compounds for the Beginner
A. M. Ingham and R. C. Henson
University of London Goldsmiths' College, New Cross, London SE14 6NW, England
A great deal of information may be gleaned from an infrared Table 1 shows a typical response in following the flow chart
or nuclear magnetic resonance spectrum by an experienced for this spectrum. The overall conclusion that a student will
chemist. A beginner, however, sees only a piece of paper with come to is that the following groups are present: OH, CH;i, and
a multitude of peaks. How is it possible to “decode” the C—O or C—C. We use infrared in conjunction with other
complicated spectrum? methods of identification. Students are encouraged to make
We have devised flow charts which successfully assist the use of all available information about the compound when
beginner to become proficient. The use of a flow chart is not drawing conclusions.
totally revolutionary but merely attempts to incorporate in Table 2. Flow Chari Responses for NMR Spectrum of p-
a systematic method the approach which might be followed
methoxybenzaldehyde
by an experienced chemist.1
See https://pubs.acs.org/sharingguidelines for options on how to legitimately share published articles.
The charts are designed primarily for the beginner. They Reference peak at 5 = 0 identified
are comparatively simple in that more complex spectra will Molecular formula CgHsOj
Downloaded via UNIV DE GUANAJUATO on November 7, 2019 at 17:03:46 (UTC).
not be encountered by the user. The student can use the chart Number of hydrogens = 8
No peaks removed by D20
to help him or her ask the correct questions and look for var-
From integrated trace calculate number of hydrogens at different chemical
ious features in the spectrum, and then to draw some con-
shf its.
clusion. He or she soon learns a method of “attacking” the Note splitting pattern tor each absorption.
interpretation. The chart will be discarded, or rather a new
chart will be built up in the student’s own mind. Chemical shift Number of hydrogens Splitting
9.8 5 1 singlet
Infrared
7.9 5 2 doublet
When studying infrared spectra, account must be taken not 7.0 5 2 doublet
only of the position of the peaks but also their sizes and shapes. 3.8 5 3 singlet
The flow chart in Figure 1 enables this to be done. Examples
can be selected to develop the student’s experience of inter-
Question
Region Box Answer Inference
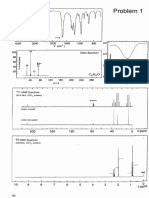
preting these features. The spectrum of propan-2-ol is shown
in Figure 2. For this compound the sepctrum above 2700 cm-1 1 no —OH, —COOH, NH absent
shows a broad intense peak (due to —OH stretch) and a sharp 3 no
peak (due to aliphatic —CH stretch). Up to 6.6 5 4 yes
Students have no difficulty in answering questions 2-4 of 5 1 Consider 9.8 5 peak first. One
the flow chart (Fig. 1). No information can be obtained in hydrogen not in region 8.0-6.6 5
so unlikely to be attached to
answer to question 5, and this is a limitation to which students’
aromatic ring. Possible CHO,
attention may be drawn. —OH or —COOH group (but
—OH and —COOH absent).
6 yes
Henson, R. C., and Stumbles, A. M., School Sci. Rev,, 60, 212, 446
1
9 yes ArH, OH, COOH or CHO group
(1979).
present (but OH, —COOH already
Table 1. Typical Student Response for Spectrum of Liquid eliminated group must be
Containing Carbon, Hydrogen, and Oxygen CHO). Subtracting CHO from
CgHg02 leaves C7H7O.
Question 11 yes Need to go round the loop again.
Region Box Answer Inference There are 2 doublets in the region
8.0-6.6 5 of 2 hyrogen each, so
above 2700 cm-1 1 yes
these are likely to be different
2 yes —OH present
hydrogens attached to a benzene
3 no / \—H or C=C absent ring, i.e., a disubstituted ring.
YJ Yh 6 yes
9 no
A yes aliphatic CH present
5 not known (broad 10 no ArH present.
absorption 8 yes p-disubstitution with different
obscures this substituents. Subtracting C6H4
region) from C7H70 leaves CH30.
