Professional Documents
Culture Documents
SCR 066
Uploaded by
Linh ĐỗOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
SCR 066
Uploaded by
Linh ĐỗCopyright:
Available Formats
Quantitative Alkaline Phosphatase ES Characterization Kit
CATALOG NUMBER: SCR066
LOT NUMBER:
QUANTITY: 1 Kit
DESCRIPTION: Embryonic stem (ES) cells are pluripotent cells derived from the inner cell mass (ICM) of
preimplantation embryos and are capable of unlimited, undifferentiated proliferation in vitro under
appropriate cell culture conditions. Undifferentiated embryonic stem cells are characterized by high
expression levels of stage-specific embryonic antigens (SSEA-1, 3 and 4), tumor rejection antigens
(TRA-1-60 and TRA-1-81), Oct-4 and alkaline phosphatase. These undifferentiated markers are
downregulated upon spontaneous or induced differentiation.
Alkaline phosphatase (AP) is a hydrolase enzyme responsible for dephosphorylating molecules
such as nucleotides, proteins, and alkaloids under alkaline conditions. The enzyme is present within
all tissues of the body but is elevated in cells of the liver, kidney, bone, placenta, embryo and under
specific disease states. Under alkaline conditions (pH>10), AP can catalyze the hydrolysis of p-
nitrophenylphosphate (p-NPP) into phosphate and p-nitrophenol, a yellow colored by-product of the
catalytic reaction. The amount of p-nitrophenol produced is proportional to the amount of alkaline
phosphatase present within the reaction. The amount of AP can thus be reliably quantified by
reading the amount of p-nitrophenol amassed after the catalytic reaction at 405 nm on a
spectrophotometer.
Alkaline Phosphatase (pH>10)
p-nitrophenylphosphate phosphate + p-nitrophenol (yellow)
Millipore’s Quantitative Alkaline Phosphatase ES Characterization Kit allows researchers to easily
quantify the amount of alkaline phosphatase present within their embryonic stem cell cultures using
a convenient 96-well colorimetric enzymatic assay. This kit can be used in conjunction with
Millipore’s Alkaline Phosphatase Detection kit (Cat. No. SCR004) to fully characterize the levels of
alkaline phosphatase present within an embryonic stem cell culture.
KIT COMPONENTS: Sufficient for 100 Reactions
p-NPP Substrate Concentrate (50X): (Part No. ES008-200UL) 200 μL
p-NPP Buffer: (Part No. ES011-100ML) 100 mL
Reaction Stop Solution: (Part No. CS200653) 5 mL
1X Wash Solution: (Part No. CS200652) 2 X 125 mL
Recombinant Alkaline Phosphatase Standard: (Part No. CS200654) 1 μg (10 μg/mL)
STORAGE/
HANDLING: All reagents are to be stored at 2° to 8°C until expiration date. p-NPP substrate concentrate and p-
NPP buffer are light sensitive and should not be exposed to light. Reaction stop solution contains
1N sodium hydroxide and is corrosive. Avoid skin contact and inhalation.
©2002 - 2010: Millipore Corporation. All rights reserved. No part of these works may be reproduced in any form without permission in writing.
USA & Canada • Phone: +1(800) 437-7500 • Fax: +1 (951) 676-9209
www.millipore.com
10-15-10/SCR066/NA/VC/LR/MM
Important Note: During shipment, small volumes of product will occasionally become entrapped in the seal of the product vial.
For products with volumes of 200 µL or less, we recommend gently tapping the vial on a hard surface or briefly
centrifuging the vial in a table-top centrifuge to dislodge any liquid in the container’s cap.
ASSAY PROTOCOL: We recommend that an 8-point standard curve for alkaline phosphatase (including blank) be
included in triplicate for every assay run. Total reaction volume = 100 μL. Recombinant alkaline
phophatase is provided in the kit.
