Professional Documents
Culture Documents
Advanced Comperhension Isomersim Que
Uploaded by
Ancient DebrisCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Advanced Comperhension Isomersim Que
Uploaded by
Ancient DebrisCopyright:
Available Formats
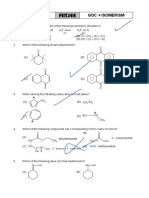
Which will show goometrical i8omcrism '!
(A l CH,Cl-1 = NOH
Q.U ..
Which of the following is/are correct mal.Cbin; ?
---
w
(A) CH,-C-OH and H-C---OCH
w
1
- Mctamers
(B) CH ,-CH,-C =CH and CH,-C =C-cH,
- Position isomers .
H,C)C=NOH
(C) H1C
(C) CH,CH,CH,NH, and CH -ctt-CH
(D) HO-N = N-OH I I J
Which of the following molecules is/are identical with NH,
that represented by - Tautomen;
(D) CH3°"½0H and (CHJ 20
- Functional isomer
Q.15 Which of the following statements arc correct:
(A) Any chiral compound with a single asynnnetric
carbon must have a positive optical rotation if the
compound has the R configuration
HrhCH3
(B) !fa strucrure has no plane of symmetry it is chiral
(A) H3CbHOH (C) All asymmetric carbons are stereocentres.
(D)Alcobol and ether are functional isomers
CH3 Q.16 The isomerism observed in alkanes is :
H3CffiH (A) metamerism
(B) chain isomerism
(C) H><;.XOH
(C) position isomerism
OH
(D) geometrical isomerism
Which conformation of n-Butane has both plane of
symmetry and centre of symmetry absent ? Co.prebemioa # I (Q. No. 17 to 19)
(A) fully eclipsed (B) Gauche Taatoaerisa :
(C) Partially eclipsed (D)Anti Structural Isomers that undergo rapid interconversions
Which of the following statements is/are not correct? and exist in dynamic equilibrium are known as
(A) Metamerism belongs to the category of structural Tautomers and relationship between them is known as
1somensm Tautomerism. Tautomers generally have different
(B) Tautomeric structures are the resonating structures functional groups.
of a molecule At equilibrium more stable tautomer is present in higher
(C) Keto form is always more stable than the enol form amount but the ratio remains same until and unless
(D) Geometrical isomerism is shown only by alkenes change is made externally. Tautomerism actually arises
due to rapid oscillation of an atom between two
3 Which of the following statements is/are correct?
(A) A meso compound has chiral centres but exhibits polyvalent atoms in a molecule.
no optical activity
(B) A meso compound has no chiral centres and thus fl OH
I
are optically inactive. TH 2- CH -..;;--- CH, = CH
(C) A meso compound has molecules which are
superirnposable on their mirror images even though H Keto form enol form
they contain chiral centres
Above is an example of Keto-enol tautomerism.
(D) A meso compound is optically inactive because
the rotation caused by any molecule is cancelled Condition for this type of keto enol tautomerism is
by an equal and opposite rotation caused by presence of a-H. Amount of enol at equilibrium is
another molecule that is the mirror image of the known as enolic content It is more if enol is more stable
first and less ifketo is more stable.
