Professional Documents
Culture Documents
Notes Solubility Parameter
Notes Solubility Parameter
Uploaded by
79Jay Sheth0 ratings0% found this document useful (0 votes)
7 views8 pagesSolubility Parameter
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentSolubility Parameter
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
7 views8 pages Notes Solubility Parameter
Notes Solubility Parameter
Uploaded by
79Jay ShethSolubility Parameter
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 8
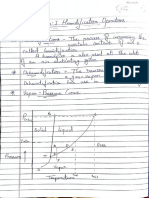
80. Relation of Structure to Chemical Properties
Intermolecular forces are generally less than 10 kcal/mole. In polymers, in the
absence of hydrogen bonding, the intermolecular force is primarily due to
dispersion effects
5.3 POLYMER SOLUBILITY
‘A chemical will be a solvent for another material if the molecules of the two
‘materials are compatible, ie. they can co-exist on the molecular scale and there
is no tendency to separate. This statement does not indicate the speed at which
solution may take place since this will depend on additional considerations such
as the molecular size of the potential solvent and the temperature, Molecules of
‘wo different species will be able to co-exist if the force of attraction between
different molecules is not less than the forces of attraction between two like
molecules of either species. Ifthe average force of attraction between dissimilar
‘molecules A and B is Fag and that between similar molecules of type B Figa and
between similar molecules of type A Faq then for compatibility Fay 2 Fag and
Fg 2 Fan. This is shown schematically in Figure 5.5 (3)
© ©
Figure 5S. Schematic representation of compatible and incompatible systems. (a) Fay 2 Fax
Fay 2 Fn: Mint compasibe (0) Fag 0 Fyy > Fas. Molecules sepurte
If either Fa, or Fyp is greater than Fay the molecules with the highest
intermolecular attraction will tend to congregate or cohere and they will expel the
dissimilar molecule with the result that two phases will be formed. These
conditions are shown in Figure 5.5 (b.
It now becomes necessary to find some suitable measure of the forces of
attraction holding molecules together. If we first consider like molecules we
‘might expect the latent heat of vaporisation L to provide a useful basis, but this
‘would exceed the value of interest to us by an amount corresponding to the
‘mechanical work done on evaporation, an amount approximating to RT where R
is the gas constant and T the absolute temperature. The value of L—RT, the
eneray of vaporisation, will also clearly depend on the molecular size, a
parameter which would not be expected to have large effect on the forces of
attraction between two dissimilar molecules. More relevant will be the terms
(L-RTIM (a measure of the energy of vaporisation per unit weight) and
L-RT
‘MID
Polymer Solubility 81
the energy of vaporisation per molar volume (where M is the molecular weight).
‘This latter term is known as the cohesive energy density and has often been
expressed in units of cal/em*. However, these units are contrary to the SI system.
‘where the units will be expressed asMPa. More commonly encountered in
‘qualitative studies is the square root of the cohesive energy density, which is
known as the solubility parameter and given the symbol 8, Le.
7
(1) Amorphous non-polar polymers and amorphous non-polar solvents.
(2) Crystalline non-polar polymers and amorphous solvents
G) Amorphous non-polar polymers and crystalline solvents.
(4) Amorphous polar polymers and solvents.
(5) Caystalline polar polymers and solvents
(6) Vuleanised rubber and thermosetting plastics.
Amorphous non-polar polymers and amorphous non-polar solvents
It is assumed in these circumstances, by analogy with gravitational and
electrostatic attraction, that Fag will be equal to the geometric mean of Fy, and
Fay. If by arbitrary definition we take Fx, > Fan then
Fan > Faw > Fos
Since we have already seen that solution will only occur when
Fag 2 Fan and Fay 2 Fog
then compatibility between amorphous non-polar polymers and solvents can only
occur when
Fan = Faw = Fon
that is, when polymer and solvent have similar solubility parameters (in practice
within about 2 MP2?)
Tables 5.4 and 5.5 predict that unvulcanised natural rubber (8
dissolved in toluene (8 = 18.2) and in carbon tetrachloride (6 = 17.5) but not in
ethanol (8 = 26.0), all values being in units of MPa”. This is found to be true.
