0% found this document useful (0 votes)
1K views7 pagesSOP for Inventory Management Process
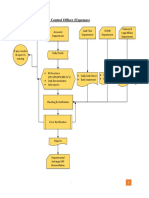
The document provides standard operating procedures for inventory management. It outlines roles and responsibilities for inventory executives, managers, field staff and vendors. Key steps include monitoring stock levels, reordering based on minimum reorder levels, conducting internal transfers between locations, discarding expired or damaged stock, recording receipt of new stock, and ensuring proper storage, stacking and labeling of inventory. The goal is to maintain appropriate stock levels to meet requirements while minimizing waste.
Uploaded by
Akkaldevi KishoreCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
1K views7 pagesSOP for Inventory Management Process
The document provides standard operating procedures for inventory management. It outlines roles and responsibilities for inventory executives, managers, field staff and vendors. Key steps include monitoring stock levels, reordering based on minimum reorder levels, conducting internal transfers between locations, discarding expired or damaged stock, recording receipt of new stock, and ensuring proper storage, stacking and labeling of inventory. The goal is to maintain appropriate stock levels to meet requirements while minimizing waste.
Uploaded by
Akkaldevi KishoreCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd
- Scope
- Inventory management
- Quality Objective
- Purposes
- Abbreviations
- Quality Characteristics
- Responsibilities