Professional Documents
Culture Documents
Report - DLC - 123220 - 11112021 - GeneralLHS 2
Uploaded by
Ambika NairOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Report - DLC - 123220 - 11112021 - GeneralLHS 2
Uploaded by
Ambika NairCopyright:
Available Formats
(123220)
NAME : Mrs. AMBIKA S NAIR COLLECTED TIME : 11-Nov-2021 10:24 am
REPORTED TIME : 11-Nov-2021 3:35 pm
LAB NO. : DLC-123220
PRINTED TIME : 11-Nov-2021 3:37 pm
AGE/SEX : 64 Years / Female
REFERRED BY : SELF
PH NO : 9986091582
PASSPORT NO :
IP/OP :
SRF ID : 539/KTM/2021116085
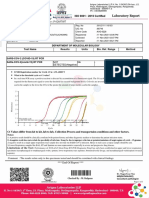
Test Description Results & Unit Reference Value
MOLECULAR BIOLOGY
COVID-19 RT PCR
Specimen Type : Nasopharyngeal/ Oropharyngeal Swab
N Gene : NOT DETECTED
RdRP/ORF 1 Ab : NOT DETECTED
SARS-COV-2 RNA : NOT DETECTED
FINAL REPORT : NEGATIVE
ICMR Reg No : DLDDPLKK
TEST PERFORMED AT: DIANOVA LABORATORIES (A UNIT OF DIANOVA DIAGNOSTICS PVT LTD) , THEVARA BUILDINGS,
NEAR FEDERAL BANK, GANDHINAGAR P.O, KOTTAYAM.
Method: Real-time PCR
This is a real-time RT-PCR test intended for the qualitative detection of nucleic acid from the 2019-nCoV in upper and lower respiratory specimens
(such as nasopharyngeal or oropharyngeal swabs, sputum, lower respiratory tract aspirates, bronchoalveolar lavage, and nasopharyngeal
wash/aspirate or nasal aspirate) collected from individuals who meet 2019-nCoV clinical and/or epidemiological criteria. The assay uses RNA
extracted from clinical samples. Using the RNA extracted, the assay performs the RT-PCR reaction by dividing it into three assays for accurate
detection of SARS-CoV-2. Each assay amplifies N gene and the COVID -19 specific target, RdRP gene, if present; thus it is designed for both the
screening and specific detection of 2019-nCoV.
Interpretation:
Positive results are indicative of the presence of SARS-CoV-2 RNA; clinical correlation with patient history and other diagnostic information is
necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses.
Negative results do not preclude SARS-CoV-2 infection and should not be used as the sole basis for patient management decisions. Negative results
must be combined with clinical observations, patient history, and epidemiological information. A false negative result may occur, if inadequate
number of organisms are present in the specimen due to improper collection, transport or handling. False negative results may also occur if
amplification inhibitors are present in the specimen. A single negative test result, particularly if this is from an upper respiratory tract specimen, does
not exclude infection. Repeat sampling and testing of lower respiratory specimen is strongly recommended in severe or progressive disease. The
repeat specimens may be considered after a gap of 2 – 4 days after the collection of the first specimen for additional testing if required.
Kindly correlate clinically
Biological reference interval pertains to the age and sex of the patient , Given reference interval values pertain to Human samples only.
Any doubt regarding the authenticity of report please contact : Mobile: +919539483092 ,E mail: dianovalabcov@gmail.com
Authorized by:
DR.LEAH THOMAS MBBS.MD Dr.Tiju Chacko MSc. PhD
MICROBIOLOGIST (Molecular Virologist)
This is an electronically authenticated report.