Simi ts found that there isa wide range of solvents for polystyrene in the
solubility parameter range 17.2-19.7 MPa"
6.5) will be
82
Relation of Structure to Chemical Properties
Table $4 Solubility parumcters of polymers
Potymer
pat? (caller)
Polyttafluroehylene 126 62
PoiyehlortriNaoroetiyne 147 2
Polymethysitoxane 49 3
iylene-propyene rubber Ie 1B
Poiisobuylene 16 19
Polyethylene 163 80
Polypropyene 163 80
Polysoprene (aura rubber) 165 81
Polybutadiene m a4
Styrene-buadiene rubber m4 a4
Poly(-buty methacrylate) 169 83
Polyi-hexyt methacrylate) 16 86
Poly-tuy! methacrylate) ns 87
Polybuty atzlate) 130 85
Polyetyl methacrylate) 133 90
Plymetylpheay soxane 183 90
Polyetylacrylate) 187 92
Polyulphide rubber 83-192 90-94
Polystyrene 187 92
Polyehoropene rubber 187-192 92-94
Polymetymetiacylte) 187 92
Polyvinyl acct) 2 oa
Polyvingl chris) wa 95
Bisshhenol A polycarbonate wa 95
Polyvinglidene chloride) 200-250 94-122
Ethyleallose 173-210 85-103
Cellulose ita) 216 oss
Polyethylene terepthalste) 218 wr
‘Acca eine 26 mt
Callulose dace 232 35
Nylon 66 28 136
Polyanethy a-eyanoueryate) 287 ta
Polya tonite 287 fl
ces fabs ie nee aid ra en oe mg
(sion he nee qa np a ge |
Polymer Solubility 83
‘Table 85 Solubility parameters and partial poares(P) of some common solvents
Polymer 8 °
pe" (eaten)?
Neo-pentane 28 63 °
Isobutlene By 87 °
snltexane 149 3 0
Diet ether 1st 74 03
Octane 135 16 °
Methyleyelonexane 159 18 °
Ethyl sobayete I 73 2
Dssopropyl ketone 163 x0 03
Methyl amlaceate 163 80 =
“Turpentine 165 aI °
Cycloerane 167 82 °
E2-Dichioropropane 67 2 =
seerAmyl acetate 169 3 =
Dipentene 13 85 ©
Amy acetate mn 83 on
Meth mba ketone 06 a6 035
Pine ei 6 86 =
Gabon tetrachloride 16 86 °
Methyl mpeopy ketone ns 82 on
Bipendine na 87 a
Xylene 180 88 °
Dimethyl eter 180 Bs =
“Toluene Iz 8S °
By cllooive 182 89 °
Dichloropropane 183 30 =
Mesity oxide 183 50 =
Tsophorone 6 Mt =
tig sutte 88 at on
Benzene 187 92 °
Diacstnealeohot 187 92 2
Chloroform 130 93 om
‘rihloroetlene 190 93 °
‘etrachloroethytene 192 94 oor
‘Telia 4 93 =
arto! 196 96 =
Meth chloride 198 97 =
Methylene debonde 198 97 =
Ethylene dehlode 200 os °
CCyelotexanone| 202 99 =
Ctsaive m2 99 =
Dioxane 202 99 oot
(Carbo disuipide 204 tno °
Accone 204 too 009
Osta 210 103 00
Buyronle 218 103 on
Henao! 218 10 8.06
84 Relation of Structure to Chemical Properties
‘Table 85 Continued
Polymer 3 A
(ealen?)"?
sec-Butana 108 u
Pyridine 109 on
Niothane mt o71
-Butanol 232 ua 010
ycloherano 233 nt 08
Isepropanl 24 us
ss Propuna 242 19 ois
Dimethonmamide 3a Bi 07
‘yarogen cyanide 2a 1B af
‘Acetic aid 283 126 030
260 27 02
mm 53 eS
2s BS =
28 43 039
Phenol 296 006
Giycerat Se 047
Water 3 OR
‘These tables are of greatest use with non-polar materials with values of 8 less
than 19.4MPa!? and where the polymers are amorphous. It will now be
necessary to discuss other systems.
Crystalline non-polar polymers and amorphous solvents
Most polymers of regular structure will crystallise if cooled below a certain
temperature, the melting point T,.. This isin accord with the thermodynamic law
(ee Section 5.3.4) thata process will only occur if there isa decrease in free energy
F in going from one state to another. This free energy change AF is related to the
heat of melting AH, the temperature T and the entropy change AS by the equation
AF =AH-TAS
Since on crystallising the AH is negative, the free energy change AF is also
negative. The process will reverse, ie. melting will occur, only when TAS
becomes equal to AH. Immersing the crystalline polymer in a liquid of similar
solubility parameter at temperatures well below Ty, will do little to change the
balance although the entropy term AS would increase slightly owing to an
increase in molecular disorder on dissolution.