1. Determine the total reaction volumes required to carry out the experiments in replicates and to
include the 8-point standard curve with blank in replicates. For example if there are 2
experimental conditions, the calculations are as follows:
2 experimental conditions X 3 replicates = 6 total reactions
8 reactions (i.e. 8-point standard curve) x 3 replicates = 24 total reactions
30 total reactions X 100 μL reaction volume = 3000 μL total volume
Assuming ~ 20% volume overage = 3000 + 600 = 3600 μL or ~ 3.6 mL total reaction
volume
2. Prepare a 2X p-NPP Substrate solution such that the total volume is one half of the total
reaction volume. Thus using the example provided above, make up 1.8 mL of a 2X p-NPP
Substrate solution by diluting 72 μL of 50X p-NPP Substrate Concentrate with 1728 μL of p-
NPP Buffer. Set aside.
Note: 2X p-NPP Substrate Solution should always be prepared fresh before each assay
run and should be protected from light.
3. Aspirate the culture medium from the ES cells or other cells to be analyzed.
4. Wash the cells with 10 mL 1X PBS to remove any residual serum.
5. Detach the ES cells to be analyzed with Accutase (Cat. No. SCR005).
6. Collect the cells in a 15 mL conical tube.
7. Centrifuge the tube at 2000 rpm for 5 minutes at room temperature to pellet the cells.
8. Remove the supernatant and resuspend the cell pellet in 2 mL 1X Wash Solution.
9. Count the number of cells using a hemacytometer.
10. Aliquot the desired number of cells (we recommend 20,000 cells per reaction) to three
Eppendorf tubes (for replicates). Do this for each of the cell sample to be analyzed.
11. Spin at 2000 rpm for 5 minutes at room temperature to pellet the cells.
12. Remove the supernatant and resuspend each cell pellet in 50 μL p-NPP Buffer. Transfer each
of the three cell suspensions to one well of the 96-well assay plate for a total of three wells.
For example, using the example provided above regading 2 experimental samples, use A4, A5,
A6 for 1 sample and B4, B5, B6 for the 2nd experimental sample. At this time, the enzymatic
reaction has not been initiated. Enzymatic reaction is initiated when 50 μL 2X p-NPP Substrate
Solution is added to the cell suspension (i.e. Step 14).
Note: It is important to include experimental replicates. Avoid plating cell suspension onto the
first 3 columns (A1-3 through H1-3) of the 96-well plate which should be reserved to run the 8-
point alkaline phosphatase standard curve.
©2002 - 2010: Millipore Corporation. All rights reserved. No part of these works may be reproduced in any form without permission in writing.
USA & Canada • Phone: +1(800) 437-7500 • Fax: +1 (951) 676-9209
www.millipore.com
10-15-10/SCR066/NA/VC/LR/MM
13. Prepare an 8-point standard curve in triplicates. This is done by serial dilutions of the AP
standard in triplicates, corresponding to 5, 2.5, 1.25, 0.625, 0.3125, 0.15625 and 0.078125 ng
AP and a blank control, as described below:
1 2 3 4 5 6 7 8 9 10 11
12
5 ng A
2.5 ng B
1.25 ng C
0.625 ng D
0.3125 ng E
0.15625 ng F
0.078125 ng G
Blank (0 ng) H
• Using a multichannel micropipette, aliquot 50 μL of p-NPP Buffer into the first three
columns (A1-3 through H1-3) of the 96-well plate. Total number = 24 wells.
• Aliquot an additional 49 μL of p-NPP Buffer into the first three wells (A1, A2, A3). Total
volume in these 3 wells after the addition is 100 μL.
• Using an appropriate micropipette and tips, transfer 1 μL of AP standard into each of the
three wells, A1, A2, A3, containing the 100 μL p-NPP Buffer. Use a fresh tip for each
transfer.
• Using a multichannel micropipette set to 50 μL, carefully mix the contents of wells A1, A2,
A3 and then transfer 50 μL to the next row of wells (B1, B2, B3).
• Repeat the mixing and transfer 50 μL to the next row of wells (C1, C2, C3). Repeat the
mixing and transfer of 50 μL, going consecutively down each row to obtain in total seven
serial dilutions of the AP standard, in triplicates, corresponding to 5, 2.5, 1.25, 0.625,
0.3125, 0.15625 and 0.078125 ng AP per well.
• Discard the excess 50 μL solution from wells G1, G2, G3.
• Wells H1, H2, and H3 should contain only the p-NPP Buffer and serve as a blank or 0 ng
AP control.