Page 1 of 1
You might also like
- 2023 NGN Ati RN Fundamentals Proctored Exam (Version 1, 2, 3, 4, 5,) With NGN Questions and Verified Answers & RationalesDocument4 pages2023 NGN Ati RN Fundamentals Proctored Exam (Version 1, 2, 3, 4, 5,) With NGN Questions and Verified Answers & Rationalesmarcuskenyatta275No ratings yet
- Recumbant DogDocument2 pagesRecumbant DogDanielle Lynn PeckNo ratings yet
- User HF59R RollscanDocument37 pagesUser HF59R RollscanWiden Carreño50% (2)
- Department of Genetics: Covid-19 RT PCRDocument1 pageDepartment of Genetics: Covid-19 RT PCRAjo Jose100% (1)
- Model of Human OccupationDocument5 pagesModel of Human OccupationPatrick IlaoNo ratings yet
- Molecular Biology: Results & Unit Reference Value Test DescriptionDocument1 pageMolecular Biology: Results & Unit Reference Value Test DescriptionLibu GeorgebabuNo ratings yet
- Report of F - CH Maribel Snigitha CiceroDocument2 pagesReport of F - CH Maribel Snigitha CiceroR.Pearlsis SophiNo ratings yet
- R8929805 Manjunath 101121084851Document1 pageR8929805 Manjunath 101121084851n girish chandra Sri Gowri and Gorav KOUNDINYANo ratings yet
- Sars-Cov2 (Covid-19) Real Time RT PCR TestDocument2 pagesSars-Cov2 (Covid-19) Real Time RT PCR TestGEO MERINNo ratings yet
- R8929806 Rajesh 101121084643Document1 pageR8929806 Rajesh 101121084643n girish chandra Sri Gowri and Gorav KOUNDINYANo ratings yet
- PdfText - 2021-11-11T202745.532Document1 pagePdfText - 2021-11-11T202745.532Murtaza AhmarNo ratings yet
- Patients Profile: Not Detected NegativeDocument2 pagesPatients Profile: Not Detected NegativeELLIE JAMES PLACIONo ratings yet
- Department of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodRïýåz Ahåmèð ShäíkNo ratings yet
- Department of Molecular Biology: Test Name Result Unit Bio. Ref. Range MethodDocument1 pageDepartment of Molecular Biology: Test Name Result Unit Bio. Ref. Range MethodJvenkat VenkatNo ratings yet
- MR - Salahudheenponneth 6e2dDocument1 pageMR - Salahudheenponneth 6e2dZATOONNo ratings yet
- R8929803 Ravi Kumar 101121084613Document1 pageR8929803 Ravi Kumar 101121084613n girish chandra Sri Gowri and Gorav KOUNDINYANo ratings yet
- Mr. MOINUDDIN ANSARI (L5835263) : Test Description Observed Value Biological Reference Range MethodDocument1 pageMr. MOINUDDIN ANSARI (L5835263) : Test Description Observed Value Biological Reference Range MethodMoinuddin AnsariNo ratings yet
- Covid 19 Sars - Cov-2 Rna: Department of Molecular BiologyDocument1 pageCovid 19 Sars - Cov-2 Rna: Department of Molecular BiologyKunal DagaNo ratings yet
- Mr. Janeesh Pal Singh: Test Description Observed Value Biological Reference RangeDocument1 pageMr. Janeesh Pal Singh: Test Description Observed Value Biological Reference RangeJaneesh Pal SinghNo ratings yet
- Arjun BabuDocument1 pageArjun Babubindu mathaiNo ratings yet
- BijuDocument1 pageBijusujith sureshNo ratings yet
- VM211511105 Masterayushgupta719848319509 20211115092858002Document2 pagesVM211511105 Masterayushgupta719848319509 20211115092858002pmirzapure420No ratings yet
- Molecular Biology and Cytogenetics - : Test Name Result UnitsDocument1 pageMolecular Biology and Cytogenetics - : Test Name Result UnitsRock McanarroNo ratings yet
- RT PCR NiteshDocument1 pageRT PCR NiteshNitesh TiwariNo ratings yet
- LabResultTempPDF CJ0304865Document2 pagesLabResultTempPDF CJ0304865Jahred EstebanNo ratings yet
- Real Time Qualitative RT-PCR Detection of 2019-nCOV RNA / Covid-19 RNADocument1 pageReal Time Qualitative RT-PCR Detection of 2019-nCOV RNA / Covid-19 RNAmanwanimuki12No ratings yet
- Torquil Dumuzi Roe JonesDocument1 pageTorquil Dumuzi Roe JonesDillen MaharjanNo ratings yet
- Specimen Nasopharyngeal Swab & Throat Swab Sars-Cov-2 Rna Target (S)Document2 pagesSpecimen Nasopharyngeal Swab & Throat Swab Sars-Cov-2 Rna Target (S)manwanimuki12No ratings yet