2000-1500 cm"1 6 no C=0, aromatic, NH, 11 no
alkene groups absent 6.6-4.5 5 12 no
1500-1100 cm-1 10 yes Above 4.5 5 13 yes Isolated CH3 group. All peaks
11 yes C—O, C—N, or C—C accounted for. This must be
present CH30—.
12 yes CH3 present
Conclusion: Groups found CH30—, CHO
13 yes CH2 or CH3 present
below 900 cm"’1 14 yes
15 no —CH2— absent,
aromatic, alkene or
monochloro C—Cl
possible CHO
704 Journal of Chemical Education
Look at region
above 2700 cm-1
NO
Look at region
2000-1500 cm-1
Look at region
1500-1100 cm-1
Fingerprint region#
NO
Aldehydes: CH stretch in the CHO group
*
near 2720 cm 1
may be doublet
# Fingerprint region
Peaks in this region are not so easy to assign.
Inferences should be confirmed from other sources.
Figure 1. Flow chart for IR interpretation.
Volume 61 Numbers August 1984 705
Figure 3 shows the proton NMR flow chart. This is some-
what longer and more comprehensive than the IR chart, but
the approach is the same. The NMR spectrum is examined
region by region starting at low field (high 5 number). From
the integrated intensities and splitting patterns within each
region the student learns to recognize the patterns which may
be attributed to definite groupings of atoms within the mol-
ecule. The exact values of chemical shifts are not used at this
stage but can be used later in confirming the structure.
The flow chart does not require a detailed knowledge of
chemical shift, but students are encouraged to refer to tables
of chemical shifts to confirm or to modify their overall con-
clusions.
Figure 4 shows the NMR spectrum of p-methoxybenzal-
dehyde and Table 2 responses to the flow chart questions for
this compound.
Using the Flow Charts
Both the IR and NMR charts have been used as part of our
teaching for four years. Compared to the groups who had not
used flow charts, those who use them are
(1) impressively quicker,
(2) more competent,
(3) more confident,
(4) and progress more quickly and more confidently with these
topics than with others of comparable difficulty where flow
charts have not been used.
Flow charts offer the advantages of enjoyment while using
them and the opportunity to work at one’s own pace. The
teacher is free to spend more time dealing with individual M f * J 6 J * > J |
problems. Figure 4. NMR spectrum of C8H802 (no peaks removed by 02O).
Figure 3. Flow chart for NMR interpretation, (continued on following page).
706 Journal of Chemical Education
Volume 61 Number 8 August 1984 707
You might also like
- Introduction To Analytical ChemistryDocument3 pagesIntroduction To Analytical ChemistryChandrashekhar ThotaNo ratings yet
- Understanding proper lubrication from a bearing's perspectiveDocument22 pagesUnderstanding proper lubrication from a bearing's perspectiveengrsurifNo ratings yet
- Advances in Circular Dichroism Spectroscopy and Its ApplicationsDocument4 pagesAdvances in Circular Dichroism Spectroscopy and Its Applicationsshaikh rizwan mustafa100% (2)
- BSC C501 Biophysics Syllabus - B.SCDocument3 pagesBSC C501 Biophysics Syllabus - B.SC16_dev5038No ratings yet
- Methods of Radar Cross-section AnalysisFrom EverandMethods of Radar Cross-section AnalysisJ.W. Jr. CrispinNo ratings yet
- Computational Chemistry Using Modern Electronic Structure Method PDFDocument7 pagesComputational Chemistry Using Modern Electronic Structure Method PDFzan99No ratings yet