14. Using a multichannel pipette, aliquot 50 μL of the 2X p-NPP Substrate Solution into each of the
wells containing the 8-point standard curve (A1-3 to H1-3) and also to each of the unknown
samples (i.e. A4, A5, A6 and B4, B5, B6) so that the final reaction volume is 100 μL. Addition
of 2X p-NPP Substrate Solution initaties the enzymatic reaction.
15. Incubate for 20 minutes at room temperature in the dark.
Note: The reaction is time dependent. Do not leave the reaction for longer than 20 minutes
(i.e. one hour),as all the samples (regardless of the amount of input AP) will then be read at the
maximum absorbance.
16. Using a multichannel pipette, add 50 μL of Reaction Stop Solution to each reaction. Total
volume per well = 150 μL.
17. Read the absorbance at 405 nm.
©2002 - 2010: Millipore Corporation. All rights reserved. No part of these works may be reproduced in any form without permission in writing.
USA & Canada • Phone: +1(800) 437-7500 • Fax: +1 (951) 676-9209
www.millipore.com
10-15-10/SCR066/NA/VC/LR/MM
DATA:
A. B.
Figure 1. Standard curve of recombinant alkaline phosphatase (A). Optimal number of cells to use per reaction was determined by
assaying discrete numbers of undifferentiated murine embryonic stem cells (Cat. No. SCR012) cultured in media containing 15%
serum and 1000 units/mL LIF/ESGRO (B).
Figure 2. Mouse ES cells (Cat No. SCR012) were cultured in media containing
15% serum and 1000 units/mL LIF/ESGRO for 2 days. After the second day,
differentiation was induced by the removal of LIF and β-mercaptoethanol (BME)
from the culture media for 9 days. Removal of LIF and BME correlates with a
decrease in alkaline phosphatase levels using SCR066. For each time point,
approximately, 20,000 cells were assayed in triplicate as per protocol instructions.
FOR RESEARCH USE ONLY; NOT FOR USE IN DIAGNOSTIC
PROCEDURES. NOT FOR HUMAN OR ANIMAL CONSUMPTION
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other
purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or
animals.
©2002 - 2010: Millipore Corporation. All rights reserved. No part of these works may be reproduced in any form without permission in writing.
USA & Canada • Phone: +1(800) 437-7500 • Fax: +1 (951) 676-9209
www.millipore.com
10-15-10/SCR066/NA/VC/LR/MM
You might also like
- PCR Lab ProtocolDocument5 pagesPCR Lab Protocolhk8atema1lNo ratings yet
- Tank FailuresDocument65 pagesTank FailuresAnonymous GfPSYi4n100% (1)
- Elite Dangerous Trader's Bible Voice AttackDocument6 pagesElite Dangerous Trader's Bible Voice AttackAaron Orion Chavez100% (1)
- Causes of Overpressurization: Understanding Common ScenariosDocument1 pageCauses of Overpressurization: Understanding Common ScenariosjargiaNo ratings yet
- Hydrocarbon Processing August 2018Document149 pagesHydrocarbon Processing August 2018VS2712100% (1)
- Atoms Bonding GuideDocument729 pagesAtoms Bonding Guide1553No ratings yet
- Chemical Agents COP 2016Document56 pagesChemical Agents COP 2016James McloughlinNo ratings yet
- Liquid ring vacuum pumps in compact designDocument7 pagesLiquid ring vacuum pumps in compact designDarwin Barra TorresNo ratings yet
- Decrease volume or increase pressure rightDocument29 pagesDecrease volume or increase pressure righthertianaNo ratings yet