49
You might also like
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- A - 2 (Isomerism, Reaction Mechantism) - Question PaperDocument14 pagesA - 2 (Isomerism, Reaction Mechantism) - Question PaperSachin DedhiaNo ratings yet
- ISI R: Organic ChemistryDocument28 pagesISI R: Organic Chemistrysarvesh goyalNo ratings yet
- Isomerism NotesDocument16 pagesIsomerism Notessurendra chowdary Makineni100% (1)
- OMFDocument16 pagesOMFDuy AnhNo ratings yet
- Latihan Soal - 1Document3 pagesLatihan Soal - 1Jonny PNo ratings yet
- CHEM201 Ch5 StereochemistryDocument38 pagesCHEM201 Ch5 Stereochemistryderinergin3No ratings yet
- Tutorial 1Document2 pagesTutorial 1Raunak KumarNo ratings yet
- Compendium On Problems in Physical-Organic ChemistryDocument27 pagesCompendium On Problems in Physical-Organic ChemistryHaryokoe buzzNo ratings yet
- Organic Chemistry IIDocument7 pagesOrganic Chemistry IIRoberto SIlvaNo ratings yet
- Isomerism DPP - With Solution PDFDocument20 pagesIsomerism DPP - With Solution PDFPiyush Agarwal50% (2)
- Organic Chemistry: TopicsDocument8 pagesOrganic Chemistry: TopicsHritwick MannaNo ratings yet
- ATP Star 2Document28 pagesATP Star 2Gowri ShankarNo ratings yet
- Test1 350 PracticeV1Document4 pagesTest1 350 PracticeV1Jennifer KelleyNo ratings yet
- Goc Stereo PDFDocument32 pagesGoc Stereo PDFDeepak GargNo ratings yet
- Tutorial 1Document5 pagesTutorial 1leftphoneforeverNo ratings yet
- 4.4, 4.5 TEST MS 1.: Observation StructureDocument4 pages4.4, 4.5 TEST MS 1.: Observation Structure3estherNo ratings yet
- 0optical Isomerism - QuizDocument3 pages0optical Isomerism - QuizSanjay Mani Tripathi50% (2)
- Stereo ChemistryDocument45 pagesStereo ChemistryFafa AlunksNo ratings yet
- Unit2 Chemical Bonding QnsDocument5 pagesUnit2 Chemical Bonding QnsArunsethupatNo ratings yet
- Stereochemistry TutorialDocument8 pagesStereochemistry TutorialfezilephathiswaNo ratings yet
- StereoDocument15 pagesStereoPrasann KatiyarNo ratings yet
- Adobe Scan 18 Mar 2021Document7 pagesAdobe Scan 18 Mar 2021KanishkaNo ratings yet
- Goal 9-1Document8 pagesGoal 9-1Koleti KoletiNo ratings yet
- Eg Ethylene (IUPAC: Ethene), CDocument16 pagesEg Ethylene (IUPAC: Ethene), CVansh JindalNo ratings yet
- OC - Stereoisomerism - E - CSDocument36 pagesOC - Stereoisomerism - E - CSHARSHIT 12ANo ratings yet
- Isomerism ReviewDocument7 pagesIsomerism Reviewayesha sheikhNo ratings yet
- CB 5Document3 pagesCB 5badisa booksNo ratings yet
- Ocd PP Special On Taut Omer Is MDocument3 pagesOcd PP Special On Taut Omer Is MKartik YadavNo ratings yet
- Set of 50 Obj in General Organic Chemistry by S.K.sinha HTTP://WWW - Openchemistry.inDocument6 pagesSet of 50 Obj in General Organic Chemistry by S.K.sinha HTTP://WWW - Openchemistry.inmyiitchemistry50% (4)
- Reaction MechanismDocument13 pagesReaction MechanismMUHAMMAD YASEENNo ratings yet
- Nuclear Magnetic Resonance (NMR) SpectrosDocument39 pagesNuclear Magnetic Resonance (NMR) SpectrosknkoradiyaNo ratings yet
- MSC Chemistry Paper-IX Unit-1Document20 pagesMSC Chemistry Paper-IX Unit-1NIKHILESH SUTRADHARNo ratings yet
