Professional Documents
Culture Documents
Coated Cotton Gauze With Ag/Zno/Chitosan Nanocomposite As A Modern Wound Dressing
Uploaded by
Rishi UpadhyayOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Coated Cotton Gauze With Ag/Zno/Chitosan Nanocomposite As A Modern Wound Dressing
Uploaded by
Rishi UpadhyayCopyright:
Available Formats
Coated Cotton Gauze with Ag/ZnO/chitosan
Nanocomposite as a Modern Wound Dressing
Mina Abbasipour1, Mohammad Mirjalili, PhD1, Ramin Khajavi, PhD2, Mohammad Mehdi Majidi, PhD1
1
Department of Textile Engineering, Yazd Branch, Islamic Azad University, Yazd IRAN
2
Department of Textile Engineering, South Tehran Branch, Islamic Azad University, Tehran IRAN
Correspondence to:
Mina Abbasipour email: abbasipour@iauyazd.ac.ir
ABSTRACT
Cotton gauze is one of the most successful wound Combining new materials, better design and complex
dressings which utilize the intrinsic properties of manufacturing are needed for achieving these goals
cotton fibers. Modern wound dressings, however, [3].
require other properties such as antibacterial and
moisture maintaining capabilities. In this study, Recently, many researchers have focused on the use
conventional cotton gauze was treated with of biological materials like chitin and chitosan [4-6].
chitosan/Ag/ZnO nanocomposite for achieving Chitosan as a biomaterial, has a great potential in
modern wound dressing properties. Cotton gauze wound healing and skin burns and regenerates normal
samples were impregnated with chitosan/Ag/ZnO skin [7, 8]. It is an antibacterial, nontoxic,
nanocomposite by the dip, dry, and cure method. biodegradable, and biocompatible polymer [9-11].
Samples were characterized by scanning electron Chitosan, along with other antibacterial materials has
microscopy (SEM), Fourier transform infrared attracted much attention during the last few years.
spectroscopy (FTIR) and UV-Vis reflective For instance, its combination with other inorganic
spectroscopy (UV-Vis); and their water absorbency agents such as Ag, Zn, CuO, TiO2 and Fe has been
ability, wicking effect, and antibacterial activity reported [12-16]. Among them, chitosan/Ag/ ZnO
determined. The results show that coated cotton nanocomposite had significantly higher antibacterial
gauze with chitosan/Ag/ZnO nanocomposite has activity than chitosan/Ag and chitosan/ZnO blend
increased drying time (78%) and water absorbency films. It was indicated that Ag and ZnO enhanced the
(38%). Furthermore, their antibacterial efficiency was antibacterial activities of chitosan [14].
96% for Escherichia coli (E. Coli) and 99% for
Staphylococcus aureus (S. aureus). The anti-bacterial and multi-functional properties of
silver nanoparticles have also been investigated [17,
Keywords: Cotton Gauze; wound Dress, Chitosan, 18]. Products containing silver nanoparticles have the
Ag, ZnO, Nanocomposite ability to exert bactericidal effects and are less
harmful to human cells than other toxic organic
INTRODUCTION antimicrobial agents [12, 19]. Tiam et al [20] found
In recent years, wound dressing has evolved that Ag nanoparticles not only have antibacterial
according to the pathogenesis of different wounds. activity, but also have wound healing properties. It
Different types of wound dressing including xerogels, can restore burnt skin to the normal skin.
charcoal cloth, alginates, chitosan, and hydrogels
have been introduced for this purpose through the last Zinc is normally required for a variety of enzymatic
decades [1]. An ideal wound dressing has the ability and cellular activities. But because of its excellent
to maintain moisture, act against microorganisms, be anti-inflammatory, drying, mild astringent and
nontoxic, non-adherent, and promote wound healing antiseptic properties, it plays a major role in wound
[2]. Modern wound dressing theory, suggests healing, especially from burns. It also supports the
promoting dynamic equilibrium between exudate demand for volume increases during the wound
absorption and optimal surface moisture at the wound healing process. For that purpose, Zinc oxide is
surface. In addition, it should be able to exchange gas widely used in skin creams. This cream can prevent
to provide the wound with sufficient oxygen tension. sunlight and ultraviolet rays from penetrating the skin
Journal of Engineered Fibers and Fabrics 124 http://www.jeffjournal.org
Volume 9, Issue 1 – 2014
and can enhance the wound healing process by (=4.8±0.1 cm) were incubated at 120 ◦C for 15 min.
delivering zinc ions to the wound and allowing them Bacterium was placed in 5 ml nutrient broth and
to remain there for an extended period of time [21]. incubated at 37 ◦C for 24 hrs. Agar Petri dishes were
Although proposed, the theory is that zinc accelerates inoculated with grown bacterium. Treated (with
re-epithelialization of the skin; the exact mechanism chitosan, chitosan/Ag, chitosan/ZnO and
is really not known [22-24]. chitosan/Ag/ZnO) and untreated samples were gently
pressed in the centre of media culture. The plates
In this study, conventional cotton gauze was coated were incubated at 37°C for 24 hours and zone of
with a nano emulsion of chitosan/Ag/ZnO to benefit inhibition observed [25, 26].
from the supreme properties of Ag, ZnO
nanoparticles and chitosan, such as prevention of AATCC-100-1998:
embedded nanoparticles agglomerations, beside the Quantitative Test-Percentage Reduction Test
intrinsic properties of cotton fibers. Some essential In quantitative (AATCC-100-1998) test method
factors of modern wound dressings like water samples (=4.8±0.1 cm) were incubated at 120°C for
absorbency, drying time (water holding time) and the 15 min [27]. The bacterial culture was grown using
amount of vertical wicking were determined for the same method as mentioned. The sterile samples
different treated samples and were compared with were placed in a 250 ml glass jar and the samples
untreated samples. were dipped in 1ml bacteria with 1000cfu/ml
concentration and were incubated at 37°C for 24
EXPERIMENTAL hours. 100 ml of sterilized distilled water was added
Materials into the jar and then shacked continuously for one
High molecular weight chitosan (DS=75%), silver min. 1 ml of solution was diluted and was placed to
nanoparticles (average size of <150 nm) were in 25 ml of nutrient agar and incubated at 37°C for 24
purchased from Sigma Aldrich Co., Ltd (USA). Zinc hours. Colonies of bacteria, recovered on the agar
oxide nanoparticles, (average particle size ≈ 20 nm) plate, were counted and the reduction percentage of
were provided by Nano Pars Lima Co., Ltd. (Iran). bacteria (R) was calculated by the Eq. (1):
Escherichia coli (ATCC 6538) and Staphylococcus
aureus (ATCC 4157) were prepared by R (%) = (B – A) ×100/ B (1)
Microorganism Lab of Azad University.
