Professional Documents
Culture Documents
Spectroscopy Foundations Questions
Uploaded by
Blohsh KeenenCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Spectroscopy Foundations Questions
Uploaded by
Blohsh KeenenCopyright:
Available Formats
1. How many joules of energy is available in one mole of photons, each with a 540nm wavelength?
2. The following questions are based on a measurement of 142Kj/mol for some photons.
a. How many Kilojoules of energy is found in each photon?
b. What is the frequency of each photon?
c. What region of the EMR spectrum do these photons belong to?
3. What is the wavelength n meters of photons with the following energies? In what region of the
EMR spectrum does each appear?
a. 4.55 x 10-2 KJ/mol
b. 4.81 x 10 4KJ/mol
4. What is the energy of the following photons in Joules/mol
a. V = 3.79 x 1011 s-1
b. V = 5.45 x 104 s-1
c. Lamda = 4.11 x 10-5 m
5. The laser light used in compact disc players has lambda=780 nm. In what region of the
electromagnetic spectrum does this light appear? What is the energy of this light in kilojoules
per mol?
6. When scotch tape is unrolled in a vacuum (but not in air), photons with a range of frequencies
around v = 2.9 x 1018 s-1 are emitted in nano second bursts.
a. What is the wavelength in meters of these photons?
b. What is the energy of one such photon?
c. What type of EMR are these?
7. What energy is associated with the following
a. 928 MHz
b. 740 nm
c. 2 x 108 Hz
8. Differentiate between electronic, rotational and vibrational energy levels.
9. State the approximate wavelength ranges of the following :
a. X-rays
b. Infrared radiation
c. Radio frequencies
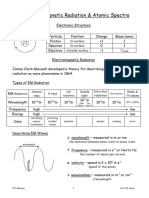
10. Label the diagram below according to energy levels of molecular orbitals. List all possible
electronic transitions for the molecular orbitals.
You might also like
- Chapter 8 The Quantum Mechanical AtomDocument18 pagesChapter 8 The Quantum Mechanical Atomsadaf yousafzaiNo ratings yet
- PS1 QaDocument4 pagesPS1 Qaogulcan kılkıyrukNo ratings yet
- Quantum Model of the Atom ChapterDocument4 pagesQuantum Model of the Atom ChapterPhạm Thái HàNo ratings yet
- AMS Tutorials 1Document2 pagesAMS Tutorials 1Mohan SinghNo ratings yet
- PBL (Chapter 2) 2022Document3 pagesPBL (Chapter 2) 2022MUHAMMAD ALIF BIN MOHD ROAIMNo ratings yet
- Homework week 3 physics electromagnetic spectrum (40 ptsDocument4 pagesHomework week 3 physics electromagnetic spectrum (40 ptsYuni AntariNo ratings yet
- Chemistry and Chemical Reactivity 9th Edition Kotz Test Bank DownloadDocument15 pagesChemistry and Chemical Reactivity 9th Edition Kotz Test Bank DownloadTodd Dean100% (23)
- Chem 442 - Chapter OneDocument26 pagesChem 442 - Chapter OneArindam DasNo ratings yet
- 001 Light Concepts & CalculationsDocument3 pages001 Light Concepts & CalculationslordkratosNo ratings yet
- Chemistry Test Enock The Village PreacherDocument2 pagesChemistry Test Enock The Village Preacherfmukuka12No ratings yet
- UV-VIS and Microwave Spectroscopy QuestionsDocument2 pagesUV-VIS and Microwave Spectroscopy QuestionsAshish KumarNo ratings yet
- Infrared SpectrosDocument50 pagesInfrared SpectrosmohammedabubakrNo ratings yet
- Chapter 7 AnswerDocument10 pagesChapter 7 AnswerAlaa TelfahNo ratings yet
- UV-VIS Spectrophotometry: A Brief Background To SpectrophotometryDocument15 pagesUV-VIS Spectrophotometry: A Brief Background To SpectrophotometrySiddh BhattNo ratings yet
- Chemistry: UNIT 1 - Module 1 Atomic StructureDocument36 pagesChemistry: UNIT 1 - Module 1 Atomic StructureAntonique HeadmanNo ratings yet
- ThesisDocument54 pagesThesisJeon ManobanNo ratings yet