- Biochemical and Pharmacological Roles of Adenosylmethionine and the Central Nervous System: Proceedings of an International Round Table on Adenosylmethionine and the Central Nervous System, Naples, Italy, May 1978From EverandBiochemical and Pharmacological Roles of Adenosylmethionine and the Central Nervous System: Proceedings of an International Round Table on Adenosylmethionine and the Central Nervous System, Naples, Italy, May 1978Vincenzo ZappiaNo ratings yet
- Users Guide For Phosphoprotein ReagentsDocument9 pagesUsers Guide For Phosphoprotein Reagentsthumita kumiNo ratings yet
- ALP Assay Kit Detects Liver and Bone BiomarkerDocument9 pagesALP Assay Kit Detects Liver and Bone BiomarkerLinh ĐỗNo ratings yet
- Protocol - 4684 096 KDocument6 pagesProtocol - 4684 096 KbnkjayaNo ratings yet
- TFS-Assets_BID_manuals_EEA002-manual-metabolic-finalDocument11 pagesTFS-Assets_BID_manuals_EEA002-manual-metabolic-finaldewiNo ratings yet
- 8-Isoprostane ELISA Kit: Package InsertDocument10 pages8-Isoprostane ELISA Kit: Package InsertJunaidy TahirNo ratings yet
- Calcium Assay Kit: Technical Data SheetDocument5 pagesCalcium Assay Kit: Technical Data SheetSujith KuttanNo ratings yet
- Alkaline Phosphatase Assay Kit InstructionsDocument3 pagesAlkaline Phosphatase Assay Kit InstructionsLinh ĐỗNo ratings yet
- MET-5125-pyruvate-assay-kit-colorimetricDocument8 pagesMET-5125-pyruvate-assay-kit-colorimetricyousrazeidan1979No ratings yet
- Estimation of Serum Alkaline Phosphatase ActivityDocument23 pagesEstimation of Serum Alkaline Phosphatase Activityshalaka.agarwalNo ratings yet
- ELISA Protocol: Buffer Preparation For ELISADocument2 pagesELISA Protocol: Buffer Preparation For ELISAJamesNo ratings yet
- West Blot Analyof Endog ProtsDocument2 pagesWest Blot Analyof Endog ProtsOvais ZargarNo ratings yet
- DNA Xpress ReagentDocument7 pagesDNA Xpress Reagenthar2dikNo ratings yet
- ALP Crestline PDFDocument2 pagesALP Crestline PDFJashmyn JagonapNo ratings yet
- Chloramphenicol ELISA Kit GuideDocument8 pagesChloramphenicol ELISA Kit Guidephyo waiNo ratings yet
- Mak461pis MKDocument5 pagesMak461pis MKAusteridad LopezNo ratings yet
- Max Efficiency Dh5 Competent Cells: 9:2, 12. 8:1, 1. Supe44 Λ-Thi-1 Gyra96 Rela1Document4 pagesMax Efficiency Dh5 Competent Cells: 9:2, 12. 8:1, 1. Supe44 Λ-Thi-1 Gyra96 Rela1Khoo Ying WeiNo ratings yet
- IP PROTOCOL FOR PROTEIN PURIFICATIONDocument6 pagesIP PROTOCOL FOR PROTEIN PURIFICATIONhalfangle100% (1)
- RNAextrc 2Document5 pagesRNAextrc 2Cecelia Dot DotNo ratings yet
- c311 ALP2 enDocument3 pagesc311 ALP2 endr. SheryarOrakzaiNo ratings yet
- CST - Protocol - Cell Signaling Technology #7976Document2 pagesCST - Protocol - Cell Signaling Technology #7976huripNo ratings yet
- AOAC99110Document1 pageAOAC99110Jhon Alexander Rincon ReinaNo ratings yet
- His-tagged Protein Purification using Ni-NTA and FPLCDocument3 pagesHis-tagged Protein Purification using Ni-NTA and FPLCRaja GunalanNo ratings yet
- Western Blot Lab PresentationDocument24 pagesWestern Blot Lab Presentationmahesh kumarNo ratings yet
- Technical Bulletin: Aspartate Aminotransferase (AST) Activity Assay KitDocument4 pagesTechnical Bulletin: Aspartate Aminotransferase (AST) Activity Assay KitbudiNo ratings yet
- 2-D Protein PreparationDocument2 pages2-D Protein PreparationAsvene SharmaNo ratings yet