Where A is the number of bacterial colonies from
Preparation treated specimen after inoculation over 24 hrs contact
Chitosan was dissolved at 0.5% (w/v) with 1% (v/v) period, and B is the number of bacterial colonies
acetic acid and then 5 ml (5% w/v) Ag and 5 ml (5% from untreated control specimen after inoculation.
w/v) ZnO nanoparticles were added to the prepared
solution. The cotton gauze was dipped in the Vertical Wicking Test
CS/Ag/ZnO colloidal solution; dried at 80 ◦C and For measuring water transport rate, the vertical
cured at 160 ◦C for 4 min. wicking test was used [28]. Treated samples with
chitosan/Ag/ZnO were cut (170 mm by 25 mm). One
Characterization end of strip was immersed about 3 mm in water. The
The presence of ZnO nanoparticles was proved by a height of water transported along the strip in different
UV-Vis spectrophotometer (JASCO-ARN-475, UK) times (1, 5 and 10 min) was then measured. Vertical
due to their reflective properties and FTIR spectrum wicking test was evaluated 5 times and the results
(Thermonicolet-Nexus 870, USA) with considering were reported by mean values.
their characteristic peaks. The morphology of the
treated cotton gauze was studied using a Philips Water Holding Time
XL30 scanning electron microscope (SEM) equipped A test method offered [28] by T-PACC (centre for
with Philips-EDAX/DX4 energy-dispersive research on textile protection and comfort) in North
Spectroscope (Netherlands). Carolina State University (NSCU) was used for
measuring drying time of samples. At first, circular
AATCC-147-1998: cuts (=3.5 inch) of samples were prepared and
Qualitative Antibacterial-Agar Diffusion Test: weighed. Then gauzes were wetted with 1cc distilled
Antibacterial proficiency of samples determined water and weighted. The reduced weight of samples
qualitatively (AATCC-147-1998) against a gram was measured every 15 min until the measured
positive organism (Staphylococcus aureus) and a weight equalled to primary weight.
gram negative bacterium (Escherichia coli). In
qualitative antibacterial-agar diffusion test, samples
Journal of Engineered Fibers and Fabrics 125 http://www.jeffjournal.org
Volume 9, Issue 1 – 2014
Water Absorption Test Figure 3 illustrates the EDX spectrum of
The water absorption was determined according to chitosan/Ag/ZnO nanocomposite. As shown in
static immersion test (BS 34491 (1990) standard) for Figure 3, ZnO and Ag elements were identified. This
fabric with high ability of water absorption [29]. The analysis verified that chitosan/Ag/ZnO
water absorption percentage (W) was calculated by nanocomposite was formed. The result agreed with
Eq. (2): the SEM as well (Figure 2b).
W= (M2-M1)/M1 (2)
where M2 is the weight of wet sample, and M1 is the
weight of dry sample.
RESULTS AND DISCUSION
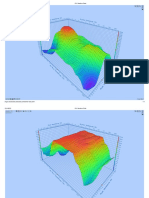
Figure 1 demonstrated the presence of ZnO on cotton
samples. According to Rayleigh’s scattering theory,
presence of nanoparticles with the particle size
between 20 and 40 nm causes wide distribution
around UV region [30].
FIGURE 3. EDX spectrum of chitosan/Ag/ZnO nanocomposite.
Figure 4a and Figure 4b depict the FTIR of chitosan
and chitosan/Ag/ZnO nanocomposite, respectively.
Chitosan FTIR shows absorption peak at 3447 cm-1,
this attributed to the combined peaks of NH2 and OH
group stretching vibration [31]. Compared with
chitosan, the broader and stronger peak moved to
lower wave number at 3390 cm-1 which indicated the
FIGURE 1. UV–vis spectra of the samples: the treated sample with interaction between this group and ZnO [32]. The
chitosan/Ag/ZnO (CAZ), the treated sample with chitosan/Ag absorption peak at 2995 is attributed to asymmetric
(CA), the treated sample with chitosan/ZnO (CZ), the treated stretching oH, CH3 and CH2 of chitosan polymer. The
sample with chitosan and untreated sample.
absorption peaks of 1637 and 1066 belong to bending
vibration of NH2 group and C-O stretching group.
Figure 2 presents SEM photographs of treated
Compared with chitosan, there is new absorption
sample with chitosan/Ag/ZnO and untreated sample
peak at 649 which belong to attachment of amide
and chitosan/Ag/ZnO nanocomposite. From SEM
group and stretching of ZnO [32]. Addition of silver
images the formed coating appears homogeneously
nanoparticles shifted the characteristic peak of amide
on the cotton gauze surface.
1637 from 1652 are ascribed to incorporate of silver
nanoparticles into the nanocomposite [33, 34].
FIGURE 2. SEM photographs of the sample: (a) untreated sample
(b) the treated sample with chitosan/Ag/ ZnO (c) chitosan/Ag/ ZnO
nanocomposite.
Journal of Engineered Fibers and Fabrics 126 http://www.jeffjournal.org
Volume 9, Issue 1 – 2014
FIGURE 5. Overall view of antibacterial test against S. aureus and
E. Coli.
FIGURE 4. FT-IR spectrum (a) chitosan and (b) chitosan/Ag/ZnO
nanocomposite.
Antibacterial Activity
The overall view of antibacterial test against S.
aureus and E. Coli is demonstrated in Figure 5.