- Electromagnetic Radiation Quantitative Relationships ws-16Document3 pagesElectromagnetic Radiation Quantitative Relationships ws-16api-368121935No ratings yet
- GenPhysics2 - Q2 Module 2Document19 pagesGenPhysics2 - Q2 Module 2Jasmin SorianoNo ratings yet
- Questions For The Er Ruler: Foundations of SpectrosDocument1 pageQuestions For The Er Ruler: Foundations of SpectrosPhiPhiNo ratings yet
- UNIVERSITY OF PETROLEUM & ENERGY STUDIES LASER PHYSICS NOTESDocument3 pagesUNIVERSITY OF PETROLEUM & ENERGY STUDIES LASER PHYSICS NOTESmanindra kumarNo ratings yet
- Worksheet 9 - Electromagnetic Radiation and The Bohr AtomDocument4 pagesWorksheet 9 - Electromagnetic Radiation and The Bohr Atomvisa1032No ratings yet
- Atoms and LightDocument4 pagesAtoms and LightIdil WarsameNo ratings yet
- CH 8Document12 pagesCH 8terasaini77No ratings yet
- 6.1 LThe Wave Nature of LightDocument10 pages6.1 LThe Wave Nature of LightShallimar AlcarionNo ratings yet
- ProblemsDocument2 pagesProblemsBest BuddyNo ratings yet
- Analitik Geometri 14 TestDocument2 pagesAnalitik Geometri 14 TestSems KrksNo ratings yet
- Homework Week 2bDocument6 pagesHomework Week 2bAlejandria SolisNo ratings yet
- Chapter 7 In-Class Problems Light Wavelength Frequency Calculations Hydrogen SpectraDocument1 pageChapter 7 In-Class Problems Light Wavelength Frequency Calculations Hydrogen SpectraBrittnay MarieNo ratings yet
- Reg QDocument78 pagesReg Qruppal42No ratings yet
- eBook+Introduction+to+Spectroscopy Pavis 32 121Document7 pageseBook+Introduction+to+Spectroscopy Pavis 32 121devi dwi rustaminingrumNo ratings yet
- Physics 1C Spring 2011: Final Exam Preparation 1Document31 pagesPhysics 1C Spring 2011: Final Exam Preparation 1Shela PotoNo ratings yet
- Numericals 11Document73 pagesNumericals 11vamshicloud14No ratings yet
- Chemistry Unit 3 ReviewDocument8 pagesChemistry Unit 3 Reviewimp3rial1100% (1)
- Serie TD 3 AnglaisDocument3 pagesSerie TD 3 AnglaisjosbenachenhouNo ratings yet
- Exam1 04Document7 pagesExam1 04Rodney SalazarNo ratings yet
- Assignment #1: Intro To Light: Information: The Electromagnetic SpectrumDocument20 pagesAssignment #1: Intro To Light: Information: The Electromagnetic SpectrumBrianss AyangssNo ratings yet
- Question Bank For Unit 3Document3 pagesQuestion Bank For Unit 3PRATHAMESH MOHILENo ratings yet
- Electromagnetic Spectrum Practice Problems Che11APDocument1 pageElectromagnetic Spectrum Practice Problems Che11APJerixan PortesNo ratings yet
- Tutorial Sheet-Unit I & IIDocument6 pagesTutorial Sheet-Unit I & IISachin DevarakondaNo ratings yet
- Chang Chemistry - Assessment Chapter 7Document10 pagesChang Chemistry - Assessment Chapter 7haha_le12No ratings yet
- Worksheet 10 PDFDocument4 pagesWorksheet 10 PDFJosh FlorentinoNo ratings yet
- Atomic StructureDocument16 pagesAtomic StructureLuna eukharisNo ratings yet
- Tut-sheet-1-PHL120-13 With Final Answers PDFDocument3 pagesTut-sheet-1-PHL120-13 With Final Answers PDFjgrgpt33No ratings yet
- Chapter 1, 2 and 3Document79 pagesChapter 1, 2 and 3ashenafiNo ratings yet
- Phy Assignment NITDocument1 pagePhy Assignment NITKartik GvrNo ratings yet
- Atomic StructureDocument6 pagesAtomic Structureramanji1021No ratings yet
- 1.1 AH CfE Chemistry NotesDocument7 pages1.1 AH CfE Chemistry Noteskira.zavyalova24No ratings yet
- Chapter-5 - Periodicity & Electronic Structure of AtomsDocument14 pagesChapter-5 - Periodicity & Electronic Structure of AtomsV KumarNo ratings yet
- SR. Physics Important QsDocument8 pagesSR. Physics Important QsKeerthanaNo ratings yet
- Tugas Pertemuan Ke 1 Sampai Ke 3 Dikumpulkan Pada Pertemuan Ke 4Document2 pagesTugas Pertemuan Ke 1 Sampai Ke 3 Dikumpulkan Pada Pertemuan Ke 4RamadhantiNo ratings yet