- MR - AJINKYA KASAR LabReportNew-4Document2 pagesMR - AJINKYA KASAR LabReportNew-4Ajinkya kasarNo ratings yet
- Department of Molecular Biology: Test Name Result Unit Bio. Ref. Range MethodDocument1 pageDepartment of Molecular Biology: Test Name Result Unit Bio. Ref. Range MethodShivam DumkaNo ratings yet
- LabreportDocument1 pageLabreportPhoto RitNo ratings yet
- NehaDocument1 pageNehaSneha PrakashNo ratings yet
- Department of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodManoj NainNo ratings yet
- Mohit Dhobe-Male24 Years-251014Document2 pagesMohit Dhobe-Male24 Years-251014SunilParjapatiNo ratings yet
- Molecular Biology Sars-Cov-2 (Covid 19) Detection (Qualitative) by Real Time RT PCRDocument1 pageMolecular Biology Sars-Cov-2 (Covid 19) Detection (Qualitative) by Real Time RT PCRShubham KumarNo ratings yet
- Ranjay Prasad Male36 Years 29089Document1 pageRanjay Prasad Male36 Years 29089Shubham KumarNo ratings yet
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Te Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Te Name Result Unit Bio. Ref. Range MethodAnirban MondalNo ratings yet
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodPritam JanaNo ratings yet
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodGirija Prasad SwainNo ratings yet
- Lab ReportDocument2 pagesLab Reportahmadsayeed68No ratings yet
- Report HYGCB 56959 12072021 GeneralLHSDocument2 pagesReport HYGCB 56959 12072021 GeneralLHSjoel pvNo ratings yet
- Some Tests Are Still in Progress. Report Will Be Available Once All Tests Are CompletedDocument3 pagesSome Tests Are Still in Progress. Report Will Be Available Once All Tests Are CompletedDheeman BaruaNo ratings yet
- Patients Profile: Not Detected NegativeDocument2 pagesPatients Profile: Not Detected NegativeELLIE JAMES PLACIONo ratings yet
- LL L LL L L L L 1111111111111111: Molecular Analysis For Qualitative Detection of Sars-Cov-2Document1 pageLL L LL L L L L 1111111111111111: Molecular Analysis For Qualitative Detection of Sars-Cov-2JaiminPatelNo ratings yet
- DGRPOPV137Document2 pagesDGRPOPV137Chandni BhaniramkaNo ratings yet
- Sars-Cov-2 by RT PCR (Qualitative) : Icmr Reg .No. - SanpalagDocument1 pageSars-Cov-2 by RT PCR (Qualitative) : Icmr Reg .No. - SanpalagHaimanti NathNo ratings yet
- MR - Saurabh Vinaykumar Shukla-1Document1 pageMR - Saurabh Vinaykumar Shukla-1KAUSHAL KUMAR SHUKLANo ratings yet
- Reshmi ReportDocument2 pagesReshmi ReportSiddhesh Vishnu GaikwadNo ratings yet
- Test Report: Ms - Ankita Ghosh (29/F)Document2 pagesTest Report: Ms - Ankita Ghosh (29/F)Aeio SavaNo ratings yet
- Mr. AMAL S - 452130670Document1 pageMr. AMAL S - 452130670SAPvioNo ratings yet
- Laboratory Report:: MR - Rohan Dhawa Name: P508466 Patient IDDocument1 pageLaboratory Report:: MR - Rohan Dhawa Name: P508466 Patient IDRohan DhawaNo ratings yet
- Qualitative Test of COVID - 19 RNA by Real Time PCRDocument1 pageQualitative Test of COVID - 19 RNA by Real Time PCRKamlesh LuharNo ratings yet
- Name Date Age/Sex Collection Date Uid No Receive Date Barcodeno Reported On Reg NoDocument1 pageName Date Age/Sex Collection Date Uid No Receive Date Barcodeno Reported On Reg NosiamitonsingNo ratings yet
- Department of Molecular Biology. The Automotive Reasearch India - Covid 19 RT PCR - Pune - Fy2122 Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. The Automotive Reasearch India - Covid 19 RT PCR - Pune - Fy2122 Test Name Result Unit Bio. Ref. Range MethodanilsgaikwadNo ratings yet
- Real Time Qualitative RT-PCR Detection of 2019-nCOV RNA / COVID-19 RNADocument1 pageReal Time Qualitative RT-PCR Detection of 2019-nCOV RNA / COVID-19 RNAHemendra RaiNo ratings yet
- RakeshJain RTPCRDocument2 pagesRakeshJain RTPCRadiNo ratings yet