- DPP # 13 Time: 30 Min.: 1. Column - I Column - IIDocument3 pagesDPP # 13 Time: 30 Min.: 1. Column - I Column - IIArjun SabnisNo ratings yet
- CML101 Major Exam-QuestionsDocument4 pagesCML101 Major Exam-QuestionsAditya AdityaNo ratings yet
- For 1-4 ClassDocument45 pagesFor 1-4 ClassNanditha ANo ratings yet
- Sheet - 02 - General Organic ChemistryDocument74 pagesSheet - 02 - General Organic ChemistrykeshavNo ratings yet
- Dasar Dasar StereokimiaDocument27 pagesDasar Dasar StereokimiaYayuk LestariNo ratings yet
- Goc FinalsheetDocument49 pagesGoc FinalsheetKartik KambleNo ratings yet
- NMR - Recall From Last WeekDocument29 pagesNMR - Recall From Last Weekteam engineerNo ratings yet
- GOC Sheet PDFDocument55 pagesGOC Sheet PDFAayush KharbandaNo ratings yet
- Chemistry QuestionDocument5 pagesChemistry QuestionPrithviraj GhoshNo ratings yet
- Work Book (Phase - IV) : SubjectiveDocument21 pagesWork Book (Phase - IV) : SubjectiveAshwani Kumar SinghNo ratings yet
- 2010 PDFDocument8 pages2010 PDFprakhar vishwakarmaNo ratings yet
- SP StereochemistryDocument63 pagesSP StereochemistryMadhumitha KatreddyNo ratings yet
- 15 Isomerism Formula Sheets QuizrrDocument6 pages15 Isomerism Formula Sheets QuizrrInertiaNo ratings yet
- End Sem2016 PDFDocument2 pagesEnd Sem2016 PDFRutul JainNo ratings yet
- 15 Isomerism Formula Sheets QuizrrDocument5 pages15 Isomerism Formula Sheets QuizrrDaniel GtsadkanNo ratings yet
- Tutorial 2. Stereochemistry PDFDocument5 pagesTutorial 2. Stereochemistry PDFMatthew PokNo ratings yet
- CH1O3 Questions PDFDocument52 pagesCH1O3 Questions PDFPrince T MashandaNo ratings yet
- Exam 1 Review 1 KOTDocument47 pagesExam 1 Review 1 KOTNoranisza MahmudNo ratings yet
- IUPAC Practice SheetDocument4 pagesIUPAC Practice Sheetdizzy057765No ratings yet
- Class Test - Structural IsomersDocument3 pagesClass Test - Structural IsomersAlex SamNo ratings yet
- Lecture 9-11 Term 3, AY 22-23Document42 pagesLecture 9-11 Term 3, AY 22-23LujainNo ratings yet
- As Mhy FG 9 SVGy 5 M Qo KC2 oDocument52 pagesAs Mhy FG 9 SVGy 5 M Qo KC2 osingharyendra175No ratings yet
- Cpet 2021Document12 pagesCpet 2021Roshan SahuNo ratings yet
- Week 6B - IsomerismDocument4 pagesWeek 6B - IsomerismMaxNo ratings yet
- Test Bank For Organic Chemistry 3rd Edition Janice SmithDocument15 pagesTest Bank For Organic Chemistry 3rd Edition Janice Smithjacobjasminekpk5No ratings yet
- Lec 2 - MCDocument22 pagesLec 2 - MCDivyam JainNo ratings yet
- Advanced Phy Level5Document1 pageAdvanced Phy Level5Ancient DebrisNo ratings yet
- Adavnced Isomerism QueDocument1 pageAdavnced Isomerism QueAncient DebrisNo ratings yet
- Isomerism Advanced QueDocument1 pageIsomerism Advanced QueAncient DebrisNo ratings yet
- Isomerism Que1Document1 pageIsomerism Que1Ancient DebrisNo ratings yet
- Isomerism Que Advanced 1Document1 pageIsomerism Que Advanced 1Ancient DebrisNo ratings yet
- 9 Coordination Compounds PDFDocument7 pages9 Coordination Compounds PDFShatabdi MahendraNo ratings yet
- Organic Chemistry Student Booklet 2Document15 pagesOrganic Chemistry Student Booklet 2Mirjeta ZymeriNo ratings yet