- PS2Document2 pagesPS2Truong CaiNo ratings yet
- Electromagnetic SpectrumDocument30 pagesElectromagnetic SpectrumyeloalmasenNo ratings yet
- Uv Visible Spectroscopy : By:-Sarika Singh. Fy M-Pharmacy (QAT)Document32 pagesUv Visible Spectroscopy : By:-Sarika Singh. Fy M-Pharmacy (QAT)aditya mhatre100% (2)
- CH 2 - Electromagnetic Radiation PDFDocument13 pagesCH 2 - Electromagnetic Radiation PDFNidhi PatelNo ratings yet
- 2023 Exam PrepDocument3 pages2023 Exam PrepBaochen TianNo ratings yet
- Optics: International Series of Monographs in Natural PhilosophyFrom EverandOptics: International Series of Monographs in Natural PhilosophyRating: 3 out of 5 stars3/5 (1)
- Fundamentals of Microwave Electronics: International Series of Monographs on Electronics and InstrumentationFrom EverandFundamentals of Microwave Electronics: International Series of Monographs on Electronics and InstrumentationNo ratings yet
- Modern Vibrational Spectroscopy and Micro-Spectroscopy: Theory, Instrumentation and Biomedical ApplicationsFrom EverandModern Vibrational Spectroscopy and Micro-Spectroscopy: Theory, Instrumentation and Biomedical ApplicationsNo ratings yet
- Rhetorical Analysis Reading 2, Cardiovascular Health in Rural HaitiDocument8 pagesRhetorical Analysis Reading 2, Cardiovascular Health in Rural HaitiBlohsh KeenenNo ratings yet
- Anatomy and Physiology (2018)Document16 pagesAnatomy and Physiology (2018)Blohsh KeenenNo ratings yet
- Samples in Spectroscopy and Beer Lamberts LawDocument1 pageSamples in Spectroscopy and Beer Lamberts LawBlohsh KeenenNo ratings yet
- Slides - Unit 1 - The Concept of CivilisationDocument47 pagesSlides - Unit 1 - The Concept of CivilisationKiller VNo ratings yet
- Creating Your TestDocument1 pageCreating Your TestBlohsh KeenenNo ratings yet
- CAPE Env. Science 2005 U2 P1Document12 pagesCAPE Env. Science 2005 U2 P1Ric Flamboyant FraserNo ratings yet
- 5th Grade Synonyms 4Document2 pages5th Grade Synonyms 4Blohsh KeenenNo ratings yet
- 5th Grade Synonyms 2Document2 pages5th Grade Synonyms 2Blohsh KeenenNo ratings yet
- Organic Chemistry Worksheet Part 2Document4 pagesOrganic Chemistry Worksheet Part 2Blohsh KeenenNo ratings yet
- Generate Expressions 1Document2 pagesGenerate Expressions 1Blohsh KeenenNo ratings yet
- 5th Grade Synonyms 3Document2 pages5th Grade Synonyms 3Blohsh KeenenNo ratings yet
- 5th Grade Synonyms 6Document2 pages5th Grade Synonyms 6Blohsh KeenenNo ratings yet
- Algebra Worksheet 2Document6 pagesAlgebra Worksheet 2Blohsh KeenenNo ratings yet
- 5th Grade Synonyms 5Document2 pages5th Grade Synonyms 5Blohsh KeenenNo ratings yet
- Solve Equations Sheet 3 AnswersDocument2 pagesSolve Equations Sheet 3 AnswersBlohsh KeenenNo ratings yet
- Generate Expressions 2Document2 pagesGenerate Expressions 2Blohsh KeenenNo ratings yet
- Solve Equations Sheet 3 AnswersDocument2 pagesSolve Equations Sheet 3 AnswersBlohsh KeenenNo ratings yet
- Algebra Worksheet 3Document6 pagesAlgebra Worksheet 3Blohsh KeenenNo ratings yet
- Algebra Worksheet 1Document7 pagesAlgebra Worksheet 1Blohsh KeenenNo ratings yet
- Algebra Worksheet SolvedDocument6 pagesAlgebra Worksheet SolvedBlohsh KeenenNo ratings yet
- Generate Expressions 3Document2 pagesGenerate Expressions 3Blohsh KeenenNo ratings yet
- Chemistry Notes Pt. 2Document124 pagesChemistry Notes Pt. 2Blohsh KeenenNo ratings yet
- Biology Notes Unit TwoDocument158 pagesBiology Notes Unit TwoBlohsh KeenenNo ratings yet
- Clase de EspañolDocument4 pagesClase de EspañolBlohsh KeenenNo ratings yet
- Biology Planning 0 Design LabDocument1 pageBiology Planning 0 Design LabBlohsh KeenenNo ratings yet
- Biology NotesDocument161 pagesBiology NotesBlohsh KeenenNo ratings yet