- ReportDocument2 pagesReportAbn.bjNo ratings yet
- Covid TestDocument1 pageCovid TestConcur ConsultancyNo ratings yet
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodDocument3 pagesDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodARUN KUMAR RNo ratings yet
- Test Report: Reg - No Age/Sex Name::: Collection:: Received Reg - DateDocument1 pageTest Report: Reg - No Age/Sex Name::: Collection:: Received Reg - Datepavan kumarNo ratings yet
- Report of Mr. Ashish VermaDocument1 pageReport of Mr. Ashish Vermaaman vermaNo ratings yet
- Molecular Biology: ICMR Registration No: RPPLPMPDocument2 pagesMolecular Biology: ICMR Registration No: RPPLPMPMaths Tricks solution Mr. G CNo ratings yet
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodAkarshNo ratings yet
- Theoretical Foundations in Nursing - 1ST YearDocument12 pagesTheoretical Foundations in Nursing - 1ST YearcelinnechelsybenitezNo ratings yet
- Titus Lithium Battery: Safety Data SheetDocument5 pagesTitus Lithium Battery: Safety Data SheetKittikun Ap UnitechNo ratings yet
- IGen Book ReviewDocument6 pagesIGen Book ReviewPedro RamosNo ratings yet
- AVTC 5 - 09 - Group 3 - Business PlanDocument9 pagesAVTC 5 - 09 - Group 3 - Business Planda zelNo ratings yet
- SDS-C12 HCA180 Chartek 1709 Part B - EngDocument11 pagesSDS-C12 HCA180 Chartek 1709 Part B - EngMinha Mubarok Ibn MasduqiNo ratings yet
- Cephalosporin UnitDocument11 pagesCephalosporin Unitviper1402No ratings yet
- Ayurveda - The Surgical InstrumentsDocument346 pagesAyurveda - The Surgical InstrumentsSumit GonNo ratings yet
- The Friendzone Research 1Document3 pagesThe Friendzone Research 1api-575715555No ratings yet
- Green Gradient Monotone Minimalist Presentation TemplateDocument20 pagesGreen Gradient Monotone Minimalist Presentation TemplateJo-ann AguirreNo ratings yet
- 02 - Establishing Background Levels - EPA - 1995Document7 pages02 - Establishing Background Levels - EPA - 1995JessikaNo ratings yet
- High Blood Pressure and DiabetesDocument4 pagesHigh Blood Pressure and DiabetesNizam HasniNo ratings yet
- NJSHA NJABA Collaborative Practice GroupDocument4 pagesNJSHA NJABA Collaborative Practice GroupBruno PontesNo ratings yet
- Dry Lab Exercise 4 EndocrineDocument4 pagesDry Lab Exercise 4 Endocrine06Fajrian RidhatunnisaNo ratings yet
- Radcool 200: Safety Data SheetDocument9 pagesRadcool 200: Safety Data SheetHendri PriyantoNo ratings yet
- Activity 3: Worksheet On Developmental Tasks of Being in Grade 11Document2 pagesActivity 3: Worksheet On Developmental Tasks of Being in Grade 11Ian BoneoNo ratings yet
- List of Empanelled Hospitals & Diagnostic Centres DGEHSDocument33 pagesList of Empanelled Hospitals & Diagnostic Centres DGEHSaaryan0% (2)
- Ethical Decision Making in ResearchDocument2 pagesEthical Decision Making in ResearchChandraKurniawanNo ratings yet
- NICU PPT Hypo and HyperthermiaDocument23 pagesNICU PPT Hypo and HyperthermiaelsawzgoodNo ratings yet
- Hospital Managment Unit 3Document62 pagesHospital Managment Unit 3seemantham123No ratings yet
- SJODR 48 555 556 CDocument2 pagesSJODR 48 555 556 Cyasser bedirNo ratings yet
- Ca1 PrelimDocument60 pagesCa1 PrelimAira EspleguiraNo ratings yet
- Walkthrough21 PDFDocument51 pagesWalkthrough21 PDFAnas SantyNo ratings yet
- Bier 2008 Neurop Rehab - newLearningInDementia +++Document33 pagesBier 2008 Neurop Rehab - newLearningInDementia +++Alexa De CatalogneNo ratings yet
- Revised PPAR-Q Aug. 2022Document2 pagesRevised PPAR-Q Aug. 2022RAY LORENZO LAGASCANo ratings yet
- KC - Program Implementation Nepal ExperienceDocument21 pagesKC - Program Implementation Nepal ExperienceDhaka2012No ratings yet
- Parents OrientationDocument2 pagesParents OrientationDonna Marie Paz CasipitNo ratings yet