- 30 413 Vidas AFP: Summary and Explanation PrincipleDocument8 pages30 413 Vidas AFP: Summary and Explanation PrincipleHafizh Widi CahyonoNo ratings yet
- Elisa KitDocument5 pagesElisa KitYani Nyiik0% (1)
- MRNAseq RibosomeProfiling ProtocolDocument13 pagesMRNAseq RibosomeProfiling ProtocolエリタノアンソネーNo ratings yet
- Corporation - 2010 - Wizard Genomic DNA Purification Kit Quick Protocol, FB022Document4 pagesCorporation - 2010 - Wizard Genomic DNA Purification Kit Quick Protocol, FB022pyangNo ratings yet
- Ankur Bhaumik 18895 UB201 Molecula Biology Lab ReportsDocument19 pagesAnkur Bhaumik 18895 UB201 Molecula Biology Lab ReportsAnkur BhaumikNo ratings yet
- Alp PDFDocument1 pageAlp PDFDhita Ariefta PNo ratings yet
- 60 Total RNA Extraction From Yeast Cells UFIR EM TDocument4 pages60 Total RNA Extraction From Yeast Cells UFIR EM TDivina Mercedes Fernando0% (1)
- Micro Array Practical MethodDocument12 pagesMicro Array Practical MethodpriyaaNo ratings yet
- Ihc ProtocolDocument8 pagesIhc ProtocolAanchal PuriNo ratings yet
- Immunoprecipitation: Immunoprecipitation Protocol Reagents NeededDocument3 pagesImmunoprecipitation: Immunoprecipitation Protocol Reagents NeededchamsoNo ratings yet
- Summary and Explanation Principle: Biomérieux Sa English - 1Document8 pagesSummary and Explanation Principle: Biomérieux Sa English - 1johnpaulcfuraggananNo ratings yet
- Western Immunoblotting ProtocolDocument8 pagesWestern Immunoblotting ProtocolJKayckeNo ratings yet
- AMD Viral RNA ExtractionDocument10 pagesAMD Viral RNA ExtractionAkibulHassanSazalNo ratings yet
- CAMPYNETDocument11 pagesCAMPYNETnadbucukNo ratings yet
- Fixation For Fish 2march2013Document3 pagesFixation For Fish 2march2013maria dulceNo ratings yet
- ECAMDocument1 pageECAMLalit LekhwaniNo ratings yet
- Report 3 and 4Document11 pagesReport 3 and 4tinanassar2004No ratings yet
- TEV Protease Purification Cell StockDocument3 pagesTEV Protease Purification Cell StockGraciela RosasNo ratings yet
- Pnas 1402658111 SappDocument25 pagesPnas 1402658111 SappAmín MoraNo ratings yet
- Molecular Biology - Amity University RajasthanDocument13 pagesMolecular Biology - Amity University Rajasthanabash_u1No ratings yet
- ATAC Seq ProtocolDocument8 pagesATAC Seq ProtocoldearbhupiNo ratings yet
- Artifact For Laboratory TechniquesDocument17 pagesArtifact For Laboratory Techniquesapi-694576377No ratings yet
- ProtocolDocument16 pagesProtocolTran Tu NguyenNo ratings yet
- Bacteriophage λ plant genomic library constructionDocument32 pagesBacteriophage λ plant genomic library constructioncynthiadajannaNo ratings yet
- SOP009 DNA& RNA Extraction From Fecal SamplesDocument6 pagesSOP009 DNA& RNA Extraction From Fecal SamplesHayleyNo ratings yet
- Allele-In-One Mouse Tail Purification-PCR KitDocument2 pagesAllele-In-One Mouse Tail Purification-PCR KitAlleleBiotechNo ratings yet
- ACPP enDocument4 pagesACPP enSyahdie FahledieNo ratings yet
- PDS R09 Aqp4e Eng.007.a EvDocument13 pagesPDS R09 Aqp4e Eng.007.a Evyousrazeidan1979No ratings yet
- Sulfenylated Protein Cell-Based Detection Kit: Item No. 600320Document11 pagesSulfenylated Protein Cell-Based Detection Kit: Item No. 600320Sergio SandovalNo ratings yet
- PCR 16S rRNA gene from bacteria coloniesDocument5 pagesPCR 16S rRNA gene from bacteria coloniesUgnė KasperavičiūtėNo ratings yet