FIGURE 6. Zones of inhibition for samples against E. coli: (a)
untreated (b) treated with chitosan (c) treated with chitosan/Ag (d)
The qualitative antibacterial assessments of samples treated with chitosan/ZnO (e) treated with chitosan/Ag/ZnO.
are shown in Figure 6 and Figure 7. As shown in
these figures, the sample treated with
chitosan/Ag/ZnO nanocomposite showed better
antibacterial effects against S. aureus and E. Coli.
Usually antibacterial activity of treated sample
against S.aureus as a gram positive bacterium is
better than E.coli as a gram negative bacterium.
The quantitative antibacterial assessments of samples
are shown in Figure 8 and Figure 9. As shown in
these figures the sample treated with
chitosan/Ag/ZnO nanocomposite showed better
antibacterial effect against S. aureus and E. Coli.
FIGURE 7. Zones of inhibition for samples against S. aureus: (a)
The results of quantitative antibacterial tests (Table I) untreated (b) treated with chitosan (c) treated with chitosan/Ag (d)
show that the treated sterile gauze with treated with chitosan/ZnO (e) treated with chitosan/Ag/ZnO.
chitosan/Ag/ZnO nanocomposite has a 99%
reduction for S. aureus and a 96% reduction for
E.coli. The other treated samples revealed a lower
percentage of reduction against S. aureus.
FIGURE 8. Quantitative (ATCC-100) test against E. Coli (a)
untreated (b) treated with chitosan/Ag/ZnO.
Journal of Engineered Fibers and Fabrics 127 http://www.jeffjournal.org
Volume 9, Issue 1 – 2014
significant difference between untreated cotton gauze
and treated one is at level of 95%. The average water
holding ability of treated gauze is 78 percent higher
than untreated ones. Holding capability of liquid
especially water is a fundamental factor for healing
the wounds [28].
TABLE III. Average drying times (water holding ability) of wet
samples.
FIGURE 9. Quantitative (ATCC-100) test against S. aureus (a) Sample type Mean time of drying (min)a
untreated (b) treated with chitosan/Ag/ZnO. Untreated 41.31
Treated with chitosan/Ag/ZnO 73.60
TABLE I. Number of colonies and their reduction percentage a
Each test was repeated five times and the mean reported
(R%) on samples.
Number of R Number R
Water Absorption Test
Sample Type S.aureus (%) of E.coli (%) The water absorption percentages of samples are
colonies colonies shown in Table IV. The difference between untreated
Untreated 1000 > 0 1000 > 0 cotton gauze and treated one is statistically
Treated with
500 50 700 30
significant (Pvalue=0). Comparing data shows that
chitosan capability in water absorption of cotton gauze had
Treated with
30 97 300 70 increased about 38 percent.
chitosan/ZnO
Treated with
2 99 250 75
When the spaces of cotton gauze between wraps and
chitosan/Ag wefts are filled with chitosan, due to channel-like
Treated with structure of chitosan [35], the number of capillaries
1 99 40 96 of cotton gauze increased in both weft and wrap
chitosan/Ag/ZnO
directions. Therefore, vertical wicking and water
Vertical Wicking Test absorption increased. This ability is highlighted for
The vertical wicking ability of treated sample with water, wound exudates, and liquid drug absorption of
chitosan/Ag/ZnO and untreated one were measured a wound dressing [29].
three different times. Each time, the test was repeated
TABLE IV. Water absorption (static immersion test) of samples.
five times and the mean value reported in Table II.
The results show that the wicking ability of treated Sample Type Mean percent of water
gauze and untreated gauze both increased though absorption (%)a
time. The results of "ANoVA" show Pvalue= 0.01 Untreated 293
which is smaller than the significant 0.5 level. So Treated with 407
difference between treated cotton gauze and untreated chitosan/Ag/ZnO
one is statistically significant (95%). a
Each test was repeated five times and the mean reported.
TABLE II. Vertical wicking ability of untreated and treated gauzes CONCLUSION
in different times.
The present study shows that treating conventional
Time Untreated Treated gauze with cotton gauze with a nanocomposite of
(min) gauze chitosan/Ag/ZnOa chitosan/Ag/ZnO will improve its wound care ability
toward modern wound dressings. Chitosan, as one of
1 1.63 2.67 the natural polysaccharides, is a nontoxic,
5 3.26 5.16 biodegradable polymer with proper biological
10 4.76 6.02
a
activities. It showed high potential for holding
Each test was repeated five times and for every time the mean
was reported nanoparticles and also for coating cotton gauze. The
emulsion of such nanocomposite can be easily
Water Holding Time applied onto textile fabrics using a simple method
Table III shows average drying time for each sample. like the dip-dry-cure process. Antibacterial efficiency
For this, the differences between untreated cotton values of treated cotton gauze with chitosan/Ag/ZnO
gauze and the treated ones were measured. The data were increased in comparison with each of the
set from "ANOVA" shows that the Pvalue= 0, so the nanoparticles separately indicating a kind of
null hypothesis is refused and there is a statistically synergetic effect for a nanocomposite of
chitosan/Ag/ZnO. Treating cotton gauze with
Journal of Engineered Fibers and Fabrics 128 http://www.jeffjournal.org
Volume 9, Issue 1 – 2014
chitosan/Ag/ZnO nanocomposite also increased [10] Wang, X.; Du, Y.; Fan, L.; Liu, H.; Hu, Y.;
drying time, wicking ability, and water absorbency; Chitosan- Metal Complexes as Antimicrobial
the main indexes of a modern wound dressing. Agent: Synthesis, Characterization and
Structure-activity Study; Polymer Bulletin;
ACKNOWLEDGEMENT 55; 2005; 105–113.
The authors want to express their thanks to Dr. M. [11] Gouda, M.; Keshk, A. S.; Evaluation of
Iman Eini and Dr. B. Shadloo from Tehran Multifunctional Properties of Cotton Fabric
University of medical science and Dr. B. Bakhshi Based on Metal/Chitosan Film; Carbohydrate
from Tarbiat Modares University of medical science Polymers; 80; 2010; 504–512.
for their useful comments. [12] Hu, Z.; Lai, W.; Shan, Y.; Nanocomposite of
Chitosan and Silver Oxide and Its
REFERENCES Antibacterial Property; Journal of Applied
[1] Edwards, J. V.; Future structure and properties Polymer Science; 108; 2008; 52–56.
of mechanism- based wound dressings, [13] Modrzejewska, Z.; Zarzycki, R.; Sielski, J.;
modified fibers with medical and specify Synthesis of silver nanoparticles in a chitosan
applications; chapter 2, 2006: 11-33. solution; progress on chemistry and
[2] Mi, F-L; Wu, Y-B; Shyu, S-S; Schoung, J-Y; application of chitin XV; 2010; 63-72.