- CONICET Digital Nro - Eb5c79d5 C6ee 4312 A855 946ace36e867 ADocument8 pagesCONICET Digital Nro - Eb5c79d5 C6ee 4312 A855 946ace36e867 AAndrea CerveraNo ratings yet
- Kubelka MunkDocument4 pagesKubelka MunkCamilo GonzalezNo ratings yet
- Cigarrete Smoke Rotational SpectrosDocument3 pagesCigarrete Smoke Rotational SpectrosJacqueline FSNo ratings yet
- Ap 3Document17 pagesAp 3Tong WuNo ratings yet
- RMNinterp PDFDocument4 pagesRMNinterp PDFJuan Emanuel VGNo ratings yet
- Madriz 2021Document5 pagesMadriz 2021pepeNo ratings yet
- 2012 - Sanchez de Armas - J Chem PhysDocument8 pages2012 - Sanchez de Armas - J Chem PhysTomas Delgado MontielNo ratings yet
- NMR Analysis of Unknowns: An Introduction To 2D NMR SpectrosDocument2 pagesNMR Analysis of Unknowns: An Introduction To 2D NMR SpectrosTirtha Mukerjee ChemistryNo ratings yet
- Acs Jchemed 8b00589Document5 pagesAcs Jchemed 8b00589john doeNo ratings yet
- The Spectral Theorem For Unitary Operators Based On The S-SpectruDocument20 pagesThe Spectral Theorem For Unitary Operators Based On The S-SpectruSasikalaNo ratings yet
- An Introduction To The Density Matrix Renormalization Group Ansatz in Quantum ChemistryDocument18 pagesAn Introduction To The Density Matrix Renormalization Group Ansatz in Quantum ChemistryAbhijit SamantaNo ratings yet
- Anand JiDocument6 pagesAnand Jibiswajit sarkarNo ratings yet
- Mosher 1992 Organic Chemistry Sixth Edition (Morrison Robert Thornton Boyd Robert Neilson)Document2 pagesMosher 1992 Organic Chemistry Sixth Edition (Morrison Robert Thornton Boyd Robert Neilson)Bhavey DhingraNo ratings yet
- Ab Initio Molecular Orbital Theory Warren HehreDocument8 pagesAb Initio Molecular Orbital Theory Warren HehrepedroNo ratings yet
- Ant Colony 4Document14 pagesAnt Colony 4T. BRENDA CHANDRAWATINo ratings yet
- J.Chem - Educ - Electrochemical Hydrogen Evolution. Sabatier's Principle and The Volcano PlotDocument5 pagesJ.Chem - Educ - Electrochemical Hydrogen Evolution. Sabatier's Principle and The Volcano PlotPianistasenderistaNo ratings yet
- BioMolSim Chapter1 Johansson Et Al 2012-3-27Document26 pagesBioMolSim Chapter1 Johansson Et Al 2012-3-27nadeem akhtarNo ratings yet
- A Technicolour Approach To The Connectome. Nat Rev Neurosci. 2008Document6 pagesA Technicolour Approach To The Connectome. Nat Rev Neurosci. 2008Kathy YuNo ratings yet
- Paper 1Document13 pagesPaper 1abdul rehman khanNo ratings yet
- Ki PHD ThesisDocument4 pagesKi PHD Thesismarialackarlington100% (2)
- Using A Matlab Implemented Algorithm For Uv-Vis SpectralDocument7 pagesUsing A Matlab Implemented Algorithm For Uv-Vis SpectralCesar FrancoNo ratings yet
- TOPIC 15E Spectroscopy and ChromatographyDocument30 pagesTOPIC 15E Spectroscopy and ChromatographyAyshath MaaishaNo ratings yet
- Vitamin-A - quantification-evo-app-note-AN54651Document6 pagesVitamin-A - quantification-evo-app-note-AN54651M Zahid SaleemNo ratings yet
- A Computational Procedure For Determining Energetically Favorable Binding Sites On Biologically Important MacromoleculesDocument9 pagesA Computational Procedure For Determining Energetically Favorable Binding Sites On Biologically Important Macromoleculeskundaningale007No ratings yet
- Materiale Didattico:: Molecular Modelling For Beginners Alan Hinchliffe 2003-Wiley-VCHDocument45 pagesMateriale Didattico:: Molecular Modelling For Beginners Alan Hinchliffe 2003-Wiley-VCHAndreaAbdelLatifNo ratings yet
- Dekhili2020 230314 174922Document10 pagesDekhili2020 230314 174922Janki BhagatNo ratings yet