- Allele-In-One Human Blood Purification-PCR KitDocument2 pagesAllele-In-One Human Blood Purification-PCR KitAlleleBiotechNo ratings yet
- Sigma AldrichDocument4 pagesSigma AldrichidownloadbooksforstuNo ratings yet
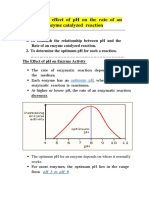
- The Effect of pH on Enzyme ActivityDocument12 pagesThe Effect of pH on Enzyme ActivityAb AbNo ratings yet
- Ch4 Lecture SlidesDocument34 pagesCh4 Lecture SlidesJad AwadNo ratings yet
- The Vegetable Oil Industry in Pakistan: August 2005Document3 pagesThe Vegetable Oil Industry in Pakistan: August 2005Nguyễn ChinhNo ratings yet
- General Instructions: Pie Matlab Assessment For Chemical EngineersDocument6 pagesGeneral Instructions: Pie Matlab Assessment For Chemical EngineersJulia RodriguezNo ratings yet
- Product FD724 EDocument1 pageProduct FD724 ERodrigo DiazNo ratings yet
- Prevent From Flower and Fruit DroppingDocument2 pagesPrevent From Flower and Fruit DroppingRachel BiostimulantsNo ratings yet
- New Expt 8 Spectroscopy Lab Chlorophyll With SpectroVisDocument12 pagesNew Expt 8 Spectroscopy Lab Chlorophyll With SpectroVisAdam Bryant PoonawalaNo ratings yet
- ASME Boiler and Pressure Vessel Code Week: February 2023Document11 pagesASME Boiler and Pressure Vessel Code Week: February 2023Mark GuevarraNo ratings yet
- Vapor Pressure of Petroleum Products (Mini Method) : Standard Test Method ForDocument9 pagesVapor Pressure of Petroleum Products (Mini Method) : Standard Test Method ForahmedNo ratings yet
- Model Paper 11th 2013 OnwardDocument93 pagesModel Paper 11th 2013 OnwardImran RashidNo ratings yet
- CH 5 - ProblemsDocument8 pagesCH 5 - ProblemsKhris Griffis89% (18)
- Material Safety Data Sheet: 1 Identification of The Substance / Preparation and of The CompanyDocument4 pagesMaterial Safety Data Sheet: 1 Identification of The Substance / Preparation and of The CompanyVirni SagitaNo ratings yet
- FTP PDFDocument1 pageFTP PDFPhilip Adom YeboahNo ratings yet
- Depressurization Rev1Document18 pagesDepressurization Rev1Muntaser YousifNo ratings yet
- Renata Industrial TrainingDocument10 pagesRenata Industrial TrainingShonar KellaNo ratings yet
- Innovative New Facade Material UsedDocument2 pagesInnovative New Facade Material UsedRafia GulzarNo ratings yet
- Calculate Flare Radiation IsoplethsDocument5 pagesCalculate Flare Radiation IsoplethsPanosMitsopoulosNo ratings yet
- Chapter 2: EmulsionsDocument52 pagesChapter 2: EmulsionsEliasNo ratings yet
- Universal cleaner for ultrasonic bathsDocument1 pageUniversal cleaner for ultrasonic bathsJuan ShunaNo ratings yet
- Tribology International: Rongping Yun, Peter Filip, Yafei LuDocument10 pagesTribology International: Rongping Yun, Peter Filip, Yafei LuRajiv Gandhi Rajiv GandhiNo ratings yet
- SC2889 PDFDocument15 pagesSC2889 PDFA MahmoodNo ratings yet
- The Progress of Nanomaterials For Carbon Dioxide Capture Via The Adsorption ProcessDocument23 pagesThe Progress of Nanomaterials For Carbon Dioxide Capture Via The Adsorption ProcessVONo ratings yet
- Traceability and Uncertainty: Cobas C 501 / C 502 / C 503 / C 303 / C 311/ C 701/ C 702 - C.F.A.S. ProteinsDocument3 pagesTraceability and Uncertainty: Cobas C 501 / C 502 / C 503 / C 303 / C 311/ C 701/ C 702 - C.F.A.S. ProteinsLaura Puentes JiménezNo ratings yet