Huang, Y-B; Tsai, Y-H; Hao, J-Y; Control of [14] Li, L. H.; Deng, J. C.; Deng, H. R.; Liu, Z. L.;
wound infections using bilayer chitosan Li, X.; Preparation, characterization and
wound dressing with sustainable antibiotic antimicrobial activities of chitosan/Ag/ZnO
delivery; J. Biomed. Mat. Res.; 59; 2002; blend films; Chemical Engineering Journal;
438-449. 160; 2010; 378–382.
[3] Seycher, M.; Lee, S. J.; Modern wound [15] Shi, L.; Zhao, Y.; Zhang, X.; Su, H.; Tan, T.;
dressings: a systematic approach to wound Antibacterial and Anti-mildew Behavior of
healing, journal of biomaterial application; 7; Chitosan/Nano-TiO2 Composite Emulsion;
1992; 142-213. Korean J. Chem. Eng; 25; 2008; 1434-1438.
[4] Jayakumar, R; prabahan, M; Kumar, P.T.; Nair, [16] Yu, D. G.; Formation of Colloidal Silver
S. V.; Furuike, T.; Tamura, H.; Biomaterials Nanoparticles Stabilized by Na+ Poly(-
based on chitin and chitosan in wound glutamic acid)–Silver Nitrate Complex via
dressing applications; Biotechnology Chemical Reduction Process; Colloids and
advances; 29; 2011; 322-337. Surfaces B: Biointerfaces; 59; 2007;171–178.
[5] Karolia, A.; Mendapara, S.; Imparting [17] Dastjerdi, R.; Mojtahedi, M. R. M.; Shoshtari,
antimicrobial and fragrance finish on cotton A. M.; Comparing the Effect of Three
using chitosan with silicon softener; Indian Processing Methods for Modification of
journal of fiber & textiles Research; 32; Filament Yarns with Inorganic
2007; 99-104. Nanocomposite Filler and Their Bioactivity
[6] Knittel, B.; Schollmeyer, E.; Chitosans for Against Staphylococcus aureus;
permanent antibacterial finish on textiles; Macromolecular Research; 17; 2009; 378–
Lenzinger Berrichte; 85; 2006;124-130. 387.
[7] Jayakumar; R.; Menon, D.; Manzoor, K.; Nair, [18] Dastjerdi, R.; Montazer, M.; A review on the
S. V.; Tamura, H.; Biomedical applications of Application of Inorganic Nano-structured
chitin and chitosan based nanomaterials-A Materials in the Modification of Textiles:
short review; Carbohydrate polymers; 82; Focus on Anti-microbial Properties; Colloids
2010; 227-232. and Surfaces B: Biointerfaces; 79; 2010; 5–
[8] Joshi, M.; Ali, S. W.; Purwar, R.; Ecofriendly 18.
antimicrobial finishing of textiles using [19] Castanon, G. A.; Nino-Martinez, N.; Gutierrez,
bioactive agents on natural products; Indian F.; Mendoza, J. R.; Ruiz, F.; Systhesis and
journal of fiber & textile research; 34; 2009; antimicrobial activity of silver nanoparticles
295-304. with different sizes; J Nanopart Res.; 10;
[9] Pillai, C. K. S.; Paul, W.; Sharma. C. P.; Chitin 2008; 1343-1348.
and Chitosan Polymers: Chemistry, Solubility [20] Tiam, J.; Wong, K. K. Y.; Ho, C. M.; Lok, C.
and Fiber Formation; Progress in Polymer N.; Yu, W-Y.; Che, C-M.; Chiu, J-F.; Tam, P.
Science; 34; 2009; 641–678. K. H.; Topical delivery of silver nanoparticles
promotes wound healing; ChemMedChem; 2;
2007;129-136.
Journal of Engineered Fibers and Fabrics 129 http://www.jeffjournal.org
Volume 9, Issue 1 – 2014
[21] Yang, L.; Mao, J.; Xue, T.; Hou, T.; Wang, L.; [32] Abdelhady, A. A.; Preparation and
Tu, M.; preparation and characteristics of characterization of chitosan oxide
Ag/nano ZnO composite antimicrobial agent; nanoparticles for imparting antimicrobial and
nanoscience; 11; 2006; 44-48. UV protection to cotton fabric; International
[22] Jones, B.; Ray, B.; Ranjit, K. T.; Manna, A. C.; journal of carbohydrate chemistry, 243;
Antibacterial activity of ZnO nanoparticles 2012; 1-7.
suspensions on broad spectrum of [33] Govindan, S.; Nivethaa, E. A. K.; Saravanan,
microorganisms; FEMS microbial Lett.; 279; R.; Narayanan, V.; Stephen, A.; Synthesis
2008; 71-76. and characterization of chitosan-silver
[23] Rajendran, R.; Balakumar, C.; Ahammed, H. A. nanocomposite; Appl. Maniocs; 2012; DOI:
M.; Jaykumal, S.; Vaideki, K.; Rajesh, M. R.; 10.1007/s13204-012-0109-5.
Use of zinc oxide nanoparticles for [34] Velmurugan, N.; Kumar, G. G.; Han, S. S.;
production of antimicrobial textiles, Nahm, K. .; Lee, Y. S.; Synthesis and
International journal of engineering & characterization of potential fungicidal silver
science technology; 2; 2010; 202-208. nano-sized particles and chitosan membrane
[24] Kathivelu, S.; Dsouza, L.; Dhurai, B.; Study of containing silver particles, Iranian polymer
antibacterial finishing of textiles using ZnO journal; 18; 2009; 383-392.
nanoparticles; IE(l) journal-TX; 90; 2009; 22- [35] Yang, J. M.; Lin, H. T.; Properties of chitosan
27. containing pp-AA-g-NIPAAM bigraft
[25] Yadav, A.; Prasad.; Kathe, A. A.; Raj, S.; nonwoven fabric for wound dressing; Journal
Yadav, D.; Sundaramoorthy, C.; of membrane science; 243; 2004; 1-7.