- PHD 2023-2026 Simulation of Neonicotinoid Properties in Different EnvironmentsDocument2 pagesPHD 2023-2026 Simulation of Neonicotinoid Properties in Different EnvironmentsJayanta L CNo ratings yet
- Keeler-2002-Understanding NMR Spectros PDFDocument210 pagesKeeler-2002-Understanding NMR Spectros PDFlaraNo ratings yet
- Carbon 09 00070Document33 pagesCarbon 09 00070ste123456789No ratings yet
- Langhals 2004Document2 pagesLanghals 2004Pranjal BajpaiNo ratings yet
- Using Degrees of Unsaturation to Solve Organic Structural ProblemsDocument4 pagesUsing Degrees of Unsaturation to Solve Organic Structural ProblemsMARIA FERNANDA PALAFOX PEREZNo ratings yet
- Combining XRD and XRF Analysis in One Rietveldlike FittingDocument7 pagesCombining XRD and XRF Analysis in One Rietveldlike FittingFauzan HzNo ratings yet
- Acs Inorgchem 9b00330Document12 pagesAcs Inorgchem 9b00330Enrique Marín RivasNo ratings yet
- The Structure of 13-Butadiene ClustersDocument20 pagesThe Structure of 13-Butadiene Clusterssof chimisteNo ratings yet
- A Guided-Inquiry Lab For The Analysis of The Balmer Series of The Hydrogen Atomic SpectrumDocument5 pagesA Guided-Inquiry Lab For The Analysis of The Balmer Series of The Hydrogen Atomic SpectrumpiloNo ratings yet
- Lifetime and Spectrally Resolved Characterization of The Photodynamics of Single Fluorophores in Solution Using The Anti-Brownian Electrokinetic TrapDocument8 pagesLifetime and Spectrally Resolved Characterization of The Photodynamics of Single Fluorophores in Solution Using The Anti-Brownian Electrokinetic TrapHui Ling MaNo ratings yet
- Simulating Interference and Diffraction in Instructional LaboratoriesDocument8 pagesSimulating Interference and Diffraction in Instructional Laboratorieskampanart chaichamongkhonNo ratings yet
- 10 1021@acs Jchemed 6b00007 PDFDocument3 pages10 1021@acs Jchemed 6b00007 PDFmiraupriyaNo ratings yet
- Quantum Dots - An Experiment W For Physical or Materials Chemistry PDFDocument3 pagesQuantum Dots - An Experiment W For Physical or Materials Chemistry PDFNicolás GrinbergNo ratings yet
- Behavior Patterns in Multiparametric Dynamical Systems: Lorenz ModelDocument14 pagesBehavior Patterns in Multiparametric Dynamical Systems: Lorenz Modelcesar abraham torrico chavezNo ratings yet
- Near-IR Spectroscopy Determines Epoxy Resin CureDocument11 pagesNear-IR Spectroscopy Determines Epoxy Resin CureRick MortyNo ratings yet
- abinit bulk crystal SHGDocument10 pagesabinit bulk crystal SHGchan yong keatNo ratings yet
- Vibrational Spectroscopy: An Integrated ExperimentDocument21 pagesVibrational Spectroscopy: An Integrated ExperimentJonathanNo ratings yet
- (I.e., The Wavelengths Proximate To: Mass SpectrometersDocument11 pages(I.e., The Wavelengths Proximate To: Mass SpectrometersMiguel Cojal AguilarNo ratings yet
- DEPARTMENT OF PHYSICS - PagenumberDocument53 pagesDEPARTMENT OF PHYSICS - Pagenumberamaranatha2007No ratings yet
- The Grignard Reaction Unraveling A Chemical PuzzleDocument11 pagesThe Grignard Reaction Unraveling A Chemical PuzzleAvinashNo ratings yet
- The Two Electron Molecular Bond Revisited: From Bohr Orbits To Two-Center OrbitalsDocument100 pagesThe Two Electron Molecular Bond Revisited: From Bohr Orbits To Two-Center Orbitalsjorge mario durango petroNo ratings yet
- Physical Methods in Modern Chemical Analysis V3From EverandPhysical Methods in Modern Chemical Analysis V3Theodore KuwanaNo ratings yet