- Organic Chemistry: Catalan (Cat) Carlos - Jaime@uab - Cat Carles Jaime CardielDocument6 pagesOrganic Chemistry: Catalan (Cat) Carlos - Jaime@uab - Cat Carles Jaime CardielNeils ArenósNo ratings yet
- Cocomp. (Repaired)Document20 pagesCocomp. (Repaired)Vinod ChaudhariNo ratings yet
- 9701 w12 QP 23Document12 pages9701 w12 QP 23poliuytrewqNo ratings yet
- 2017 FY13CE Chemistry Detailed SolutionDocument32 pages2017 FY13CE Chemistry Detailed Solutionlaukkeas50% (2)
- Chemistry - Haloalkanes and HaloarenesDocument34 pagesChemistry - Haloalkanes and HaloarenesKaran VermaNo ratings yet
- SCH 1201 - Inorganic Chemistry Ii - Transition Metal ChemistryDocument305 pagesSCH 1201 - Inorganic Chemistry Ii - Transition Metal ChemistrysanelisofuturemoyoNo ratings yet
- Drug Design and IsomarismDocument25 pagesDrug Design and IsomarismModern Institutes IndoreNo ratings yet
- 9.coordination CompoundsDocument46 pages9.coordination CompoundsSeenu MNo ratings yet
- Module 4a - Unsaturated Hydrocarbons (Alkenes and Alkynes)Document12 pagesModule 4a - Unsaturated Hydrocarbons (Alkenes and Alkynes)meyaNo ratings yet
- CH.1.05 Part 2 IsomersmDocument21 pagesCH.1.05 Part 2 IsomersmgamexooobolgNo ratings yet
- Carbohydrate NomenclatureDocument8 pagesCarbohydrate NomenclatureAwais Naseer100% (3)
- Goc + IsomerismDocument5 pagesGoc + IsomerismRohail HussainNo ratings yet
- Problemas ICHO28 A ICHO24Document40 pagesProblemas ICHO28 A ICHO24Leonardo FagundesNo ratings yet
- Test Bank For Organic Chemistry 9Th Edition Wade Test Bank For Organic Chemistry 9Th Edition WadeDocument23 pagesTest Bank For Organic Chemistry 9Th Edition Wade Test Bank For Organic Chemistry 9Th Edition WadeAshleyNo ratings yet
- Org ChemDocument2 pagesOrg ChemSabbyAlonzoNo ratings yet
- Organic Chemistry For Aspiring Pharmacists PART 1Document95 pagesOrganic Chemistry For Aspiring Pharmacists PART 1Jessica GutierrezNo ratings yet
- 978-1305780170 Organic ChemistryDocument9 pages978-1305780170 Organic ChemistryReccebacaNo ratings yet
- AQA A Level Chemistry Student Book 1Document378 pagesAQA A Level Chemistry Student Book 1Mahdiya75% (8)
- Organic Chemistry 7th Edition Carey Test BankDocument15 pagesOrganic Chemistry 7th Edition Carey Test Bankmanuelhuynhv2zNo ratings yet
- Unit-4 Stereochemistry-FinalDocument100 pagesUnit-4 Stereochemistry-FinalJATIN DALMIANo ratings yet
- 14Document18 pages14phanisaiNo ratings yet
- Test Bank For Organic Chemistry 3rd Edition by KleinDocument36 pagesTest Bank For Organic Chemistry 3rd Edition by Kleinuprightdrollerjit3t100% (30)
- CHEM-E2130 Polymer Properties: Steve SpoljaricDocument91 pagesCHEM-E2130 Polymer Properties: Steve SpoljaricSivasankar JeyabaskaranNo ratings yet
- Organic Chemistry - 103 - Lecture 1Document41 pagesOrganic Chemistry - 103 - Lecture 1Abdus SubhanNo ratings yet
- Test1 350 v4 AnswersDocument5 pagesTest1 350 v4 AnswersCARLOS ALBERTO OSORIO MARTINEZNo ratings yet
- Organic Chemistry 7th Edition Carey Test BankDocument15 pagesOrganic Chemistry 7th Edition Carey Test BankEunice Cheslock100% (30)