Vigneshwaran, N.; Functional finishing in
cotton fabrics using zinc oxide nanoparticles; AUTHORS’ ADDRESSES
Bull. Matter. Sci.; 29; 2006; 641-645. Mina Abbasipour
[26] Veni, k.; Mani, A.; Preparation and Mohammad Mirjalili, PhD
characterization of medicinal herb Ramin Khajavi, PhD
glycyrrhiza glabra and a study of anti- Mohammad Mehdi Majidi, PhD
microbial and thermal properties on cotton Yazd Branch
fabric for eye syndromes; Journal of textile Islamic Azad University
and apparel, technology and management; 7; Safayeh Street
2012; 1-9. Yazd 8916871967
[27] Yazdanshenas, M. E.; Damerchely, R.; Rashidi, IRAN
A. S.; Khajavi, R.; Bioactive nano-composite
multifilament yarns, Journal of engineered
fibers and fabrics, 7; 2012; 69-78.
[28] Meftahi, A.; Khajavi, R.; Rashidi, A.; Sattari,
M.; Yazadanshenas, M. E.; Torbi, M.; The
effect of cotton gauze coating with microbial
cellulose; Cellulose; 17; 2010;199-204.
[29] Montalvo, J. G.; Von Hoven, T. M.; Textile
technology, review of standard test methods
for moisture in lint cotton; The journal of
cotton science; 12; 2008; 33-47.
[30] Wong, Y. W.H.; Yuen, C .W. M.; Leung, M. Y.
S.; Ku, S. K.A.; Lam, H. L. I.; Selected
Applications of Nanotechnology in Textiles;
AUTEX Research Journal; 6; 2006; 1-8.
[31] Slam, M. M. F.; Masum, S. Md.; Rahman, M.
M.; Islam-Molla, A. Md.; Shaikh, A. A.; Roy,
S. K.; Preparation of chitosan from shrimp
shell and investigation of its properties;
International journal of Basic and applied
science; 11; 2011;116-130.
Journal of Engineered Fibers and Fabrics 130 http://www.jeffjournal.org
Volume 9, Issue 1 – 2014
You might also like
- Ijftr 40 (1) 25-30Document6 pagesIjftr 40 (1) 25-30SahibZadaNo ratings yet
- (Autex Research Journal) Single-Step Antimicrobial and Moisture Management Finishing of PC Fabric Using Zno NanoparticlesDocument4 pages(Autex Research Journal) Single-Step Antimicrobial and Moisture Management Finishing of PC Fabric Using Zno NanoparticlesMuhammad Bilal QadirNo ratings yet
- EJCHEM-Volume 62-Issue Special Issue (Part 2) Innovation in Chemistry - Page 797-817Document21 pagesEJCHEM-Volume 62-Issue Special Issue (Part 2) Innovation in Chemistry - Page 797-817esliNo ratings yet
- 03 The Evaluation of Antibacterial Activity of Fabrics Impregnated With Dimethyltetradecyl (3 - (Trimethoxysilyl) Propyl) Ammonium ChlorideDocument8 pages03 The Evaluation of Antibacterial Activity of Fabrics Impregnated With Dimethyltetradecyl (3 - (Trimethoxysilyl) Propyl) Ammonium Chloridedharmayanti976No ratings yet
- Synthesis and Application of Zinc Oxide Nanoparticles On Nylon Fabric by Layer by Layer Technique As Antimicrobial PropertyDocument8 pagesSynthesis and Application of Zinc Oxide Nanoparticles On Nylon Fabric by Layer by Layer Technique As Antimicrobial PropertyÖzkan YaparNo ratings yet
- DSC SericinDocument16 pagesDSC SericinĐặng Minh AnhNo ratings yet
- Tejido Funcionalizado Con AgNps-QuitosanoDocument6 pagesTejido Funcionalizado Con AgNps-QuitosanoDE LA CRUZ PUMA ROSA MARÍANo ratings yet
- Rajendra N 2013Document5 pagesRajendra N 2013Radhai.RNo ratings yet
- Durable multifunctional cotton fabric using in situ nano-ZnODocument12 pagesDurable multifunctional cotton fabric using in situ nano-ZnOSrikrishna NNo ratings yet
- Antibacterial Activity of Cupric Oxide Nanoparticles Against Pathogenic BacteriaDocument4 pagesAntibacterial Activity of Cupric Oxide Nanoparticles Against Pathogenic BacteriaNILTHON FRANCO POMA HUARINGANo ratings yet
- Novel biological scaffold with green nanoparticles for artificial skinDocument7 pagesNovel biological scaffold with green nanoparticles for artificial skinjsofv5533No ratings yet
- Antibacterial Effect of Nanosized Silver Colloidal Solution On Textile FabricsDocument6 pagesAntibacterial Effect of Nanosized Silver Colloidal Solution On Textile Fabricsapi-3733260No ratings yet
- DEVELOPMENT OF DOUBLE CLOTH WITH FUNCTIONAL FINISHESDocument35 pagesDEVELOPMENT OF DOUBLE CLOTH WITH FUNCTIONAL FINISHESBhuva_janaNo ratings yet
- 08 AkibaiyoDocument5 pages08 AkibaiyoDeepak RajendranNo ratings yet
- Agri Sci - Ijasr-Application of Nanoparticles Bio-Synthesized From Asoka - Polyalthia Longifolia - Leaves On Cotton TextilesDocument10 pagesAgri Sci - Ijasr-Application of Nanoparticles Bio-Synthesized From Asoka - Polyalthia Longifolia - Leaves On Cotton TextilesTJPRC PublicationsNo ratings yet
- 2 29 1645708157 2ijtftjun20222Document8 pages2 29 1645708157 2ijtftjun20222TJPRC PublicationsNo ratings yet
- Tela Funcionalizada Con AgNps-pérdida de Efectividad AntibacterianaDocument9 pagesTela Funcionalizada Con AgNps-pérdida de Efectividad AntibacterianaDE LA CRUZ PUMA ROSA MARÍANo ratings yet
- Var WWW HTML CCT HTML Authors 3 3749 3749 2023-09-28 3 1695908592Document13 pagesVar WWW HTML CCT HTML Authors 3 3749 3749 2023-09-28 3 1695908592wjohn6182No ratings yet