- Olmesartan ReviewDocument46 pagesOlmesartan ReviewDivyesh KuduvaNo ratings yet
- NMR imaging estimates blood flowDocument19 pagesNMR imaging estimates blood flowHoracio Dorantes ReyesNo ratings yet
- Protein-Ligand Interactions and Their Analysis: Babur Z. ChowdhryDocument17 pagesProtein-Ligand Interactions and Their Analysis: Babur Z. ChowdhrycarlosNo ratings yet
- Catalysts 13 00344Document22 pagesCatalysts 13 00344bruno barrosNo ratings yet
- Catalyst Characterization: Characterization TechniquesDocument21 pagesCatalyst Characterization: Characterization Techniquestatianibl6090No ratings yet
- 4.20 TYBSc Chemistry PDFDocument30 pages4.20 TYBSc Chemistry PDFShivam Mishra0% (1)
- BS ChemistryDocument73 pagesBS Chemistryawais gujjarNo ratings yet
- Improving Grief Support for StudentsDocument29 pagesImproving Grief Support for StudentsEng-Abdirahman Haji Mohamed NurNo ratings yet
- IR, NMR, and MS data for organic compoundsDocument50 pagesIR, NMR, and MS data for organic compoundsMuthi KhairunnisaNo ratings yet
- Assignment 1-Spectroscopy - Oct2020-Feb2021Document7 pagesAssignment 1-Spectroscopy - Oct2020-Feb2021Amirah AzlanNo ratings yet
- 400MHz Solid State NMRDocument3 pages400MHz Solid State NMRHafizi Abu BakarNo ratings yet
- How To Set Up An MQMAS On Topspin GuideDocument16 pagesHow To Set Up An MQMAS On Topspin GuideStephen DayNo ratings yet
- NMR Spectroscopy: by Darshan R. Telange, KNCP, Butibori (Nagpur)Document60 pagesNMR Spectroscopy: by Darshan R. Telange, KNCP, Butibori (Nagpur)team engineerNo ratings yet
- MP Pharmaceutics Batch 2017-19 - 09 - 2019Document33 pagesMP Pharmaceutics Batch 2017-19 - 09 - 2019Abhishek RampalNo ratings yet
- Sophisticated Analytical Instrumentation Facility Panjab University ChandigarhDocument2 pagesSophisticated Analytical Instrumentation Facility Panjab University ChandigarhNasirNo ratings yet
- Mechanical Resonance - Theory and ApplicationsDocument10 pagesMechanical Resonance - Theory and ApplicationsEbrahim Abd El HadyNo ratings yet
- 3 - 1H NMRDocument3 pages3 - 1H NMRAbhishek kumarNo ratings yet
- Composition and Chemical Variability of The Oleoresin of Pinus Nigra Ssp. Laricio From CorsicaDocument9 pagesComposition and Chemical Variability of The Oleoresin of Pinus Nigra Ssp. Laricio From CorsicaRifa MuhammadNo ratings yet
- Product Brief 2022Document28 pagesProduct Brief 2022Dyah Ayu DaratikaNo ratings yet
- Dosy BrukerDocument24 pagesDosy BrukeryulliarperezNo ratings yet
- Caracterización Del Agua de MasaDocument9 pagesCaracterización Del Agua de MasaSaenz GutierrzNo ratings yet
- Cooper1979 PDFDocument3 pagesCooper1979 PDFJ Venkat RamanNo ratings yet
- InterpretingNMR r4 Overview1Document1 pageInterpretingNMR r4 Overview1pdaneseNo ratings yet
- Vanadio NMRDocument7 pagesVanadio NMRsergioodin4851No ratings yet
- Shigemi Toda (A) 111117 Action PPT (Rev.4) Tokaicarbon TodaDocument16 pagesShigemi Toda (A) 111117 Action PPT (Rev.4) Tokaicarbon Todachinmoyd1No ratings yet
- New nor-triterpene from Shorea robusta resinDocument2 pagesNew nor-triterpene from Shorea robusta resinSaya AfieraNo ratings yet
- Introduction To 2D-NMR - 10.6 2. Cosy - 10.7: A. Overview of COSY B. How To Read COSY SpectraDocument12 pagesIntroduction To 2D-NMR - 10.6 2. Cosy - 10.7: A. Overview of COSY B. How To Read COSY SpectraraisameongNo ratings yet
- Syllabus MSC Nit WarangalDocument30 pagesSyllabus MSC Nit WarangalAshish ChahalNo ratings yet