Tere are thus no solvents at room temperature for polyethylene, polypropyl-
ene, poly-4 methylpent-I-ene, polyacetals and polytetrafluoroethylene. However,
as the temperature is raised and approaches Ty the TAS term becomes greater
than AH and appropriate solvents become effective, Swelling will, however,
‘occur in the amorphous zones of the polymer in the presence of solvents of
similar solubility parameter, even at temperatures well below Ty,
Polymer Solubility 85
‘Amorphous non-polar polymers and crystallising solvents
‘This situation is identical to the previous one and occurs for example when
paraffin wax is mixed into rubber above the melting point of the wax. On
cooling, the wax starts to crystallise, some of it forming a bloom on the rubber
surface. Such a bloom assists in protecting a diene rubber from ozone attack,
Amorphous polar polymers and solvents
As already mentioned molecules cohere because of the presence of one or more
‘of four types of forces, namely dispersion, dipole, induction and hydrogen
bonding forces. In the case of aliphatic hydrocarbons the dispersion forces
predominate. Many polymers and solvents, however, ae said to be polar because
they contain dipoles and these can enhance the total intermolecular attraction. It
is generally considered that for solubility in such cases both the solubility
parameter and the degree of polarity should match. This latter quality is usually
expressed in terms of partial polarity which expresses the fraction of total forces
due to the dipole bonds, Some figures for partial polarities of solvents are given
in Table 5.5 but there is a serious lack of quantitative data on polymer partial
polarities. At the present time a comparison of polarities has to be made on a
‘commonsense rather than a quantitative approach,
‘An alternative approach, due to Hansen, isto partition the solubility parameter
into three components, 8,, 8, and 8), due t0 contributions from dispersion
forces, dipole-dipole forces and hydrogen bonding forces respectively. The three
components may be represented by co-ordinates in three-dimensional space.
Partitioning can, at least in theory, be carried out for the solubility parameters of
both solvents and polymers. It may be argued that for each polymer there is a
characteristic radius originating from its point in space which encloses the points
forall liquids that are solvents for the polymer. AS a very rough guide this radius
is about 2 SI units. Whilst data for many solvents have been presented there is
‘only limited information on polymers. Values for some solvents and polymers are
given in Table 5.6.
Table 56 Parone values ofthe solubity paramcter (after Hansen!)
5. &, 8
eavem®)"? | eave)? | Geaem®y!®
Hexane bs o ° Bas
Benzene fet tos 05 Lo ois
Terahydrofuran | az 28 39 932
‘Acetone 138 st a 977
Dionane 930 oo as 0.00
Dimethjformamide = | 82 67 55 ius
Methanol 7a 60. 109 i438
Ethylene glycol { a se | oat | i630
water | soo an 2350
Polythene a o | a | at
Polystyrene 89s os te} on
Polymetiyl methacrylate) @ 40 py || ey
crn
86 Relation of Structure to Chemical Properties
Crystalline polar polymers and solvents
It has already been pointed out that crystalline non-polar polymers do not
rormally have solvents well below their crystalline melting point and the same
‘comment can apply to a large number of polar crystalline polymers.
It has, however, been possible to find solvents for some polar crystalline
polymers such as the nylons, poly(vinyl chloride) and the polycarbonates. This is
because of specific interactions between polymer and solvent that may often
‘occur, for instance by hydrogen bonding.
For example, nylon 66 will dissolve in formic acid, glacial acetic acid, phenol
and cresol, four solvents which not only have similar solubility parameters but
also ate capable of acting as proton donors whilst the carbonyl groups on the
nylon act as proton acceptors (Figure 5.6).
3
:
j ono)
Y
A more interesting example is given with PVC and the polycarbonate of bis-
phenol A, both slightly crystalline polymers. It is noticed here that whilst
methylene dichloride is a good solvent and tetrahydrofuran a poor solvent for the
polycarbonate the reverse is true for PVC yet all four materials have similar
solubility parameters. It would seem that the explanation is that a form of
hydrogen bonding occurs between the polycarbonate and methylene dichloride
and between PVC and tetrahydrofuran (Figure 5.7). In other words there is a
specific interaction between each solvent pair.
i
oh i
cco a-F-a
cH, ‘cH, —CH, a
i
Figure 57
Many studies have been made to try to assess the propensity to hydrogen
bonding of chemical structures, As a result the following broad generalisations
may be made!
(1) Proton donors include highly halogenated compounds such as choloroform
and pentachlorethane. Less halogenated materials are weaker donors.
(2) Polar acceptors include, in roughly descending order of strength, amines,
ethers, ketones, aldehydes and esters (with aromatic materials usually being
‘more powerful than aliphatics).
Polymer Solubility 87
(3) Some materials such as water, alcohols, carboxylic acids and primary and
secondary amines may be able to act simultancously as proton donors and
acceptors. Cellulose and poly(vinyl alcohol) are two polymers which also
function in this way.
(4) A number of solvents such as the hydrocarbons, carbon disulphide and
carbon tetrachloride are quite incapable of forming hydrogen bonds,
Vulcanised rubber and thermosetting plasties
‘The conventionally covalently cross-linked rubbers and plastics cannot dissolve
‘without chemical change. They will, however, swell in solvents of similar
solubility parameter, the degree of swelling decreasing with increase in cross-link
density, The solution properties of the thermoelastomers which are two-phase
‘materials are much more complex, depending on whether or not the rubber phase
‘and the resin domains are dissolved by the solvent.