- Antimicrobial Finishing On Cotton Fabric With Mint Stem ExtractDocument8 pagesAntimicrobial Finishing On Cotton Fabric With Mint Stem ExtractMohammed Atiqul Hoque ChowdhuryNo ratings yet
- Synthesis and Characterization of Agtio 2 Core Shell Nanoparticles and Study of Its Antibacterial ActivityDocument11 pagesSynthesis and Characterization of Agtio 2 Core Shell Nanoparticles and Study of Its Antibacterial ActivityJean Marco PortalNo ratings yet
- Comparative Evaluation of Antibacterial Efficacy and Effect On Dentin Microhardness of Triple Antibiotic Paste Modified With Nano Zinc Oxide and Nano Titanium DioxideDocument5 pagesComparative Evaluation of Antibacterial Efficacy and Effect On Dentin Microhardness of Triple Antibiotic Paste Modified With Nano Zinc Oxide and Nano Titanium DioxideInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Primary Study of Cellulose Acetate Hollow FiberDocument7 pagesPrimary Study of Cellulose Acetate Hollow FiberUjak KimiaNo ratings yet
- Journalof Material ScienceDocument8 pagesJournalof Material ScienceFahim Bin Abdur RahmanNo ratings yet
- Advanced Powder Technology: M. Sundrarajan, S. Ambika, K. BharathiDocument6 pagesAdvanced Powder Technology: M. Sundrarajan, S. Ambika, K. Bharathitamirat lamaroNo ratings yet
- Functionalization of A Cotton Fabric Surface With Titania Nanosols: Applications For Self-Cleaning and UV-Protection PropertiesDocument6 pagesFunctionalization of A Cotton Fabric Surface With Titania Nanosols: Applications For Self-Cleaning and UV-Protection PropertiesJaime FrancoNo ratings yet
- Synthesis, Characterization and Antibacterial Activity of Aluminium Oxide NanoparticlesDocument4 pagesSynthesis, Characterization and Antibacterial Activity of Aluminium Oxide NanoparticlesArka GhoshNo ratings yet
- Self-Cleaning Cotton FabricsDocument6 pagesSelf-Cleaning Cotton FabricsAliAkbarPamungkasNo ratings yet
- Multifunctional Finishing of Cotton FabricDocument10 pagesMultifunctional Finishing of Cotton FabricJaime GomezNo ratings yet
- 227469-Article Text-553863-1-10-20220630Document7 pages227469-Article Text-553863-1-10-20220630Girmaw YeshanbelNo ratings yet
- Research ArticleDocument11 pagesResearch ArticleInas HafizhahNo ratings yet
- Articulos Tejido EpitelialDocument29 pagesArticulos Tejido EpitelialDariana FigueroaNo ratings yet
- Use of Chitosan in Mosquito Repellent Finishing For Cotton Textiles 2165 8064.1000162Document3 pagesUse of Chitosan in Mosquito Repellent Finishing For Cotton Textiles 2165 8064.1000162Mbonea MbmNo ratings yet
- Antibacterial Activity of Hydrophobic Composite MaDocument7 pagesAntibacterial Activity of Hydrophobic Composite MaHumberto FujitaNo ratings yet
- Effect of Nano Tio Pretreatment On Functional Properties of Cotton FabricDocument6 pagesEffect of Nano Tio Pretreatment On Functional Properties of Cotton FabricIJERDNo ratings yet
- Cinnamic Acid Encapsulation in Chitosan Nanoparticles for Improved Antibacterial ActivityDocument5 pagesCinnamic Acid Encapsulation in Chitosan Nanoparticles for Improved Antibacterial ActivityDella LestariNo ratings yet
- Biopolymer-ZnO NanocompositeDocument12 pagesBiopolymer-ZnO NanocompositeDeepika GuptaNo ratings yet
- Clinical Potential of A Silk Sericin-Releasing Bioactive Wound Dressing For The Treatment of Split-Thickness Skin Graft Donor SitesDocument14 pagesClinical Potential of A Silk Sericin-Releasing Bioactive Wound Dressing For The Treatment of Split-Thickness Skin Graft Donor SitesAgus DniNo ratings yet
- Preparation and Characterization of Microfiltration Membrane Embedded With Silver Nano-ParticlesDocument7 pagesPreparation and Characterization of Microfiltration Membrane Embedded With Silver Nano-ParticlesdahliaNo ratings yet
- Biochemical Engineering JournalDocument7 pagesBiochemical Engineering JournalCici Suci MaulinaNo ratings yet
- 2011 Article 9641Document11 pages2011 Article 9641hrnisaaNo ratings yet
- Microbial Cellulose As Support Material For The Immobilization of Denitrifying BacteriaDocument6 pagesMicrobial Cellulose As Support Material For The Immobilization of Denitrifying BacteriaBoorin DbtNo ratings yet
- J Ijbiomac 2012 04 003Document6 pagesJ Ijbiomac 2012 04 003nezarahayuNo ratings yet
- Effect of Metallic Salts On Dye Ability of Cotton FabricsDocument13 pagesEffect of Metallic Salts On Dye Ability of Cotton Fabricsshunde barasoNo ratings yet
- Zno-Modified Hybrid Polymers As An Antibacterial Finish For TextilesDocument12 pagesZno-Modified Hybrid Polymers As An Antibacterial Finish For TextilesShagun SinhaNo ratings yet
- Antifungal Activity of Cinnamaldehyde Against Candida AlbicansDocument9 pagesAntifungal Activity of Cinnamaldehyde Against Candida Albicanstuanda222No ratings yet
- PublicationDocument6 pagesPublicationrajya lakshmiNo ratings yet
