Professional Documents
Culture Documents
General Chemistry 2E2
Uploaded by
ronneil joy obina0 ratings0% found this document useful (0 votes)
8 views1 pageThis document provides instructions for two exercises on significant figures and calculations. The first exercise asks students to identify the number of significant figures in 20 different numbers. The second exercise provides 14 calculation problems and asks students to calculate the answer to the appropriate number of significant figures. The exercises are meant to help students practice skills in identifying significant figures and performing calculations while maintaining the correct number of significant figures.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document provides instructions for two exercises on significant figures and calculations. The first exercise asks students to identify the number of significant figures in 20 different numbers. The second exercise provides 14 calculation problems and asks students to calculate the answer to the appropriate number of significant figures. The exercises are meant to help students practice skills in identifying significant figures and performing calculations while maintaining the correct number of significant figures.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
8 views1 pageGeneral Chemistry 2E2
Uploaded by
ronneil joy obinaThis document provides instructions for two exercises on significant figures and calculations. The first exercise asks students to identify the number of significant figures in 20 different numbers. The second exercise provides 14 calculation problems and asks students to calculate the answer to the appropriate number of significant figures. The exercises are meant to help students practice skills in identifying significant figures and performing calculations while maintaining the correct number of significant figures.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
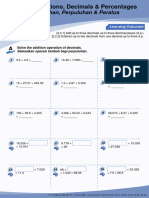
General Chemistry 2
Exercise
I. How many significant figures do the following numbers have?
1. 956 11. 3670000
2. 2.1390 12. 0.0000620
3. 4390 13. 96
4. 0.500 14. 678.02400
5. 500 15. 30000
6. 5.9 × 104 16. 0.002
7. 0.40001 17. 91630
8. 1.7 × 10−3 18. 0.000400
9. 650. 19. 6.0
10. 4.150 × 10−4 20. 352
II. Calculate the answer to the appropriate number of significant figures.
1. 32.567 + 135.0 + 1.4567 =
10. 0.2 / 0.0005 =
2. 246.24 + 238.278 + 98.3 =
11. 7.895 + 3.4 =
3. 658.0 + 23.5478 + 1345.29 =
12. 350.0 – 200 =
4. 23.7 × 3.8 =
13. 27.68 – 14.369 =
5. 45.76 × 0.25 =
11.34−9.63
14. ( ) × 100.0 =
11.34
6. 81.04 × 0.010 =
2.0265−2.02
7. 6.47 × 64.5 = 15. ( 2.0265
) × 100.0 =
8. 0.005 – 0.0007 =
9. 7.895 / 34 =
You might also like
- Significant Digits PracticeDocument1 pageSignificant Digits PracticemarleighNo ratings yet
- Decimal MultiplyDocument3 pagesDecimal Multiplyapi-3705412840% (1)
- Bryce Tuesday Sept 27Document10 pagesBryce Tuesday Sept 27Teacher MaedelNo ratings yet
- PT Assignment Question 1Document6 pagesPT Assignment Question 1Pheletso Andrias MoloantoaNo ratings yet
- WS - Significant FiguresDocument3 pagesWS - Significant FiguresJuan Nicolás Vizcaya MolinaNo ratings yet
- Maths Worksheet (Answer Key)Document2 pagesMaths Worksheet (Answer Key)Linh HaNo ratings yet