53.1 Plasticisers
thas been found that the addition of certain liquids (and in rare instances solids)
to a polymer will give a non-tacky product with a lower processing temperature
and which is softer and more flexible than the polymer alone, As an example the
addition of 70 parts of di-iso-octyl phthalate to 100 parts of PVC will convert the
polymer from a hard rigid solid at room temperature to a rubber-like material
Such liquids, which are referred 10 as plasticisers, are simply high boiling
solvents for the polymer. Because itis important that such plasticisers should be
‘non-volatile they have a molecular weight of at least 300. Hence because of their
size they dissolve into the polymer only at a very slow rate at room temperature.
For this reason they are blended (fuxed, gelled) with the polymer at elevated
temperatures or in the presence of volatile solvents (the latter being removed at
some subsequent stage of the operation).
For a material to act as a plasticiser it must conform to the following
requirements:
(1) It should have a molecular weight of at least 300,
(2) It should have a similar solubility parameter to that of the polymer,
(3) Ifthe polymer has any tendency to crystallise, it should be capable of some
specific interaction with the polymer.
(4) It should not be a crystalline solid at the ambient temperature unless it is
capable of specific interaction with the polymer,
‘The solubility parameters of a number of commercial plasticisers ate given in
Table 5.7
From Table 5.7 it will be seen that plasticisers for PVC such as the octyl
phthalates, titolyl phosphate and dioctyl sebacate have solubility parameters
within I egs unit of that of the polymer. Dimethyl phthalate and the paraffinic oils
which are not PVC plasticisers fall outside the range. It will be noted that tritolyl
phosphate which gels the most rapidly with PVC has the closest solubility
parameter tothe polymer. The sebacates which gel more slowly but give products,
which are flexible at lower temperatures than corresponding formulations from
tritoly! phosphate have a lower solubility parameter. It is, however, likely that
any difference in the effects of phthalate, phosphate and sebacate plasticisers in
You might also like
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (843)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5808)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1092)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (346)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- 9 Sanitary LandfillDocument12 pages9 Sanitary Landfill79Jay ShethNo ratings yet
- 2 - Wastewater Treatment TechniqesDocument37 pages2 - Wastewater Treatment Techniqes79Jay Sheth100% (1)
- 18 02 2023 CombinedDocument139 pages18 02 2023 Combined79Jay ShethNo ratings yet
- Wire Enamel - 4Document22 pagesWire Enamel - 479Jay ShethNo ratings yet
- Phospheric Acid Report Phase-2 PDFDocument75 pagesPhospheric Acid Report Phase-2 PDF79Jay ShethNo ratings yet
- Wire Enamel - 3Document18 pagesWire Enamel - 379Jay ShethNo ratings yet
- Notes Glass Transition TemperatureDocument23 pagesNotes Glass Transition Temperature79Jay ShethNo ratings yet
- 1 MTO-2 NotesDocument189 pages1 MTO-2 Notes79Jay ShethNo ratings yet
- Notes Molecular WeightDocument13 pagesNotes Molecular Weight79Jay ShethNo ratings yet
- Notes Addition PolymerizationDocument26 pagesNotes Addition Polymerization79Jay ShethNo ratings yet
- Notes - Thermodynamics of Polymer Solution and Solubility ParameterDocument6 pagesNotes - Thermodynamics of Polymer Solution and Solubility Parameter79Jay ShethNo ratings yet
- Report On Numerical 1Document10 pagesReport On Numerical 179Jay ShethNo ratings yet
- Practical Exam Quiz MarksDocument6 pagesPractical Exam Quiz Marks79Jay ShethNo ratings yet
- Report of NumericalsDocument7 pagesReport of Numericals79Jay ShethNo ratings yet
- 10 Landfill LeachateDocument15 pages10 Landfill Leachate79Jay ShethNo ratings yet
- 11 Radioactive and Nuclear WasteDocument19 pages11 Radioactive and Nuclear Waste79Jay ShethNo ratings yet
- FFO Numericals Report FinalDocument14 pagesFFO Numericals Report Final79Jay ShethNo ratings yet
- Practical Exam Quiz SolutionDocument5 pagesPractical Exam Quiz Solution79Jay ShethNo ratings yet
- 12 IncinerationDocument7 pages12 Incineration79Jay ShethNo ratings yet
- 6 Solid Waste and ClassificationDocument11 pages6 Solid Waste and Classification79Jay ShethNo ratings yet
- 7 Solid Waste Mngt.Document11 pages7 Solid Waste Mngt.79Jay ShethNo ratings yet
- 5 AP Abatement TechniquesDocument16 pages5 AP Abatement Techniques79Jay ShethNo ratings yet