- 1 s2.0 S0141813021000076 MainDocument8 pages1 s2.0 S0141813021000076 MainhawNo ratings yet
- Ewing Dian Setyadi - Artikel Ilmiah - PpjpiDocument17 pagesEwing Dian Setyadi - Artikel Ilmiah - PpjpiewingsetyadiNo ratings yet
- Efficacy of Super-Oxidized Water Fogging in Environmental DecontaminatioDocument5 pagesEfficacy of Super-Oxidized Water Fogging in Environmental DecontaminatioMehrshad GhasemabadiNo ratings yet
- 2502-Article Text-11861-1-10-20211130Document4 pages2502-Article Text-11861-1-10-20211130Anisa RatnasariNo ratings yet
- 2020-Sol-Gel Based Coatings For The Protection of Cultural Heritage TextilesDocument9 pages2020-Sol-Gel Based Coatings For The Protection of Cultural Heritage TextilesVictoria Gonzalez FriasNo ratings yet
- Effects of Salivary or Serum Pellicles On The Candida Albicans Growth and Biofilm Formation On Soft Lining MaterialsDocument12 pagesEffects of Salivary or Serum Pellicles On The Candida Albicans Growth and Biofilm Formation On Soft Lining MaterialsSaravanan ThangarajanNo ratings yet
- 3 Application of Thiourea Polyurethane-Copper Sulfide Composite ForDocument6 pages3 Application of Thiourea Polyurethane-Copper Sulfide Composite ForMervatNo ratings yet
- Zinc Oxide Nano Particles Coating On Polyester Fabric Functionalized Through Alkali TreatmentDocument18 pagesZinc Oxide Nano Particles Coating On Polyester Fabric Functionalized Through Alkali TreatmentAlexander Fabián GNo ratings yet
- A Novel Antibacterial Titania Coating Metal Ion Toxicity and in Vitro Surface ColonizationDocument6 pagesA Novel Antibacterial Titania Coating Metal Ion Toxicity and in Vitro Surface ColonizationkompostoNo ratings yet
- 1286 6225 1 PBDocument10 pages1286 6225 1 PBRassya taniaNo ratings yet
- Antimicrobial Finishing of Cotton Fabrics Based On Gamma Irradiated Carboxymethyl Cellulose Poly Vinyl Alcohol TiO2 NanocompositesDocument9 pagesAntimicrobial Finishing of Cotton Fabrics Based On Gamma Irradiated Carboxymethyl Cellulose Poly Vinyl Alcohol TiO2 NanocompositesMK ChemistNo ratings yet
- AlginatesDocument24 pagesAlginatesClaudia HollausNo ratings yet
- Roadmap to Sustainable Textiles and Clothing: Eco-friendly Raw Materials, Technologies, and Processing MethodsFrom EverandRoadmap to Sustainable Textiles and Clothing: Eco-friendly Raw Materials, Technologies, and Processing MethodsNo ratings yet
- Bacterial Cellulose: Sustainable Material for TextilesFrom EverandBacterial Cellulose: Sustainable Material for TextilesNo ratings yet
- Facette to unveil COLLECTION 2023 at INDIA DESIGN SHOWDocument3 pagesFacette to unveil COLLECTION 2023 at INDIA DESIGN SHOWRishi UpadhyayNo ratings yet
- Land Use Pattern - UttarakhandDocument1 pageLand Use Pattern - UttarakhandRishi UpadhyayNo ratings yet
- Logo ModelDocument1 pageLogo ModelRishi UpadhyayNo ratings yet
- Authorization LetterDocument7 pagesAuthorization LetterRishi UpadhyayNo ratings yet
- Climate GraphDocument14 pagesClimate GraphRishi UpadhyayNo ratings yet
- F GH 2 Xuw 8 T8 LCC 5 FFDocument3 pagesF GH 2 Xuw 8 T8 LCC 5 FFRishi UpadhyayNo ratings yet
- An Hoteliers Perspective Analysis of Eco-Resort Concept: A Study of Sri Lankan Eco ResortsDocument12 pagesAn Hoteliers Perspective Analysis of Eco-Resort Concept: A Study of Sri Lankan Eco ResortsRishi UpadhyayNo ratings yet
- DBAR VerDocument1 pageDBAR VerRishi UpadhyayNo ratings yet
- Course Handout: Amity University RajasthanDocument3 pagesCourse Handout: Amity University RajasthanRishi UpadhyayNo ratings yet
- How To Open Nfo FilesDocument1 pageHow To Open Nfo FilesChiara DavidNo ratings yet
- Ac W4yb45yq4y4Document8 pagesAc W4yb45yq4y4Rishi UpadhyayNo ratings yet
- Course Handout: Amity University RajasthanDocument3 pagesCourse Handout: Amity University RajasthanRishi UpadhyayNo ratings yet
- Delhi Jaipur MR Rishi UpadhyayDocument2 pagesDelhi Jaipur MR Rishi UpadhyayRishi UpadhyayNo ratings yet
- SpiceJet flight booking confirmation for Mahindra KumarDocument2 pagesSpiceJet flight booking confirmation for Mahindra KumarRishi UpadhyayNo ratings yet
- Produced by An Autodesk Educational ProductDocument1 pageProduced by An Autodesk Educational ProductRishi UpadhyayNo ratings yet
- CorelDRAW Graphics Suite X5 With KeygenDocument2 pagesCorelDRAW Graphics Suite X5 With KeygenRishi UpadhyayNo ratings yet
- Subject-Declaration Letter For ANDC-2016Document1 pageSubject-Declaration Letter For ANDC-2016Rishi UpadhyayNo ratings yet
- Resumea FE - Mehak AgarwalDocument3 pagesResumea FE - Mehak AgarwalRishi UpadhyayNo ratings yet
- Department of Urban Planning, Chandigarh Admn.: The Senior Town Planner, U.T. ChandigarhDocument1 pageDepartment of Urban Planning, Chandigarh Admn.: The Senior Town Planner, U.T. ChandigarhRishi UpadhyayNo ratings yet
- Subject-Declaration Letter For ANDC-2016Document1 pageSubject-Declaration Letter For ANDC-2016Rishi UpadhyayNo ratings yet