- t2 M 1250 Multiplying and Dividing Decimals by 10 100 1000 Activity Sheets - Ver - 5Document6 pagest2 M 1250 Multiplying and Dividing Decimals by 10 100 1000 Activity Sheets - Ver - 5Choy Mun WeiNo ratings yet
- WorksheetsDocument25 pagesWorksheetschanyuanwang1No ratings yet
- 1 Review and Applications of Basic Mathematics: Exercise 1.1Document9 pages1 Review and Applications of Basic Mathematics: Exercise 1.1ivonneNo ratings yet
- Chapter 9 Study GuideDocument2 pagesChapter 9 Study Guideapi-299844682No ratings yet
- MultdeciDocument1 pageMultdeciapi-69947919No ratings yet
- RS Agarwal Decimal Fractions Chapter - UPSCjob PDFDocument5 pagesRS Agarwal Decimal Fractions Chapter - UPSCjob PDFdeepu4303100% (1)
- Performance Task 2.1Document1 pagePerformance Task 2.1actdesquitadoNo ratings yet
- Decimal Fractions: Important Facts and FormulaeDocument12 pagesDecimal Fractions: Important Facts and FormulaeVivek KumarNo ratings yet
- WS - Significant FiguresDocument3 pagesWS - Significant FiguresCheap GoatsNo ratings yet
- Grade 6 Multiply Two Decimals 0 4 Decimal Digits Column CDocument2 pagesGrade 6 Multiply Two Decimals 0 4 Decimal Digits Column CPrincess SerratoNo ratings yet
- Math KSSR Weekly Practice Y4-Week11Document2 pagesMath KSSR Weekly Practice Y4-Week11WK CheeNo ratings yet
- Mathrathon - DecimalsDocument3 pagesMathrathon - DecimalsKervin Fernandez SegarraNo ratings yet
- Math KSSR Weekly Practice Y4-Week12Document2 pagesMath KSSR Weekly Practice Y4-Week12WK CheeNo ratings yet
- Find The Value of Each of The Following and State Your Answer in Standard Form. 1 2Document6 pagesFind The Value of Each of The Following and State Your Answer in Standard Form. 1 2kym poonNo ratings yet
- PHYSICS1Document2 pagesPHYSICS1Bernadette PitogoNo ratings yet
- Assignment 1Document4 pagesAssignment 1Bhavik BohraNo ratings yet
- Significant Figures Multiplication and DivisionDocument1 pageSignificant Figures Multiplication and DivisionErcan KizilbogaNo ratings yet
- 3 ChapDocument5 pages3 Chapsameermohanty6369No ratings yet
- Table A-4: Properties of Saturated LiquidsDocument13 pagesTable A-4: Properties of Saturated Liquidsكرار عبدالحسين قاسم مسائي 23 BNo ratings yet
- Apparatus and MaterialDocument9 pagesApparatus and Materialamiruddin sharifNo ratings yet
- Pozo 3Document5 pagesPozo 3ania olsuuarNo ratings yet
- Grade 5 Adding Decimals 3 Digit ADocument2 pagesGrade 5 Adding Decimals 3 Digit ATammam AlelaiwiNo ratings yet
- Chem Lab 8Document4 pagesChem Lab 8Norayr GulumainNo ratings yet
- Graficas (Recuperado)Document7 pagesGraficas (Recuperado)Luis GuerreroNo ratings yet
- Assignment On Chapter 11Document3 pagesAssignment On Chapter 11SarveshSailNo ratings yet
- Graficas (Recuperado)Document7 pagesGraficas (Recuperado)Luis GuerreroNo ratings yet
- 1.2 Intuitive Limits CWDocument3 pages1.2 Intuitive Limits CWmia samperNo ratings yet
- 1000c+ Sin (/2) : 5.117× 10 Mol CMDocument4 pages1000c+ Sin (/2) : 5.117× 10 Mol CMkasun1237459No ratings yet
- 1630442094approximations Assignments 01Document4 pages1630442094approximations Assignments 01sabir aliNo ratings yet
- E1 SolutionDocument8 pagesE1 Solution66 SB jay gotiNo ratings yet
- Experiment: Elasticity of CantileverDocument8 pagesExperiment: Elasticity of CantileverHarshul SingalNo ratings yet
- RectDocument2 pagesRectMacedo S OliveiraNo ratings yet