- Typical Office Ground - Sixth Floor PlanDocument1 pageTypical Office Ground - Sixth Floor PlanRishi UpadhyayNo ratings yet
- Project Report On Export Documentation and Procedure 2Document92 pagesProject Report On Export Documentation and Procedure 2anish_10677953No ratings yet
- SIP Reporfdbsdfgat ProperDocument47 pagesSIP Reporfdbsdfgat ProperRishi UpadhyayNo ratings yet
- The Site Is Located Near GhansoliDocument1 pageThe Site Is Located Near GhansoliRishi UpadhyayNo ratings yet
- Concept Sheet ModelDocument1 pageConcept Sheet ModelRishi UpadhyayNo ratings yet
- Geometric Trapezoid Chevrons Sunburst Motifs Symmetrical Aluminum Stainless Steel Bakelite Chrome Stained Glass Inlays LacquerDocument1 pageGeometric Trapezoid Chevrons Sunburst Motifs Symmetrical Aluminum Stainless Steel Bakelite Chrome Stained Glass Inlays LacquerRishi UpadhyayNo ratings yet
- TedDocument1 pageTedkedungjambalNo ratings yet
- History Notes: Islamic Architecture: Origin and TypesDocument14 pagesHistory Notes: Islamic Architecture: Origin and TypesRishi UpadhyayNo ratings yet
- Pages From (Architecture Ebook) Building Services Handbook-3eargh Ergqrgsg WERGTWet GDocument1 pagePages From (Architecture Ebook) Building Services Handbook-3eargh Ergqrgsg WERGTWet GRishi Upadhyay0% (1)
- Primary Maths Dissertation ExamplesDocument8 pagesPrimary Maths Dissertation ExamplesPaperWritersAlbuquerque100% (1)
- Kitne PakistanDocument2 pagesKitne PakistanAnkurNo ratings yet
- AMS 2750 E Heat Treatment Standards ComplianceDocument3 pagesAMS 2750 E Heat Treatment Standards ComplianceQualidadeTFNo ratings yet
- Sap GlossaryDocument324 pagesSap GlossaryNikos TataliasNo ratings yet
- EnrollNOLA Daily Seat Availability ReportDocument2 pagesEnrollNOLA Daily Seat Availability ReportAdvocateOnlineNo ratings yet
- Mac 2009Document60 pagesMac 2009Ridwan Pramudya100% (1)
- UX5HPDocument2 pagesUX5HPNazih ArifNo ratings yet
- Demolition and excavation worksDocument30 pagesDemolition and excavation worksHafizan Hanafiah100% (3)
- (Oxford Studies in Digital Politics) Jack Parkin - Money Code Space - Hidden Power in Bitcoin, Blockchain, and Decentralisation-Oxford University Press (2020)Document297 pages(Oxford Studies in Digital Politics) Jack Parkin - Money Code Space - Hidden Power in Bitcoin, Blockchain, and Decentralisation-Oxford University Press (2020)berpub0% (1)
- Business Ethics Q3 Mod2 Foundations of The Principles of Business1Document5 pagesBusiness Ethics Q3 Mod2 Foundations of The Principles of Business1Julie CabusaoNo ratings yet
- KORT RENZO C. BESARIO BS NURSING LESSON REVIEWDocument3 pagesKORT RENZO C. BESARIO BS NURSING LESSON REVIEWDummy AccountNo ratings yet
- Manual THT70 PDFDocument54 pagesManual THT70 PDFwerterNo ratings yet
- Roke TsanDocument53 pagesRoke Tsanhittaf_05No ratings yet
- Basics of ECG Recording GuideDocument21 pagesBasics of ECG Recording GuideghoziNo ratings yet
- Owner'S Manual: Dell Poweredge T110 Ii SystemsDocument130 pagesOwner'S Manual: Dell Poweredge T110 Ii SystemsDonNo ratings yet
- 3 Huang2015Document10 pages3 Huang2015kikoNo ratings yet
- Communalism in India - Causes, Incidents and MeasuresDocument5 pagesCommunalism in India - Causes, Incidents and Measures295Sangita PradhanNo ratings yet
- 40 Inventive Principles Applied to Service OperationsDocument16 pages40 Inventive Principles Applied to Service Operationssina yadegariNo ratings yet
- Jumping EventsDocument12 pagesJumping EventsPrecious Khyla SantosNo ratings yet
- Festivals WorksheetDocument8 pagesFestivals WorksheetlurdesNo ratings yet
- Implementing Cisco Application Centric Infrastructure: (Dcaci)Document2 pagesImplementing Cisco Application Centric Infrastructure: (Dcaci)radsssssNo ratings yet
- Michigan English TestDocument22 pagesMichigan English TestLuisFelipeMartínezHerediaNo ratings yet
- Ground Penetrating Radar For Locating Buried Utilities: Operation ManualDocument75 pagesGround Penetrating Radar For Locating Buried Utilities: Operation ManualArmando BiringkanaeNo ratings yet
- 3rd Simposium On SCCDocument11 pages3rd Simposium On SCCNuno FerreiraNo ratings yet
- AJPMenu Bar MCQDocument8 pagesAJPMenu Bar MCQAkNo ratings yet
- Types of FlowersDocument3 pagesTypes of FlowersAurea Jasmine DacuycuyNo ratings yet
- Personal Information SheetDocument4 pagesPersonal Information SheetLenny PangNo ratings yet
- Lucky TextileDocument5 pagesLucky TextileSaim Bin RashidNo ratings yet
- Macalloy Corporate Brochure September 2018 LR PDFDocument12 pagesMacalloy Corporate Brochure September 2018 LR PDFsampathkumarNo ratings yet
- DBR Gensets DPL120UK Installation, Operation and Maintenance Manual For YN 513510 (MANUAL-I - 2958658 - 1 - A) - 1Document282 pagesDBR Gensets DPL120UK Installation, Operation and Maintenance Manual For YN 513510 (MANUAL-I - 2958658 - 1 - A) - 1RaymondNo ratings yet