- Decimals Multiplication xx02 Various Hundredths 001.1516225812Document2 pagesDecimals Multiplication xx02 Various Hundredths 001.1516225812Anonymous jHgxmwZNo ratings yet
- 0 1 and 0 01 SOLUTIONSDocument1 page0 1 and 0 01 SOLUTIONS412160120No ratings yet
- Standard Form WorksheetDocument1 pageStandard Form WorksheetBenedictAnthonyHughGilkesNo ratings yet
- Trayectoria: Y Vs XDocument6 pagesTrayectoria: Y Vs XAlvaro HernandezNo ratings yet
- Example 11.3-2Document3 pagesExample 11.3-2shella168No ratings yet
- Multiplying Powers of TenDocument1 pageMultiplying Powers of TensedfhjoklkNo ratings yet
- Decimal Operations 2 MrsDocument2 pagesDecimal Operations 2 MrsLynn DeLeonNo ratings yet
- OblongDocument3 pagesOblongMacedo S OliveiraNo ratings yet
- Dividing Decimals by 10, 100 & 1,000Document2 pagesDividing Decimals by 10, 100 & 1,000WanJingNo ratings yet
- Grade 6 Multiplying Decimals by 10 100 or 1000 eDocument2 pagesGrade 6 Multiplying Decimals by 10 100 or 1000 eD MacNo ratings yet
- Decimal Operations 2Document1 pageDecimal Operations 2Ampedio Jr SulpotNo ratings yet
- Decimal Operations 2Document1 pageDecimal Operations 2Jacob Kap Khan KhualNo ratings yet
- CHM256 C2 PDFDocument65 pagesCHM256 C2 PDFDanial JuniorNo ratings yet
- Stat Is TikaDocument9 pagesStat Is TikamirantiNo ratings yet
- 1A, 1B, GraficasDocument4 pages1A, 1B, GraficasLazo DayelyNo ratings yet
- Mental Maths ActivityDocument1 pageMental Maths ActivitygovendervenoNo ratings yet
- Prealgebra 4Th Edition Tom Carson Solutions Manual Full Chapter PDFDocument36 pagesPrealgebra 4Th Edition Tom Carson Solutions Manual Full Chapter PDFcarriboo.continuo.h591tv100% (7)
- Prealgebra 4th Edition Tom Carson Solutions ManualDocument15 pagesPrealgebra 4th Edition Tom Carson Solutions Manualletitiaesperanzavedhd100% (29)
- 1st Worksheet BM 2019Document3 pages1st Worksheet BM 2019Aira LeeNo ratings yet
- Mathematics: Quarter 2 - Module 11: Dividing Decimal With Up To 2 Decimal PlacesDocument9 pagesMathematics: Quarter 2 - Module 11: Dividing Decimal With Up To 2 Decimal PlaceszytwnklNo ratings yet
- New Microsoft Excel WorksheetDocument2 pagesNew Microsoft Excel WorksheetTousif MahmoodNo ratings yet
- (Week 2) (Pre-Calculus) Practice Set 1 - Classifying Equations As ConicsDocument3 pages(Week 2) (Pre-Calculus) Practice Set 1 - Classifying Equations As Conicsronneil joy obinaNo ratings yet
- Reviewe1 1Document3 pagesReviewe1 1ronneil joy obinaNo ratings yet
- X LegDocument1 pageX Legronneil joy obinaNo ratings yet
- MathematicsDocument2 pagesMathematicsronneil joy obinaNo ratings yet
- DOCTYPE htm1Document2 pagesDOCTYPE htm1ronneil joy obinaNo ratings yet
- General Chemistry 2 ExerciseDocument2 pagesGeneral Chemistry 2 Exerciseronneil joy obinaNo ratings yet
- Statistics and ProbabilitHT1Document4 pagesStatistics and ProbabilitHT1ronneil joy obinaNo ratings yet
- General Chemistry 2E1Document2 pagesGeneral Chemistry 2E1ronneil joy obinaNo ratings yet
- UntitledDocument1 pageUntitledronneil joy obinaNo ratings yet
- Basiccalculuse3 1Document5 pagesBasiccalculuse3 1ronneil joy obinaNo ratings yet
- Basiccalculus E2Document1 pageBasiccalculus E2ronneil joy obinaNo ratings yet
- Basic Calculus Exercise I. Limit of A Function: Complete The Table. 1. 2 5 3Document4 pagesBasic Calculus Exercise I. Limit of A Function: Complete The Table. 1. 2 5 3ronneil joy obinaNo ratings yet